Back to Journals » Journal of Pain Research » Volume 13
Electroacupuncture Inhibits Hyperalgesia by Alleviating Inflammatory Factors in a Rat Model of Migraine
Authors Zhao L , Liu L, Xu X, Qu Z , Zhu Y, Li Z, Zhao J, Wang L, Jing X, Li B
Received 31 July 2019
Accepted for publication 24 December 2019
Published 13 January 2020 Volume 2020:13 Pages 75—86
DOI https://doi.org/10.2147/JPR.S225431
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Luopeng Zhao, 1, 2,* Lu Liu, 1, 3,* Xiaobai Xu, 1 Zhengyang Qu, 1 Yupu Zhu, 1 Zhijuan Li, 1 Jingxia Zhao, 2 Linpeng Wang, 1 Xianghong Jing, 3 Bin Li 1
1Acupuncture and Moxibustion Department, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Key Laboratory of Acupuncture Neuromodulation, Beijing, People’s Republic of China; 2Beijing Key Laboratory of Clinic and Basic Research with Traditional Chinese Medicine on Psoriasis, Beijing Institute of Traditional Chinese Medicine, Beijing, People’s Republic of China; 3Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Li; Xianghong Jing Email [email protected]; [email protected]
Objective: Acupuncture has a therapeutic effect similar to that of prophylactic drugs and can be considered a treatment option for migraineurs. However, the mechanism of acupuncture treatment’s effect on migraine is uncertain. An approach based on anti-inflammatory effects is an important treatment strategy for migraine because non-steroidal anti-inflammatory drugs (NSAIDs) are usually used during migraine attacks. Meningeal inflammation is thought to be responsible for the activation of the trigeminovascular system. Our previous study found that electroacupuncture (EA) decreased neurogenic inflammation mediator expression in the trigeminal ganglion (TG) and alleviated hyperalgesia. The present study examined whether EA would inhibit hyperalgesia by alleviating neurogenic inflammatory factors.
Methods: A rat model of migraine was established using dural electrical stimulation (DES). Five groups were analyzed in this study. The Model group received DES three times to mimic migraine attacks, a Control group had sham DES, and three groups received electroacupuncture after DES: a Non-Acu group at a non-acupuncture point, a GB20 group at GB20, and a GB20/34 group at GB20 and GB34 acupuncture points. We evaluated mechanical hyperalgesia using an electronic von Frey esthesiometer in the awake state. After sacrifice, the dura mater was analyzed using immunofluorescence. Serum calcitonin gene-related peptide, cyclooxygenase-2, brain-derived neurotrophic factor, IL-1β, IL-6, and TNF levels were determined using enzyme-linked immunosorbent assays to evaluate the anti-inflammatory effect of acupuncture.
Results: After repeated DES, we observed facial and hind paw mechanical hyperalgesia, which was inhibited by electroacupuncture. Electrical stimulation increased the number of mast cells and macrophages and serum levels of inflammatory factors. GB20 and GB20/34 electroacupuncture significantly decreased the number of mast cells and macrophages and serum levels of inflammatory factors. Moreover, electroacupuncture at GB20/34 was superior to that at GB20 alone in inhibiting hyperalgesia and alleviating inflammatory factors.
Conclusion: Electroacupuncture inhibits DES-induced hyperalgesia by alleviating inflammatory factors. Inhibition of dural mast cells, macrophages, and serum inflammatory factors may be one of the mechanisms involved in acupuncture treatment’s effect on migraine.
Keywords: migraine, acupuncture, GB20, GB34, neurogenic inflammation, hyperalgesia, inflammatory
Introduction
Migraine, a recurrent headache disorder, is frequently associated with nausea, vomiting, or increased light/sound sensitivity.1 It is not just an episodic disorder, with some migraineurs experiencing headaches very frequently, and 2% of episodic migraine patients transform into chronic migraine patients.2,3 Insufficient acute treatment efficacy is closely related to an increased risk of developing chronic migraine.4 Therefore, the purpose of treatment should be to not only relieve pain and improve patients’ ability to function, but also to prevent disease progression to chronic migraine.5 Although pharmacologic treatment is the mainstay of treatment of migraine, many problems, such as medication contraindications, limited response to medications, side effects, and acute medication overuse, are problematic. Non-pharmacological approaches may potentially offer an alternative for migraineurs who hope to avoid the side effects of pharmacological therapies.
It is well known that acupuncture, a non-pharmacological treatment, has been used worldwide for pain relief as one of the complementary treatments. A Cochrane meta-analysis study of acupuncture and migraine prophylaxis indicated that acupuncture has a similar therapeutic effect to that of prophylactic drugs and can be considered a treatment option for patients.6 However, the mechanism of acupuncture treatment’s effect on migraine is uncertain and there is a need for further elucidation. Previous studies indicated acupuncture could exert its therapeutic effect via an anti-inflammatory effect.7 Acupuncture could alleviate migraine by decreasing matrix metalloproteinase-2 activity and modulating inflammatory mediators by affecting the CB1 receptor.8 Our previous study found that EA at the GB20 acupoint decreases calcitonin gene-related peptide (CGRP) expression in the trigeminal ganglion (TG) and alleviates electronic stimulation-induced hyperalgesia.9 However, the effect of acupuncture on CGRP-mediated neurogenic inflammation has not been studied.
