Back to Journals » Medical Devices: Evidence and Research » Volume 12
Electrical stimulation in the treatment of bladder dysfunction: technology update
Authors Coolen RL, Groen J, Blok BFM
Received 16 May 2019
Accepted for publication 24 July 2019
Published 11 September 2019 Volume 2019:12 Pages 337—345
DOI https://doi.org/10.2147/MDER.S179898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
RL Coolen, J Groen, BFM Blok
Department of Urology, Erasmus Medical Center, Rotterdam 3015 GD, The Netherlands
Correspondence: BFM Blok
Department of Urology, Erasmus Medical Center, Room SK-1270, Doctor Molewaterplein 40, Rotterdam 3015 GD, The Netherlands
Tel +31 10 703 2414
Fax +31 10 703 5632
Email [email protected]
Abstract: The urinary bladder has two functions: urine storage and voiding. Clinically, two major categories of lower urinary tract symptoms can be defined: storage symptoms such as incontinence and urgency, and voiding symptoms such as feeling of incomplete bladder emptying and slow urinary stream. Urgency to void with or without incontinence is called overactive bladder (OAB). Slow urinary stream, hesitancy, and straining to void with the feeling of incomplete bladder emptying are often called underactive bladder (UAB). The underlying causes of OAB or UAB can be either non-neurogenic (also referred to as idiopathic) and neurogenic, for example due to spinal cord injury or multiple sclerosis. OAB and UAB can be treated conservatively by lifestyle intervention or medication. In the case that conservative treatment does not provide sufficient benefit, electrical stimulation can be used. Sacral neurostimulation or neuromodulation (SNM) is offered as a third-line therapy to patients with non-neurogenic OAB or UAB. In SNM, the third or fourth sacral nerve root is stimulated and after a test period, a neuromodulator is implanted in the buttock. Until recently only a non-rechargeable neuromodulator was approved for clinical use. However, nowadays, a rechargeable sacral neuromodulator is also on the market, with similar safety and effectiveness to the non-rechargeable SNM system. The rechargeable device was approved for full body 1.5T and 3T MRI in Europe in February 2019. Regarding neurogenic lower urinary tract dysfunction, electrical stimulation only seems to benefit a selected group of patients.
Keywords: lower urinary tract symptoms, neurogenic bladder, electrical stimulation, neuromodulation, sacral neuromodulation, tibial nerve stimulation
Introduction
The urinary bladder has two functions: urine storage and voiding. Patients experience lower urinary tract symptoms (LUTS) in the case of storage and/or voiding dysfunction. These symptoms can be divided into two categories: storage symptoms and (post-)voiding symptoms. Storage symptoms are: increased daytime frequency, nocturia, urgency, urgency urinary incontinence, stress urinary incontinence, and mixed urinary incontinence. Voiding symptoms are: slow stream, intermittency, hesitancy, straining to void, feeling of incomplete bladder emptying, and post-micturition dribble. Storage symptoms are the most prevalent and the most bothersome for patients.1,2 Urgency to void with or without incontinence is called overactive bladder (OAB) and has a prevalence of 11.8%.1 Slow urinary stream, hesitancy, and straining to void with the feeling of incomplete bladder emptying are often called underactive bladder (UAB).3 In our view, UAB with significant post void residue (PVR), which necessitates regular drainage of the bladder, is clinically much more important than UAB without significant PVR. The underlying causes of OAB and UAB can be grouped into non-neurogenic (also referred to as idiopathic) and neurogenic causes. Neurogenic causes are, for example, spinal cord injury, Parkinsonism, multiple sclerosis, and spina bifida. The division between non-neurogenic and neurogenic causes is important for the choice of treatment.
Table 1 gives an overview of the treatment modalities for OAB and UAB. Patients with non-neurogenic OAB or UAB are initially treated with lifestyle intervention, physical therapy, or biofeedback. Pharmacotherapy is offered as a second-line therapy. When these treatment modalities fail, sacral neuromodulation (SNM) or intradetrusor botulinum toxin A (onabotulinumtoxinA or abotulinumtoxinA) injections can be offered. Urinary incontinence due to neurogenic OAB or UAB, on the other hand, is initially treated with pharmacotherapy. Secondly, intradetrusor botulinum toxin A injections can be offered. When botulinum toxin A injections fail in the treatment of neurogenic OAB, surgical interventions such as bladder augmentation and urinary diversion can be suggested. UAB due to neurogenic lower urinary tract dysfunction resulting in significant PVR is treated with clean intermittent catheterization. Secondly, an indwelling catheter may be given or a surgical intervention known as sphincterotomy can be performed. Electrical stimulation is considered an option only in selected cases of neurogenic OAB or UAB. This review provides an overview of the latest research regarding electrical stimulation in the treatment of non-neurogenic and neurogenic OAB and UAB.
 |
Table 1 Treatment options for overactive and underactive bladder |
Firstly, a summary of clinically approved modes of electrical stimulation in the treatment of non-neurogenic OAB and UAB is provided. In the second section, the non-approved modes of electrical stimulation in the treatment of OAB and UAB are discussed. In the final section, the possible modes of electrical stimulation in the treatment of neurogenic OAB and UAB are summarized.