Meningeal inflammation is thought to be largely responsible for migraine pain, which is the result of multiple neuromodulators and inflammatory factors. CGRP is classified as the most important neuromodulator in this process.10 CGRP not only dilates the intracranial vessels but also mediates neuroinflammation by mediating the release of various neuroinflammatory factors. In the context of inflammation, CGRP released from primary sensory nerve terminals could activate peripheral target cells, such as mast cells, immune cells, and vascular smooth muscle cells, producing pro-inflammatory mediators.11 Previous clinical observations have shown that increased serum levels of related neuromodulators, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNFα), during migraine attacks.12 In parallel, numerous animal studies have shown that exogenous IL-1β, IL-6, and TNFα can sensitize the nociceptors in animal models.12 This idea that migraine is an inflammatory disorder is supported by the efficacy of non-steroidal anti-inflammatory drugs (NSAIDs) in migraine therapy and the increased intracranial levels of inflammatory mediators during migraine attacks.13,14 In addition, persistent inflammation will sensitize meningeal nociceptors, resulting in sensitization.15 Therefore, anti-inflammatory therapy is an important treatment strategy for migraine treatment.
We hypothesized that EA would alleviate hyperalgesia by decreasing neurogenic inflammatory factors in a migraine rat model. To test this hypothesis, a previously used model was used to induce recurrent migraine attack and hyperalgesia.9 We then evaluated mechanical hyperalgesia using an electronic von Frey esthesiometer; analyzed dural immune cells using immunofluorescence; and measured CGRP, cyclooxygenase-2 (COX-2), brain-derived neurotrophic factor (BDNF), IL-1β, IL-6, and TNFα levels in jugular vein blood using an enzyme-linked immunosorbent assay (ELISA) to evaluate the anti-inflammatory effect of acupuncture in a model of migraine. Moreover, we also evaluated the effect of EA at GB20 and GB34 in comparison to EA at GB20 alone.
Materials and Methods
Animals
All experimental procedures in this study were performed in accordance with the International Association for the Study of Pain guidelines and Guideline for the Care and Use of Laboratory Animals (State Council of China, 2013). This experiment was approved by the Beijing Institutional Review Board for Animal Experiments (Use Committee of Capital Medical University, Beijing; Approval number: AEEI 2015–075). All surgeries were conducted under anesthesia and every effort was made to minimize animal suffering.
Fifty male, pathogen-free Sprague Dawley rats, weighing 200±10 g, were utilized in this study. Rats were individually maintained in a climate-controlled laboratory environment (room temperature, 21 ± 2°C; humidity, 40–70%) on a 12 hr light/dark cycle with free access to water and food. The rats were acclimated to the new environment for 1 week before undergoing surgery to implant the electrodes required for electrical stimulation.
Group Assignment
After an acclimation period of seven days, the 50 animals were randomly divided into the following five groups (n = 10 per group): a control group (C), which received electrode implantation without stimulation; a model group (M), which only received electrical stimulation; a GB20 group (GB20), which received EA at GB20 after electrical stimulation; a GB20/34 group (GB20+GB34), which received EA at GB20 and GB34 after electrical stimulation, and a non-acupuncture point electroacupuncture group (NA), which received EA at a distant non-acupuncture point (approximately 10 mm above the iliac crest) after electrical stimulation.16 The experiment lasted for 7 days after the end of recovery. Electrical stimulation was given to the M, GB20, GB20+GB34, and NA groups with a stimulator (YC-2 stimulator; Chengdu Instrument Factory, Chengdu, China) every other day (on days 1, 3, and 5) over a total of three sessions. The GB20, GB20+GB34, and NA groups received electroacupuncture after electrical stimulation every day from day 1 to day 5, over a total of five sessions. A diagram of the experimental protocol is shown in Figure 1.
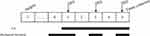 |
Figure 1 Diagram of the experimental protocol. |
Establishment of the Rat Model of Conscious Migraine
As described in a previous study, a pair of electrode fixtures (Beijing Jiandeer, Beijing, China) were fixed into the cranial holes such that they contacted the dura around the superior sagittal sinus.9 Based on previous studies, dural electrical stimuli (DES) consisting of 0.5-ms monophasic square-wave pulses with a pulse duration of 0.5 ms (1.8–2.0 mA, 20 Hz) were given to the rats in the GB20, GB20+GB34, NA, and M groups for a 15 min period every other day over a total of three sessions.17 During the course of stimulation, it was observed that exploratory behavior decreased and there was a significant increase in freezing-like resting and grooming behavior. These behavioral changes suggest success in the model induction. Rats in the control group were connected to the stimulator without stimulation.
EA
Each rat had their movement restricted by a modified tubular textile, which is equivalent to the size of a rat, without causing pain and suffering. The human GB20 is located “in the anterior region of the neck, inferior to the occipital bone, in the depression between the origins of the sternocleidomastoid and trapezius muscles” and GB34 is located “on the fibular aspect of the leg, in the depression anterior and distal to the head of the fibula.”18 The location of GB20 in rats is defined as 3 mm lateral to the midpoint of a line joining the two ears at the back of the head according to a previous study.19 GB34 is located in the depression anterior and distal to the head of the fibula of the rat. For rats in the GB20 group, two stainless steel acupuncture needles (diameter, 0.25 mm; length, 25 mm; Suzhou Medical Appliance Factory, Suzhou, China) were inserted at GB20 bilaterally to a depth of 2–3 mm in the direction of the opposite eye (Figure 2A). The needle handle was then connected to an electrical stimulator (Han’s acupuncture point nerve stimulator HNAS-200E; Nanjing, China) for 15 mins/day. For rats in the GB20+GB34 group, EA was given with a connection to GB20 and GB34 bilaterally (Figure 2B). The EA stimulator was set at a frequency of 2/15 Hz (amplitude-modulated wave) and an intensity of 0.5–1.0 mA without causing the rat to struggle.17 For rats in the NA group, needles were inserted bilaterally at distant non-acupuncture points (approximately 10 mm above the iliac crest) to a depth of 2–3 mm and EA was performed using the same parameters as those in the EA group. Animals in the control and model groups were similarly placed into fixtures for 20 mins, but no acupuncture was performed.
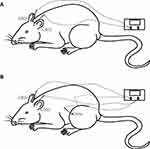 |
Figure 2 Diagram of the acupuncture points used in the GB20 group (A) and GB20/34 (B) group in a rat. |
Mechanical Threshold
A mechanical threshold test was performed as in our previous study.9 An electronic von-Frey anesthesiameter (Model 2390, IITC Life Science, Woodland Hills, CA, USA) was employed to evaluate withdrawal thresholds of the face and hind paw after EA. The von Frey anesthesiameter probe was applied vertically to the skin of the face or hind paw until the occurrence of an escape movement and we recorded the values on the screen. All measurements were made by the same operator and repeated three times at five-minute intervals for each test.
Jugular Vein Blood Sampling
After anesthesia induction, the neck skin of the rat was prepared using pet hair clippers and a transverse incision was made using a scalpel. The jugular vein was exposed at the angle between the clavicle and sternocleidomastoid muscle. Blood samples were collected using a 2.5-mL syringe. The blood sample was transferred to a blood collection tube with an anticoagulant and aprotinin. These blood samples were centrifuged at 3000 rpm and 4°C for 20 mins. The serum was then transferred to a sterile container and stored at −80 ° C waiting for further tests.
Harvesting Dura Mater
After anesthesia, the animals were sacrificed, perfused intracardially with 37°C normal saline followed by cold 4% paraformaldehyde in PBS. A piece of the dura around the superior sagittal sinus was removed. The dura mater was then cut into smaller pieces (0.5–1 cm) and processed as wholemount preparations on glass slides. Immediately after perfusion-fixation, the specimens were further fixed in paraformaldehyde for 1 to 3 hrs and washed in PBS pH 7.2 containing 0.3% Triton X-100 (PBST) prior to immunohistochemistry.
Immunohistochemistry
Rat dura mater whole mounts were washed for 10 mins in PBST. Non-specific binding of the primary antibodies was blocked by incubating in 0.1 MPBS containing 5% bovine serum albumin and 0.3% Triton-X. After blocking, the dura was incubated for 72 h at 4°C with a primary antibody against a given mast cell antibody (Anti-Mast Cell Tryptase, ab2378, Abcam, 1:200) or macrophage cell antibody (CD163 Monoclonal Antibody (ED2), MA5-166561:200). After incubation with primary antibodies, specimens were washed with PBST for 10 mins. The dura was then incubated in PBST containing secondary antibody (Cy3-labeled Goat Anti-Mouse IgG (H+L), A0521, 1:500, Alexa Fluor 555-labeled Donkey Anti-Mouse IgG (H+L), A0460, 1:500). The specimens were subsequently washed with PBST for 10 mins and mounted with an antifading mounting medium containing nuclei-staining DAPI.
Microscopic Analysis
Specimens were examined, and images were obtained using an epifluorescence microscope. These images were captured using a Leica semiautomatic light microscope (Leica DM5500B). For the analysis of immunofluorescence (IF) results, images at 40× magnification of the dura mater were randomly selected using a microscope. The average integrated density of images was recorded as the result. Image analyses were conducted using Image J 1.52a.