Clinically approved modes of electrical stimulation in the treatment of non-neurogenic OAB and UAB
In this section, SNM with non-rechargeable and rechargeable systems is discussed, after which posterior tibial nerve stimulation is addressed. In Table 2, the chronology of introduction and approval of these devices is given.
 |
Table 2 Introduction and approval of sacral neuromodulation and tibial nerve stimulation |
Sacral neuromodulation
SNM may improve both the storage and voiding function of the bladder. It is therefore used both in patients with OAB syndrome presenting with symptoms such as urgency urinary incontinence and urgency-frequency as well as in patients with UAB. An SNM system consists of a lead with four electrodes stimulating the S3 or S4 sacral nerve root. The lead is connected to a subcutaneous pulse generator. The lead is usually placed on one side in one of the sacral foramina. It is thought that SNM activates afferent pathways which modulate forebrain structures involved in awareness and alertness.4,5 This mechanism is probably similar in, for example, tibial nerve stimulation and pudendal nerve stimulation. However, SNM recruits 1000-times more axons than stimulation of a single nerve.
Non-rechargeable SNM systems
Until recently, the only commercially available devices were the voltage-driven InterStim and InterStim II (Medtronic, Minneapolis, USA). The InterStim II is a smaller, lighter version of the InterStim device.6 It weighs 22 g and is 51 by 44 by 7.7 mm in size. The pulse generator can deliver pulses at a rate of 2.1–130 Hz with a pulse width of 60–450 µs, at a maximum amplitude of 8.5 V. A significant improvement of the initially developed lead was introduced in 2002. This so-called tined lead eliminated the need to fixate the lead to the surrounding tissue since the tines ensure that the lead is anchored properly. This made the implant procedure faster and less invasive. Due to battery depletion patients require revision surgeries to replace the battery. Increasing battery life has been a goal for some time. There is a trade-off between size and battery longevity, a smaller device has a shorter lifespan. The InterStim and the smaller InterStim II have a battery life of approximately 5.5–9.2 and 2.9–5.4 years, respectively, depending on the parameter settings.7 In practice, the pulse width ranges from 180 to 220 ms, the frequency lies between 10 and 20 Hz and the amplitude is smaller than 3.5 V.8 No differences concerning efficacy have been observed between continuous and cyclic stimulation.9
The effectiveness of SNM in women with refractory idiopathic urgency urinary incontinence was evaluated after 5 years follow-up.10 Eighty per cent of the patients still used the SNM system after 5 years. At 1-month follow-up, 87% had a 50% or higher reduction of incontinence episodes or daily pad use. This number decreased to 62% at 5 years. In 15% of the patients, complete continence was achieved throughout the follow-up period. In a clinical study of 27 patients with non-obstructive urinary retention, 83% of the patients had a 50% or higher reduction of symptoms at a median follow-up duration of 5.7 (±3.2) years.11 However, 42% of the patients required one or more surgical revisions. In a randomized trial comparing intradetrusor onabotulinumtoxinA injections of 200 U to SNM in patients with urgency urinary incontinence refractory to lifestyle intervention and pharmacotherapy options, a significant decrease in symptoms was reported in both groups.12,13 However, the onabotulinumtoxinA group had a greater reduction urgency urinary incontinence episodes during the first 6 months of follow-up, urgency urinary incontinence episodes decreased with 3.9 a day compared to a decrease of 3.3 in the SNM group. This significant difference in symptom decrease is of unknown clinical importance. The extent to which these results can be related to clinical practice is limited by the fact that the applied dosage of 200 U onabotulinumtoxinA is not approved by the FDA or CE. The effects of the 200 U onabotulinumtoxinA extend longer than the FDA approved dose of 100 U for idiopathic OAB.14 However, the 200 U dose is associated with more complications than the 100 U dose. In clinical practice, after repeat onabotulinumtoxinA injections of 100 U patients often ask for a more definitive solution, and SNM is offered to these patients.