ELISA
We measured serum CGRP (CSB-E08211r, CUSABIO TECHNOLOGY LLC, Houston, Texas.), IL-1β (RLB00, R&D, Minnesota, USA), IL-6 (R6000B, R&D, Minnesota, USA), TNFα (RTA00, R&D), COX-2 (CSB-E13399r, CUSABIO TECHNOLOGY LLC), and BDNF (DBNT00, R&D) levels using ELISA according to the method recommended by the kit manufacturer.
Statistical Analysis
Data are shown as the mean ± SD. Withdrawal thresholds were analyzed using repeated measures analysis of variance (ANOVA). When the interaction between the intervention and time resulted in a P-value less than 0.05, the data were analyzed using a one-way ANOVA. All other data were analyzed using one‐way ANOVA using SPSS v18.0 software (SPSS, Chicago, IL, USA). Post-hoc testing was performed using a Bonferroni (homogeneity of variance) or Tamhane (heterogeneity of variance) test. Differences with P-values less than 0.05 were considered significant.
Results
EA Alleviated Hyperalgesia After Dural Stimulation (Figure 3)
The results of the facial withdrawal threshold testing did not meet Mauchly’s test of Sphericity (P=0.035). Tests of within-subjects effects revealed an interaction between Group and Time (P<0.001). The data were normally distributed and analyzed using a one-way ANOVA. For the facial withdrawal threshold (Figure 3A), we did not observe any significant differences at baseline between the five groups (D0: P>0.05). After DES, the facial withdrawal threshold of the model group had significantly decreased (D1: P<0.001; D3: P<0.001; D5: P<0.001). When compared with the model group, the GB20 and GB20/34 group had a significantly higher withdrawal threshold (D1: vs GB20 P<0.001, vs GB20/34 P<0.001; D3: vs GB20 P<0.001, vs GB20/34 P<0.001; D5: vs GB20 P<0.001, vs GB20/34 P<0.001). The facial withdrawal threshold in the NA group did not differ significantly from that in the model group (D1/D3/D5: P>0.05). The GB20+GB34 group withdrawal threshold was significantly higher than that of the GB20 group (D3: P=0.019; D5: P=0.048).
The results of hind paw withdrawal threshold testing met Mauchly’s test of Sphericity (P=0.431). Tests of within-subjects effects revealed an interaction between Group and Time (P<0.001). The data were normally distributed and analyzed using a one-way ANOVA. Regarding the hind paw withdrawal threshold (Figure 3B), no significant difference was observed at baseline between the five groups (D0: P>0.05). However, after dural electrical stimulation, the hind paw withdrawal threshold was significantly lower in the Model group than in the Control group (D1: P=0.004, D3/D5: P<0.001). The hind paw withdrawal threshold was significantly higher in the GB20 and GB20+GB34 groups than in the model group, suggesting that EA attenuated the decrease in withdrawal threshold induced by dural electrical stimulation (D1: P>0.05; D3: vs GB20: P=0.006, vs GB20/34: P<0.001; D5: vs GB20: P=0.002, vs GB20/34: P<0.001). The hind paw withdrawal thresholds in the NA and model groups did not differ significantly (D1/D3/D5: P>0.05). The GB20/34 group withdrawal threshold was significantly higher than that in the GB20 group (D3: P=0.046; D5: P=0.03).
EA Alleviated Immunohistochemistry of Mast Cell and Macrophage (Figure 4, 5)
To observe dural mast cells (Figure 4) and macrophages after dural stimulation (Figure 5), we analyzed immunofluorescence for tryptase and CD163 to measure mast cell and macrophage levels, respectively. The number of cells significantly increased after stimulation compared with that in the Control group (mast cells: P=0.014 [Figure 4] and macrophages: P<0.001). EA significantly decreased the number of cells (mast cells: vs GB20: P<0.001, vs GB20/34: P=0.029 [Figure 4] and macrophages vs GB20: P=0.052, vs GB20/34, P=0.004 [Figure 5]). However, we observed no significant difference between the GB20/34 and GB20 groups (mast cells: P>0.05; macrophages: P>0.05).