Rechargeable SNM systems
A rechargeable current-driven SNM system presumably has a longer life span than non-rechargeable devices, lasting up to 15 years, leading to fewer replacement surgeries. The Axonics r-SNM is a rechargeable sacral neuromodulator (Figure 1).15 This device is smaller in size compared to non-rechargeable devices: it is 5 cc in volume whereas the InterStim II is 14 cc in volume. At the last follow-up visit in a clinical study, the average amplitude was 1.7 (±1.1) mA, the frequency was 14.3 (±1.6) Hz, the pulse width was 210.6 (±11.6) microseconds, and the impedance was 1201 (±214) ohms.16 These parameter settings are comparable to those of the InterStim system. The Axonics r-SNM device requires frequent recharging. This is done transcutaneously and takes approximately 1 hr. In a clinical study, the recharge interval for most patients (69%) was 14 days or more.16 Almost all patients were able to recharge their device 3 months after implantation. Rechargeable systems are probably less costly than non-rechargeable systems, due to the fact that device replacement is an important cost factor in the total costs of SNM therapy.17 The Axonics r-SNM has been shown to be effective and improves quality of life in patients with OAB during a follow-up period of 1 year.18,19 The possible advantage of a current driven system over a voltage drive neuromodulator is that fewer adjustments in the outpatient clinic are necessary due to the fact that the system gives a constant current to the nerve fibers independent of the resistance of the surrounding tissue. Furthermore, in February 2019, the system was approved for full body 1.5T and 3T MRI (CE mark) in Europe, being the first implantable SNM system that was granted this approval.
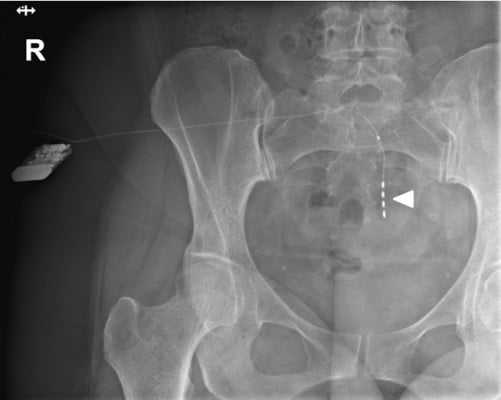
 |
Figure 1 Anterior fluoroscopy of an implanted rechargeable SNM. The arrowhead points to the four electrodes.Abbreviaton: SNM, sacral neuromodulation. |
Posterior tibial nerve stimulation
This technique involves one-sided stimulation of the posterior tibial nerve, 5 cm above the medial malleolus of the ankle. Stimulation can be carried out transcutaneously with a surface electrode, or percutaneously by placing a needle through the skin. The signal is retrogradely conducted through nerve fibers originating from L4 to S3. Percutaneous tibial nerve stimulation has been FDA approved and has a CE mark for the treatment of OAB.
Percutaneous and transcutaneous stimulation are carried out once or twice a week for 30 mins with a non-implantable device. A disadvantage of these methods is the fact that patients need weekly hospital visits. A comparison between the transcutaneous and the percutaneous methods has been made for patients with OAB.20 Transcutaneous stimulation consisted of sessions of 30 mins once a week for 12 weeks using biphasic square waves at a 20 Hz frequency with 200 cycles per second. Percutaneous stimulation parameters were the same. Both methods decreased voiding frequency and gave a similar improvement in the quality of life. The number of axons that are activated by tibial nerve stimulation is a 1000-fold less than the number activated by SNM, so tibial nerve stimulation is probably less effective than SNM.4 In addition, SNM provides continuous stimulation whereas tibial nerve stimulation is carried out once a week initially and once every 2 weeks at a later stage.
The BlueWind RENOVA (BlueWind Medical, Herzliya, Israel) is a relatively new implantable tibial nerve stimulator and has a volume of 0.3 cc and a diameter of 3.4 mm.21 Implantation of the device is done under local or general anesthesia. Patients themselves apply stimulation with an external control unit worn around the ankle. In a study by Heesakkers et al, OAB patients stimulated during the first 3 months for 30 mins a day, 6 days a week and, thereafter, during 3 days a week for 3 months.22 Stimulation was performed at a frequency of 40 Hz with a pulse width up to 800 µs at a maximum amplitude of 9 mA. During follow-up, 70.6% of the patients experienced a 50% or higher reduction of symptoms and quality of life significantly improved. Recently, the coin-sized implantable tibial nerve stimulator eCoin (Valencia Technologies Corporation, Valencia, USA) was tested in 46 patients with refractory OAB symptoms during a follow-up period of 6 months.23 The eCoin is a leadless implant that has a diameter of 23 mm and a thickness of 2.4 mm and comes with an external controller. The devices give a constant current pulse of 20 Hz with a pulse width of 0.2 ms with an amplitude of 0.5–15 mA. The amplitude is adjustable by the external controller. During 12 weeks, 30 mins simulation sessions were elicited by the device every 2 days; thereafter, simulation sessions were carried out every 15 days. The eCoin is implanted under local anesthesia. There was a 71% median reduction of urgency urinary incontinence episodes during follow-up with 4.2 urgency urinary incontinence episodes per day at baseline and 1.5 at 6 months. One serious adverse event was reported related to the implantation procedure. After all, this device seems promising, however, there are no data of the possible longevity of this battery-powered non-rechargeable device.
Non-approved modes of electrical stimulation in the treatment of non-neurogenic OAB and UAB
Intravesical stimulation
During intravesical stimulation, a neutral electrode is placed on the skin and an active electrode is placed transurethrally into the bladder. In a clinical study of 17 patients with OAB, intravesical stimulation was given twice a week for 4 weeks.24 Twelve weeks after the start of treatment, voiding frequency was significantly reduced and health-related quality of life had significantly improved compared to baseline. However, urgency urinary incontinence did not significantly change. In a retrospective study of 89 patients with UAB stimulation sessions were performed 5 days a week for 30 mins until bladder function was normal or no further improvement was achieved.25 Stimulation parameters were as follows: 1–30 mA pulse amplitude, 10–25 Hz pulse frequency, and a 200–800 µs pulse width. On average, 27 (±25) treatment sessions were performed on each patient, with a range of 5–181. Intravesical stimulation significantly decreased retention, 47% had a more than 40% reduction of PVR. Also, voiding efficiency significantly increased. However, research on long-term effects of intravesical stimulation is lacking, and comparative research on stimulation settings is limited.
Pudendal nerve stimulation
The pudendal nerve is usually accessed percutaneously through a posterior approach during a surgery under general anesthesia. A needle is placed medial to the ischial tuberosity where after the lead is placed. Radiographs are made to assess the lead position. Animal studies have shown various effects of pudendal nerve stimulation on bladder function. Bladder capacity was shown to increase by sensory pudendal nerve stimulation in an OAB rat model.26 Furthermore, animal studies have revealed alterations in the expression of proteins as a consequence of electrical stimulation. Jiang et al, studied a rat model with stress urinary incontinence and found that stimulation promotes neuronal regeneration, possibly through upregulation of brain-derived neurotrophic factor in motorneurons.27 In a clinical study by Peters et al, a comparison was made between SNM and pudendal nerve stimulation to treat bladder dysfunction in 37 patients with OAB symptoms and three patients with urinary retention.28 They reported an overall reduction in symptoms of 63% with pudendal nerve stimulation and 46% with SNM. Patients reported greater improvement of pelvic pain, urgency, frequency, and bowel dysfunction with pudendal nerve stimulation as opposed to SNM. However, the different effects on voiding frequency did not appear from voiding diaries.
Saphenous nerve stimulation
The saphenous nerve is a sensory nerve innervating the medial part of the skin between the ankle and the knee. In a study regarding 15 healthy participants, electrical stimulation of the saphenous nerve was performed with two surface electrodes.29 Stimulation was carried out during 1-hr session at a frequency of 20 Hz with a pulse width of 200 µs. The nerve was activated when the amplitude of stimulation was 25.7 (±7.4) mA and the highest tolerable level of activation was at an amplitude of 47.7 (±9.3) mA. This result indicates that transcutaneous stimulation can achieve saphenous nerve activation at a tolerable level. However, this study did not consider outcome measures related to bladder function.
In a pilot study in 18 female patients with idiopathic OAB, the saphenous nerve was stimulated percutaneously once a week during 3 months.30 Stimulation sessions lasted 30 mins. Stimulus pulses were 200 µs wide and were applied at a frequency of 20 Hz. The amplitude was set at the highest tolerable level to achieve the maximum therapeutic effect. After 3 months significant decreases in urgency, urgency urinary incontinence and nocturia were reported and a significant increase in the overall health-related quality of life was observed. Frequency, however, was not significantly different compared to baseline.
Transcutaneous electrical nerve stimulation
Transcutaneous electrical nerve stimulation (TENS) is carried out with surface electrodes, and therefore, provides a non-invasive alternative to other stimulation modalities. Above we described TENS of the tibial nerve. Other sites suitable for TENS include the suprapubic, sacral, penile/clitoral, vaginal, and rectal areas. Effectiveness of TENS has been demonstrated in patients with idiopathic bladder dysfunction.31 Areas of stimulation were the dermatomes of S2 and S3 and the thigh area. Stimulation frequency was 20–50 Hz, and the pulse width was 200 µs. Stimulation was carried out daily during 2–6 weeks. Only short-term clinical improvement was shown. There are no data on long-term efficacy.