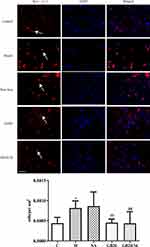 |
Figure 4 Electroacupuncture (EA) treatment decreased the number of dural mast cells. Scale bar = 50 μm. Model group vs Control group (*P<0.05), GB20, GB20/34 group vs Model group (##P<0.01). |
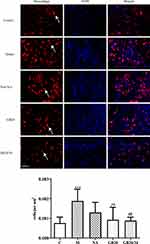 |
Figure 5 Electroacupuncture (EA) treatment decreased the number of dural macrophages. Scale bar = 50 μm. Model group vs Control group (***P<0.001), GB20, GB20/34 group vs Model group (##P<0.01). |
EA Reduced the Serum Concentration of IL-1β, IL-6, TNF-α, and COX-2 (Figure 6)
Dural stimulation significantly increased serum IL-1β, IL-6, TNF-α, and COX-2 levels compared with those in the control group (IL-1β: P<0.001; IL-6: P<0.001; TNF-α: P<0.001; COX-2: P<0.001). EA significantly decreased the serum levels of IL-1β, IL-6, TNF-α, and COX-2 (IL-1β: GB20: P<0.001, GB20/34: P<0.001; IL-6: GB20: P<0.001, GB/34: P<0.001; TNF-α: GB20: P=0.023, GB20/34: P<0.001; COX-2: P<0.001, GB20/34: P<0.001). Moreover, the plasma levels of IL-1β, IL-6, TNF-α, and COX-2 in the GB20+GB34 group were significantly lower than those in the GB20 group (IL-1β: P=0.034; IL-6: P=0.006; TNF-α: P=0.045; COX-2: P>0.05).
EA Alleviated Changes in CGRP and BDNF Serum Concentration (Figure 6)
DES significantly increased serum CGRP and BDNF levels as compared to those in the control group (CGRP, P<0.001; BDNF, P<0.001). EA at GB20 or GB20/34 treatment significantly decreased serum CGRP and BDNF levels (CGRP: vs GB20: P=0.026, vs GB20+GB34: P=0.004; BDNF: vs GB20: P=0.001, vs GB20+GB34: P<0.001). CGRP levels of the GB20/34 group were lower than those of the GB20 group. However, no significant difference was observed between the GB20 and GB20/34 groups with respect to CGRP and BDNF levels (CGRP: P>0.05; BDNF: P>0.05).
Discussion
In this study, we evaluated hyperalgesia; dural mast cells and macrophages; and the serum levels of CGRP, IL-1β, IL-6, TNFα, COX-2, and BDNF. The current findings show that EA alleviates hyperalgesia in a recurrent migraine model and reduces CGRP, IL-1β, IL-6, COX-2, TNFα, and BDNF levels in the jugular vein blood of this rat model of migraine. Furthermore, this study explored the effect of acupuncture on mast cells and macrophages in the dura mater in a model of migraine headache.
In the present study, we chose common acupuncture points based on the clinical treatment of migraine. Acupuncture is based on the theory of meridians. According to traditional acupuncture theory, dysfunction of the Gallbladder Meridian is the basic pathophysiology underlying migraine. Therefore, acupuncture points in this meridian, especially GB20 and GB34, are utilized most frequently in the clinical treatment for migraine. In clinical practice, combination acupuncture points are used because operators expect to obtain synergistic effects with different acupuncture points. GB20 is close to the region where migraine headache occurs, exerting a therapeutic effect from an adjacent region, while GB34 exerts distant effects because it is distant from the headache site.18 From the perspective of traditional acupuncture theory, the synergistic effect is caused by the enhancement of communication, which normalizes dysfunction of the Gallbladder Meridian. In the present study, we observed that combined GB20+GB34 acupuncture was superior to GB20 acupuncture alone in alleviating hyperalgesia. The results revealed that acupuncture in the GB20+GB34 group produced a superior therapeutic effect compared to GB20 alone in modulating serum neuroinflammatory factors. Generally, GB20 is chosen as the main acupuncture point and GB34 is used to strengthen the effect of GB20. Our previous research also found that the EA at GB20 is better than at GB34 when used alone.20 Therefore, we did not use GB34 alone in this study.
We observed DES-induced hyperalgesia in the face and hind paw, which mimics cephalic and extracephalic mechanical allodynia, a common feature of migraine attacks, which reflects the sensitization of secondary and higher neurons of the trigeminovascular system under non-noxious stimuli. Functional magnetic resonance imaging shows activation of the trigeminal ganglion, spinal trigeminal nucleus, and thalamus during the course of cutaneous allodynia after migraine.21 As in our previous study, we observed that acupuncture at GB20 has an anti-hyperalgesia effect. In the present study, we observed enhancement of efficacy when GB20 was used in combination with a distant acupuncture site, GB34. Yu and colleagues found an EA-induced negative feedback inhibitory activity of convergent neurons after visceral nociceptive stimulation.22 Therefore, we speculate that acupuncture at GB20 combined with GB34 may strengthen the EA-induced negative feedback against DES-induced nociceptive signals. We then tested inflammatory factors in peripheral blood finding that related neurogenic inflammatory responses were also inhibited.