Electrical stimulation in the treatment of neurogenic OAB and UAB
Electrical stimulation in the treatment of neurogenic lower urinary tract dysfunction is not approved by the FDA and has no CE mark.
Sacral neuromodulation
The results regarding the effectiveness of SNM in patients with neurogenic OAB and UAB are conflicting. It seems that SNM cannot reliably suppress detrusor overactivity due to neurogenic causes.32 The results in patients with spinal cord injury are inconclusive.33 In a study in patients with multiple sclerosis, however, voided volume significantly increased, PVR significantly decreased, voiding frequency significantly decreased, and incontinence significantly decreased during a follow-up period of 3 years.34 The effects of SNM seem to be lost after time which could be a consequence of neurological deterioration due to the underlying disease.
Brindley stimulator
The Brindley stimulator (Finetech Medical Ltd., Welwyn Garden City, UK) is meant to restore bladder emptying in patients with a spinal cord lesion.35 The implant procedure consists of the bilateral placement of electrodes over the ventral roots of S2–S4, combined with a rhizotomy of the dorsal roots of these nerves. The dorsal rhizotomy is necessary to abolish reflexive contraction of the bladder. However, sensory information conveyed by the dorsal roots is consequently also abolished. For that reason, the Brindley stimulator is solely used in patients with a complete spinal cord lesion. The electrodes, placed extradurally or intrathecally, are connected to an internal receiver placed subcutaneously below the ribs in the abdomen. The patient can void by placing an external transmitter block, which is connected to a digital controller, over the skin where the internal receiver is situated. Stimulation parameters can be adjusted within certain limits: the frequency ranges from 2 to 53 Hz, the amplitude ranges from 10 to 40 V, and the pulse width ranges from 0 to 720 µs. In addition to bladder emptying, defecation and erection can be induced depending on parameter settings. In patients with complete spinal cord injury and detrusor overactivity, a 68% reduction of urinary tract infections, a 54% improvement in social life, and a 54% improvement in continence has been reported.36 The effectiveness of the procedure has been reported in several studies with a maximum follow-up of 12 years.37 Despite the beneficial effects, the Brindley stimulator is currently no longer being implanted.
Pudendal nerve stimulation
In patients with neurogenic lower urinary tract dysfunction, the overall symptom score was reduced by 52% post pudendal nerve stimulation.38 In this study, pudendal nerve stimulation was carried out during 12 sessions spread out over 4 consecutive weeks. Electrical stimulation was conducted with two pairs of needles (located 1 cm bilateral to the sacrococcygeal joint and 1 cm bilateral to the tip of the coccyx) at a frequency of 2.5 Hz with an amplitude of 25–35 mA for 45 mins. Filling problems, including incontinence, and voiding problems were markedly reduced. The long-term efficacy of pudendal nerve stimulation in the treatment of neurogenic lower urinary tract dysfunction is yet to be studied.
Tibial nerve stimulation
Tibial nerve stimulation provides a non-invasive (transcutaneous) or minimally invasive (percutaneous) option in the treatment of neurogenic lower urinary tract dysfunction. Multiple clinical studies have been conducted in patients with neurogenic lower urinary tract dysfunction. These studies indicate that tibial nerve stimulation might be effective and safe for these patients.39 In a study conducted in multiple sclerosis patients with an OAB, stimulation was applied once a week for 12 weeks.40 Patients who responded well continued the treatment, at first, once every 2 weeks during 3 months. Subsequently, the interval was enlarged to once every 3 weeks during 3 months followed by once every 4 weeks during 3 months. Stimulation was carried out percutaneously with a frequency of 20 Hz and a pulse width of 200 µs. The amplitude was set at the maximum tolerable level for each patient. At the 1-year endpoint, symptoms were significantly improved. Although promising, the study is a retrospective non-controlled study. In another study, conducted in patients with Parkinson’s Disease (percutaneous stimulation weekly during 12 weeks, 200 Hz, 200 s), LUTS and urodynamic parameters improved significantly.41
Transcutaneous electrical nerve stimulation
A systematic review of the literature regarding TENS for neurogenic bladder dysfunction was performed by Gross et al, in 2016.42 Suprapubic or sacral stimulation in the identified studies was performed at a frequency of 20, 50, or 75 Hz with a pulse width of 200 µs at an amplitude of 16 or 20 mA or at a comfortable sensation. The duration of stimulation sessions in the different studies was 15, 30, and 180 mins per day for a duration of 30–720 days. Clitoral/penile stimulation was carried out at a frequency of 5–25 Hz with a pulse width of 200–1500 µs and an amplitude which was the maximal tolerable or at 1.5 or 2 times the level at which certain reflexes were elicited. The duration of stimulation ranged from 20 mins to less than 2 hrs per day during 1–84 days. Vaginal/rectal stimulation was carried out at a frequency of 8 or 10 Hz, with a pulse width of 400 or 450 µs, for 30 or 40 mins a day during 63 or 84 days. One RCT, in which the sacral area was stimulated, reported a significant difference between the treatment and control group regarding leaks per day during treatment.43 Gross et al, concluded that TENS has potential, though there is a need for more reliable data.42
Transcranial magnetic stimulation (TMS)
The effects of repetitive TMS on LUTS have been investigated in patients with Parkinson Disease, multiple sclerosis, and incomplete spinal cord injury. Eight patients with Parkinson Disease and urinary symptoms such as urgency and increased frequency received inhibitory TMS over the motor cortex for 2 weeks at 1 Hz.44 Two weeks after stimulation ended symptoms significantly reduced compared to baseline. Two weeks later, symptoms returned to baseline levels. Ten multiple sclerosis patients received excitatory TMS over the motor cortex for 2 weeks at 5 Hz.45 Six patients suffered detrusor underactivity, three patients had detrusor overactivity, and one patient had a combination of both. Urodynamic evaluation was performed 3 days after completion of the 2-week TMS treatment. Nine patients reported improvement of voiding symptoms, but there was no improvement of storage symptoms. These subjective outcomes measures corresponded with the urodynamic parameters. Thus, this stimulation protocol might only be effective in patients with UAB. In 12 out of 23 patients with incomplete spinal cord injury an neurogenic bladder symptoms, excitatory TMS over the motor cortex 20 ms before transcutaneous pudendal nerve stimulation, facilitated the pudendal anal reflex, indicating a possible effect on the urethral sphincter.46 However, no direct measures related to bladder function were investigated. In conclusion, TMS has shown positive effects on bladder symptoms in the short term in selected groups of patients.
Conclusions and future perspectives
We have discussed multiple electrical stimulation modalities and the effects on bladder function. Stimulation parameters (Table 3) vary between the different modalities. The optimal settings depend on effectiveness and the highest tolerable level for each patient. Comparative research on parameter settings for each modality is lacking. Regarding the effectiveness of SNM, SNM with a non-rechargeable device has been shown to be an effective treatment option for idiopathic OAB and UAB. However, nowadays also a rechargeable sacral neuromodulator is on the market with similar safety and effectiveness as the non-rechargeable SNM system. The rechargeable device has been approved for full body 1.5T and 3T MRI in Europe. Tibial nerve stimulation is a less invasive but also less effective alternative. Regarding the rechargeable SNM device, only short-term efficacy has been reported. There is a need for larger studies with a longer duration of follow-up to study efficacy and to optimize the configuration of the devices. Regarding neurogenic lower urinary tract dysfunction, electrical stimulation seems to benefit a selected group of patients only.
 |
Table 3 Stimulation parameters of electrical stimulation modalities for bladder dysfunction |
Disclosure
The authors report no conflicts of interest in this work.
References
1. Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306–1315. doi:10.1016/j.eururo.2006.09.019
2. Agarwal A, Eryuzlu LN, Cartwright R, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. Eur Urol. 2014;65:1211–1217. doi:10.1016/j.eururo.2014.01.019
3. Uren AD, Cotterill N, Harding C, et al. The development of the ICIQ-UAB: a patient reported outcome measure for underactive bladder. Neurourol Urodyn. 2019;38(3):996–1004. doi:10.1002/nau.23947
4. Blok BF. Sacral neuromodulation for the treatment of urinary bladder dysfunction: mechanism of action and future directions. Bioelectron Med. 2018;1:85–94. doi:10.2217/bem-2017-0003
5. Blok BF, Groen J, Bosch JL, et al. Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int. 2006;98:1238–1243. doi:10.1111/j.1464-410X.2006.06521.x
6. Sacral neuromodulation Therapy, Implant manual, 2018. Available from: http://professional.medtronic.com/pt/uro/snm/prod/index.htm.