Electrical stimulation of the dura mater is regarded as a good method to activate and sensitize the trigeminovascular system.23 The innervation of the dura mater is primarily via the ophthalmic branch of the trigeminal nerve. Stimulation of the dura mater causes elevated plasma neuroinflammatory factors, which indicates the existence of an inflammatory state.24 The emergence of inflammation is the result of interactions between immune cells, meningeal afferents, and the dural vasculature. Neuropeptides released from sensory nerve endings contribute to vasodilation, plasma protein extravasation (PPE), and activation of immune cell traffic.25
We evaluated dural mast cells and macrophages, which play a critical role in the generation of dural inflammation. Dural mast cell activation and degranulation can lead to the release of powerful pro-inflammatory mediators. These inflammatory molecules further activate and sensitize meningeal nociceptors to facilitate migraine attacks.26,27 Macrophages, which are derived from peripheral blood monocytes, can produce various cytokines and growth factors to modulate inflammation.28 Neuropeptides from nerve terminals and inflammatory factors released from these immune cells were evaluated in jugular vein blood.12 Dural macrophages line blood vessels and connective tissues, responding to inflammatory stimuli immediately.29 A previous study found that cortical spreading depression-induced activation of pial and dural macrophages is more likely to increase inflammation in which meningeal nociceptors function.29 In this study, we observed that EA significantly decreased the number of immune cells. Therefore, we detected a reduction in neuropeptides and inflammatory factors using ELISA.
The TG is the main source of CGRP in the jugular vein blood, which is released from the peripheral terminals of trigeminal neurons. Our previous study indicated that electrical stimulation increased CGRP expression in the TG. In the peripheral trigeminovascular system, release of CGRP is involved in the activation of meningeal nociceptors and in the formation of inflammation by dilating intracranial arteries, triggering the release of pro-inflammatory substances from mast cells, immune cells, and endothelial cells.11,24,30 A state of neurogenic inflammation alters the microenvironment of meningeal nociceptors, which results in the sensitization and activation of trigeminal meningeal nociceptors.10,13 In this study, we observed hyperalgesia in a rat migraine model induced after electronic stimulation. At the same time, we found an increase in serum neuroinflammatory factors, including IL-1β, TNFα, and IL-6. After GB20 or GB20/34 treatment, the normalization of CGRP, IL-1β, TNFα, and IL-6 corresponded with behavioral improvement.
IL-1β, IL-6, and TNFα are the main inflammatory factors that are increased during migraine attacks. These cytokines were observed in the internal jugular blood of migraine patients without aura during the first 2 hrs after attack onset.12 These mediators are released primarily by resident and infiltrating immune cells, contributing to the activation and sensitization of nociceptors.31,32 IL-1β and IL-6 are considered possible pain mediators in neurogenic inflammation and, therefore, they may cause the generation of migraine pain and sensitization of nociceptors.31 A previous in vivo study suggested that IL-1β can promote powerful activation and mechanical sensitization of meningeal nociceptors and direct application of IL-6 to the dura produced a dose-dependent facial and hind-paw allodynia.13,33 Another study demonstrated that local administration of TNF-α promoted an increase in the responses of meningeal nociceptors to mechanical stimulation of their dural receptive field and neuronal discharges.34 In in vitro studies, it has been observed that TNF-α can promote the expression of CGRP and BDNF in trigeminal neurons.35,36 In this study, we observed increased IL-1β, IL-6, and TNF-α levels in the jugular vein blood, reflecting the inflammatory state of the intracranial circulation. EA significantly decreased the serum levels of IL-1β, IL-6, and TNF-α, which is consistent with hyperalgesia.
In addition to inflammatory factors, we measured the level of COX-2. COX-2 is an inducible enzyme that is expressed at sites of inflammation and is responsible for the formation of inflammatory mediators called prostaglandins.37 A previous clinical trial reported that the serum level of COX-2 is significantly increased during migraine attacks.38 It is a drug target for migraine treatment because COX-2 inhibitors could abort migraine attacks and delay the process of sensitization.37 In this study, we found that serum levels of COX-2 increased after dural stimulation. A previous labeling study revealed COX-2 expression in dural macrophages and dural axons. The co-localization of COX-2 and CGRP is observed in some dural nerve fibers, which suggests that COX-2 may be related to the function of CGRP.39 It has been shown that CGRP release is mediated by a COX-2-dependent pathway and COX-2 expression in trigeminal ganglion cells could contribute to the development of pain in trigeminally mediated headaches.40,41 The attenuation of CGRP release by COX-2 inhibition could at least in part explain the mechanism of COX inhibitors as migraine therapy.37 In this study, we observed the effect of EA in reducing the plasma COX-2 level.
Episodic migraine tends to develop into chronic migraine, which may involve cytokine-mediated neural plasticity and nociceptor sensitization. Therefore, we tested serum BDNF, which is increased during a migraine attack and inversely related to headache intensity.42,43 BDNF is an important neurotrophic factor involved in the chronic transformation of pain in a variety of diseases.44 A recent study demonstrated that primary afferent-derived BDNF is involved in the transition from acute to chronic pain.45 It acts as a pain modulator at both the peripheral and central level and is involved in modulating the activation of glutamatergic, N-methyl-d-aspartate (NMDA) receptors.15 Therefore, BDNF is important in the development of central sensitization.46 It has been demonstrated that CGRP is co-expressed with BDNF in trigeminal ganglion neurons in rats.47 Previous studies demonstrated that CGRP promotes the release of BDNF from trigeminal neurons and this may be one downstream mechanism by which administration of CGRP triggers migraine.15 Like with CGRP, we observed the serum level of BDNF significantly increased after DES. EA reduced the BDNF levels in the jugular vein blood.