7. Rittenmeyer H. Sacral nerve neuromodulation (InterStim). Part II: review of programming. Urol Nurs. 2008;28:21–25.
8. Peeren F, Hoebeke P, Everaert K. Sacral nerve stimulation: interstim therapy. Expert Rev Med Devices. 2005;2:253–258. doi:10.1586/17434440.2.3.253
9. Price DM, Noblett K. Prospective randomized crossover trial comparing continuous and cyclic stimulation in InterStim therapy. Female Pelvic Med Reconstr Surg. 2015;21:355–358. doi:10.1097/SPV.0000000000000188
10. Groen J, Blok BF, Bosch JL. Sacral neuromodulation as treatment for refractory idiopathic urge urinary incontinence: 5-year results of a longitudinal study in 60 women. J Urol. 2011;186:954–959. doi:10.1016/j.juro.2011.04.059
11. Mehmood S, Altaweel WM. Long-term outcome of sacral neuromodulation in patients with idiopathic nonobstructive urinary retention: single-center experience. Urol Ann. 2017;9:244–248. doi:10.4103/UA.UA_165_16
12. Amundsen CL, Komesu YM, Chermansky C, et al. Two-year outcomes of sacral neuromodulation versus onabotulinumtoxinA for refractory urgency urinary incontinence: a randomized trial. Eur Urol. 2018;74:66–73. doi:10.1016/j.eururo.2018.02.011
13. Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women: a randomized clinical trial. JAMA. 2016;316:1366–1374. doi:10.1001/jama.2016.14617
14. Blok BF. OnabotulinumtoxinA vs sacral neuromodulation for urgency incontinence. JAMA. 2017;317:534–535. doi:10.1001/jama.2016.19560
15. The Axonics r-SNM System, 2019. Available from: http://www.axonicsmodulation.com/product/.
16. Blok BF, Van Kerrebroeck P, de Wachter S, et al. Programming settings and recharge interval in a prospective study of a rechargeable sacral neuromodulation system for the treatment of overactive bladder. Neurourol Urodyn. 2018;37:S17–S22. doi:10.1002/nau.23476
17. Noblett KL, Dmochowski RR, Vasavada SP, et al. Cost profiles and budget impact of rechargeable versus non-rechargeable sacral neuromodulation devices in the treatment of overactive bladder syndrome. Neurourol Urodyn. 2017;36:727–733. doi:10.1002/nau.23008
18. Blok BF, Van Kerrebroeck P, de Wachter S, et al. Three month clinical results with a rechargeable sacral neuromodulation system for the treatment of overactive bladder. Neurourol Urodyn. 2018;37:S9–S16. doi:10.1002/nau.23465
19. Blok BF, Van Kerrebroeck P, de Wachter S, et al. A prospective, multicenter study of a novel, miniaturized rechargeable sacral neuromodulation system: 12-month results from the RELAX-OAB study. Neurourol Urodyn. 2019;38:689–695. doi:10.1002/nau.23892
20. Ramirez-Garcia I, Blanco-Ratto L, Kauffmann S, et al. Efficacy of transcutaneous stimulation of the posterior tibial nerve compared to percutaneous stimulation in idiopathic overactive bladder syndrome: randomized control trial. Neurourol Urodyn. 2018;38:261–268. doi:10.1002/nau.23843
21. Physicians, Overactive Bladder, 2018. Available from: http://www.bluewindmedical.com/bluewind-renova.
22. Heesakkers JPFA, Digesu GA, van Breda J, et al. A novel leadless, miniature implantable tibial nerve neuromodulation system for the management of overactive bladder complaints. Neurourol Urodyn. 2018;37:1060–1067. doi:10.1002/nau.23401
23. MacDiarmid S, Staskin DR, Lucente V, et al. Feasibility of a fully implanted, nickel sized and shaped tibial nerve stimulator for the treatment of overactive bladder syndrome with urgency urinary incontinence. J Urol. 2019;201(5):967–972. doi:10.1016/j.juro.2018.10.017
24. Yune JJ, Shen JK, Pierce MA, et al. Intravesical electrical stimulation treatment for overactive bladder: an observational study. Investig Clin Urol. 2018;59:246–251.
25. Deng H, Liao L, Wu J, et al. Clinical efficacy of intravesical electrical stimulation on detrusor underactivity: 8 years of experience from a single center. Medicine (Baltimore). 2017;96:e8020. doi:10.1097/MD.0000000000008020
26. Hokanson JA, Langdale CL, Sridhar A, et al. Stimulation of the sensory pudendal nerve increases bladder capacity in the rat. Am J Physiol Renal Physiol. 2018;314:F543–F550. doi:10.1152/ajprenal.00373.2017
27. Jiang HH, Song QX, Gill BC, et al. Electrical stimulation of the pudendal nerve promotes neuroregeneration and functional recovery from stress urinary incontinence in a rat model. Am J Physiol Renal Physiol. 2018;315:1555–1564. doi:10.1152/ajprenal.00431.2017
28. Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–647. doi:10.1002/nau.20174
29. Sharan E, Hunter K, Hassouna M, et al. Characterizing the transcutaneous electrical recruitment of lower leg afferents in healthy adults: implications for non-invasive treatment of overactive bladder. BMC Urol. 2018;18:10. doi:10.1186/s12894-018-0322-y.