Given the vital role of the dura mater in migraine pathophysiology, two animal studies have examined dura mater involvement in the acupuncture treatment for migraine.8,20 Zhang and colleagues found that the upregulated CB1 receptor is involved in the inhibition of dura mater PPE production by EA.8 Xu’s study focused on the effect of EA at GB20/34 on dural and serum vasoactive neurotrophic factors, including substance P, vasoactive intestinal peptide, neuropeptide Y, pituitary adenylate cyclase-activating polypeptide, nitric oxide, and endothelin-1.20 This study concentrated on the role of dural mast cells and macrophages, serum inflammatory factors, and nerve growth factor in the positive effects of EA on migraine rats. Moreover, similar to the previous study, we observed that the use of a combination of acupuncture points is more effective than the use of a single point. In further studies, we will verify more acupoint combinations through experiments and clinical observation.
Conclusion
In the present study, we observed electroacupuncture treatment ameliorates hyperalgesia by decreasing plasma levels of IL-1β, COX-2, IL-6, and TNFα. We also observed the synergistic effect of GB20 and GB34 in alleviating hypersensitization and modulating plasma neuroinflammatory factors. However, further research is required on the mechanism of acupoint combinations.
Abbreviations
BDNF, brain derived neurotrophic factor; CGRP, Calcitonin gene-related peptide; COX-2, Cyclooxygenase-2; DES, dural electrical stimulation; EA, electrical acupuncture; IL-1β, interleukin-1β; IL-6, interleukin-6; NSAIDs, non-steroidal anti-inflammatory drugs; TG, trigeminal ganglion; TNFα, tumor necrosis factor-α.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
References
1. Headache classification committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi:10.1177/0333102417738202.
2. Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. doi:10.1002/ana.v63:2
3. Louter MA, Bosker JE, van Oosterhout WP, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain. 2013;136:3489–3496. doi:10.1093/brain/awt251
4. Lipton RB, Fanning KM, Serrano D, et al. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. 2015;84:688–695. doi:10.1212/WNL.0000000000001256
5. Lipton RB, Pan J. Is migraine a progressive brain disease? JAMA. 2004;291:493–494.
6. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:Cd001218.
7. McDonald JL, Cripps AW, Smith PK. Mediators, receptors, and signalling pathways in the anti-inflammatory and antihyperalgesic effects of acupuncture. Evid Based Complement Alternat Med. 2015;2015:975632.
8. Zhang H, He S, Hu Y, et al. Antagonism of cannabinoid receptor 1 attenuates the anti-inflammatory effects of electroacupuncture in a rodent model of migraine. Acupunct Med. 2016;34:463–470.
9. Zhao LP, Liu L, Pei P, et al. Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine. Neural Regen Res. 2017;12:804–811.
10. Burgos-Vega C, Moy J, Dussor G. Meningeal afferent signaling and the pathophysiology of migraine. Prog Mol Biol Transl Sci. 2015;131:537–564.
11. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533–552. doi:10.1146/annurev-pharmtox-010814-124701
12. Sarchielli P, Alberti A, Baldi A, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46:200–207. doi:10.1111/hed.2006.46.issue-2
13. Yan J, Melemedjian OK, Price TJ, et al. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6). Mol Pain. 2012;8:6. doi:10.1186/1744-8069-8-6
14. Becker WJ. Acute migraine treatment in adults. Headache. 2015;55:778–793. doi:10.1111/head.12550
15. Burgos-Vega CC, Quigley LD, Avona A, et al. Dural stimulation in rats causes brain-derived neurotrophic factor-dependent priming to subthreshold stimuli including a migraine trigger. Pain. 2016;157:2722–2730. doi:10.1097/j.pain.0000000000000692
16. Li QQ, Shi GX, Yang JW, et al. Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiol Behav. 2015;139:482–490. doi:10.1016/j.physbeh.2014.12.001
17. Pei P, Liu L, Zhao L, et al. Effect of electroacupuncture pretreatment at GB20 on behaviour and the descending pain modulatory system in a rat model of migraine. Acupunct Med. 2016;34:127–135. doi:10.1136/acupmed-2015-010840
18. World Health Organization. Regional Office for the Western Pacific. WHO Standard Acupuncture Point Locations in the Western Pacific Region; Available from: https://apps.who.int/iris/handle/10665/206952. Accessed 7 January 2020.
19. Siu FK, Lo SC, Leung MC.Electro-acupuncture potentiates the disulphide-reducing activities of thioredoxin system by increasing thioredoxin expression in ischemia-reperfused rat brains. Life Sci. 2005;77:386–399. doi:10.1016/j.lfs.2004.10.069
20. Xu X, Liu L, Zhao L, et al. Effect of electroacupuncture on hyperalgesia and vasoactive neurotransmitters in a rat model of conscious recurrent migraine. Evid Based Complement Alternat Med. 2019;2019:9512875.
21. Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl 1):S44–53. doi:10.1016/j.pain.2013.07.021
22. Yu L, Wang W, Li L, et al. Inhibition of electroacupuncture on nociceptive responses of dorsal horn neurons evoked by noxious colorectal distention in an intensity-dependent manner. J Pain Res. 2019;12:231–242. doi:10.2147/JPR
23. Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiological study. Brain. 1996;119(Pt1):249–256. doi:10.1093/brain/119.1.249
24. Rua R, McGavern DB. Advances in meningeal immunity. Trends Mol Med. 2018;24:542–559. doi:10.1016/j.molmed.2018.04.003
25. Levy D, Labastida-Ramirez A, MaassenVanDenBrink A. Current understanding of meningeal and cerebral vascular function underlying migraine headache. Cephalalgia. 2018;39:333102418771350.
26. Baun M, Pedersen MH, Olesen J, et al. Dural mast cell degranulation is a putative mechanism for headache induced by PACAP-38. Cephalalgia. 2012;32:337–345. doi:10.1177/0333102412439354
27. Forsythe P. Mast cells in neuroimmune interactions. Trends Neurosci. 2019;42(1):43–55. doi:10.1016/j.tins.2018.09.006
28. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi:10.2174/1568010054022024
29. Schain AJ, Melo-Carrillo A, Borsook D, et al. Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann Neurol. 2018;83:508–521. doi:10.1002/ana.25169
30. Hendriksen E, van Bergeijk D, Oosting RS, et al. Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev. 2017;79:119–133. doi:10.1016/j.neubiorev.2017.05.001
31. Zhang X, Burstein R, Levy D. Local action of the proinflammatory cytokines IL-1beta and IL-6 on intracranial meningeal nociceptors. Cephalalgia. 2012;32:66–72. doi:10.1177/0333102411430848
32. Yucel M, Kotan D, Gurol Ciftci G, et al. Serum levels of endocan, claudin-5 and cytokines in migraine. Eur Rev Med Pharmacol Sci. 2016;20:930–936.
33. Neeb L, Hellen P, Hoffmann J, et al. Methylprednisolone blocks interleukin 1 beta induced calcitonin gene related peptide release in trigeminal ganglia cells. J Headache Pain. 2016;17:19. doi:10.1186/s10194-016-0609-x
34. Zhang XC, Kainz V, Burstein R, et al. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2011;152:140–149. doi:10.1016/j.pain.2010.10.002
35. Bowen EJ, Schmidt TW, Firm CS, et al. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65–77. doi:10.1111/j.1471-4159.2005.03524.x
36. Balkowiec-Iskra E, Vermehren-Schmaedick A, Balkowiec A. Tumor necrosis factor-alpha increases brain-derived neurotrophic factor expression in trigeminal ganglion neurons in an activity-dependent manner. Neuroscience. 2011;180:322–333. doi:10.1016/j.neuroscience.2011.02.028
37. Silberstein SD, Stirpe JC. COX inhibitors for the treatment of migraine. Expert Opin Pharmacother. 2014;15:1863–1874. doi:10.1517/14656566.2014.937704
38. Li C, Zhu Q, He Q, et al. Plasma levels of cyclooxygenase-2 (COX-2) and visfatin during different stages and different subtypes of migraine headaches. Med Sci Monit. 2017;23:24–28. doi:10.12659/MSM.899269
39. Zhang XC, Kainz V, Jakubowski M, et al. Localization of COX-1 and COX-2 in the intracranial dura mater of the rat. Neurosci Lett. 2009;452:33–36. doi:10.1016/j.neulet.2009.01.032
40. Mozaffari E, Doosti A, Arshi A, et al. Association of COX-2 promoter polymorphisms −765G/C and −1195A/G with migraine. Iran J Public Health. 2016;45:1625–1635.
41. Neeb L, Hellen P, Boehnke C, et al. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One. 2011;6:e17360.
42. Fischer M, Wille G, Klien S, et al. Brain-derived neurotrophic factor in primary headaches. J Headache Pain. 2012;13:469–475.
43. Tanure MT, Gomez RS, Hurtado RC, et al. Increased serum levels of brain-derived neurotropic factor during migraine attacks: a pilot study. J Headache Pain. 2010;11:427–430.
44. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538.
45. Sikandar S, Minett MS, Millet Q, et al. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain. 2018;141:1028–1039.
46. Nijs J, Meeus M, Versijpt J, et al. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin Ther Targets. 2015;19:565–576.
47. Lemos C, Mendonca D, Pereira-Monteiro J, et al. BDNF and CGRP interaction: implications in migraine susceptibility. Cephalalgia. 2010;30:1375–1382.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