30. MacDiarmid SA, John MS, Yoo PB. A pilot feasibility study of treating overactive bladder patients with percutaneous saphenous nerve stimulation. Neurourol Urodyn. 2018;37:1815–1820. doi:10.1002/nau.23531
31. Slovak M, Chapple CR, Barker AT. Non-invasive transcutaneous electrical stimulation in the treatment of overactive bladder. Asian J Urol. 2015;2:92–101. doi:10.1016/j.ajur.2015.04.013
32. Wollner J, Krebs J, Pannek J. Sacral neuromodulation in patients with neurogenic lower urinary tract dysfunction. Spinal Cord. 2016;54:137–140. doi:10.1038/sc.2015.124
33. Ren J, Chew DJ, Biers S, et al. Electrical nerve stimulation to promote micturition in spinal cord injury patients: a review of current attempts. Neurourol Urodyn. 2016;35:365–370. doi:10.1002/nau.22730
34. Engeler DS, Meyer D, Abt D, et al. Sacral neuromodulation for the treatment of neurogenic lower urinary tract dysfunction caused by multiple sclerosis: a single-centre prospective series. BMC Urol. 2015;15:105.
35. Bladder Stimulator Leaflet, 2018. Available from: https://finetech-medical.co.uk/products/finetech-brindley-bladder-control-system/.
36. Vastenholt JM, Snoek GJ, Buschman HP, et al. A 7-year follow-up of sacral anterior root stimulation for bladder control in patients with a spinal cord injury: quality of life and users’ experiences. Spinal Cord. 2003;41:397–402. doi:10.1038/sj.sc.3101465
37. Li LF, Ka-Kit Leung G, Lui WM. Sacral nerve stimulation for neurogenic bladder. World Neurosurg. 2016;90:236–243. doi:10.1016/j.wneu.2016.02.108
38. Li T, Feng X, Lv J, Cai T, Wang S. Short-term Clinical efficacy of electric pudendal nerve stimulation on neurogenic lower urinary tract disease: a pilot research. Urology. 2018;112:69–73. doi:10.1016/j.urology.2017.10.047
39. Schneider MP, Gross T, Bachmann LM, et al. Tibial nerve stimulation for treating neurogenic lower urinary tract dysfunction: a systematic review. Eur Urol. 2015;68:859–867. doi:10.1016/j.eururo.2015.07.001
40. Canbaz Kabay S, Kabay S, Mestan E, et al. Long term sustained therapeutic effects of percutaneous posterior tibial nerve stimulation treatment of neurogenic overactive bladder in multiple sclerosis patients: 12-months results. Neurourol Urodyn. 2017;36:104–110. doi:10.1002/nau.22868
41. Kabay S, Canbaz Kabay S, Cetiner M, et al. The clinical and urodynamic results of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson’s disease. Urology. 2016;87:76–81. doi:10.1016/j.urology.2015.09.026
42. Gross T, Schneider MP, Bachmann LM, et al. Transcutaneous electrical nerve stimulation for treating neurogenic lower urinary tract dysfunction: a systematic review. Eur Urol. 2016;69:1102–1111. doi:10.1016/j.eururo.2016.01.010
43. Guo ZF, Liu Y, Hu GH, et al. Transcutaneous electrical nerve stimulation in the treatment of patients with poststroke urinary incontinence. Clin Interv Aging. 2014;9:851–856. doi:10.2147/CIA.S61084
44. Brusa L, Finazzi Agro E, Petta F, et al. Effects of inhibitory rTMS on bladder function in Parkinson’s disease patients. Mov Disord. 2009;24(3):445–448. doi:10.1002/mds.22434
45. Centonze D, Petta F, Versace V, et al. Effects of motor cortex rTMS on lower urinary tract dysfunction in multiple sclerosis. Mult Scler. 2007;13(2):269–271. doi:10.1177/1352458506070729
46. Vasquez N, Balasubramaniam V, Kuppuswamy A, et al. The interaction of cortico-spinal pathways and sacral sphincter reflexes in subjects with incomplete spinal cord injury: a pilot study. Neurourol Urodyn. 2015;34(4):349–355. doi:10.1002/nau.22554
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
