Back to Journals » International Journal of Women's Health » Volume 11
Elbasvir/grazoprevir in women with hepatitis C virus infection taking oral contraceptives or hormone replacement therapy
Authors Hézode C, Kwo P, Sperl J , Hwang P, Long J, Talwani R, Robertson MN, Haber BA
Received 26 January 2019
Accepted for publication 1 August 2019
Published 20 November 2019 Volume 2019:11 Pages 617—628
DOI https://doi.org/10.2147/IJWH.S203022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Christophe Hézode,1 Paul Kwo,2 Jan Sperl,3 Peggy Hwang,4 Jianmin Long,4 Rohit Talwani,4 Michael N Robertson,4 Barbara A Haber4
1Service d’Hépatologie, Hôpital Henri-Mondor, AP-HP, Université Paris-Est, INSERM U955, Créteil, France; 2Division of Gastroenterology/Hepatology, Department of Medicine, Stanford University School of Medicine, Palo Alto, CA, USA; 3Department of Hepatogastroenterology, Institut Klinické a Experimentální Medicíny (IKEM), Prague, Czech Republic; 4Department of Infectious Disease, Merck & Co., Inc., Kenilworth, NJ, USA
Correspondence: Christophe Hézode
Service d’Hepatologie, Hôpital Henri Mondor, 51, avenue du Maréchal de Lattre de Tassigny, Créteil 94010, France
Tel +33 14 981 2111
Email [email protected]
Introduction: Some direct-acting antiviral regimens for hepatitis C virus (HCV) infection pose safety or efficacy concerns if coadministered with drugs containing ethinyl estradiol. The present analysis was conducted to examine the impact of concomitant oral contraceptive pills (OCP) or hormone replacement therapy (HRT) during treatment with elbasvir (EBR)/grazoprevir (GZR) in women with HCV genotype (GT)1 or GT4 infection.
Methods: This is a post hoc, integrated retrospective analysis of female participants with HCV GT1 or GT4 infection who received EBR 50 mg/GZR 100 mg once daily for 12 weeks in phase 2/3 clinical trials. The primary end point was sustained virologic response at 12 weeks after therapy completion (SVR12). For this analysis, participants were stratified according to whether they received OCP or HRT during the original treatment study.
Results: A total of 1,022 women with HCV GT1 or GT4 infection were included (receiving OCP/HRT, n=81; not receiving OCP/HRT, n=941). Most participants receiving OCP/HRT were treatment-naive (79%), noncirrhotic (91.4%), and aged >35 years (71.6%). SVR12 rates were similar in women receiving OCP/HRT and those not receiving OCP/HRT (95.1% vs 96.3%). SVR12 rates remained high across all subgroups within the population receiving OCP/HRT: SVR12 rates were 94.6%, 100%, and 100% in participants with GT1a, GT1b, and GT4 infection, and all women aged 18–35 years achieved SVR (21/21). Treatment-related adverse events occurred in 40.7% (33/81) and 30.1% (283/941) of women receiving and those not receiving OCP/HRT, respectively.
Conclusion: The efficacy and safety of EBR/GZR administered for 12 weeks was similar in women receiving OCP/HRT and those not on OCP/HRT. These data indicate that EBR/GZR can be safely used for the treatment of HCV GT1 or GT4 infection in women receiving concomitant OCP/HRT.
Keywords: clinical trial, ethinyl estradiol, levonorgestrel, NS5A inhibitor, NS3/4A protease inhibitor
In recent years, there has been a dramatic increase in the number of newly reported cases of hepatitis C virus (HCV) infection worldwide, driven largely by the increase in injection drug use in younger adults.1,2 As a result of this increase in HCV infection among younger adults, the prevalence of HCV infection has also increased notably among women of child-bearing potential. The number of women of reproductive age with HCV infection in the United States National Notifiable Diseases Surveillance System increased from 15,550 in 2006 to 31,039 in 2014.3 Furthermore, estimates also suggest that between 2011 and 2014, the national rate of HCV detection among women of childbearing age in the United States increased by 22% (from 139 to 169 per 100,000 women) and the proportion of infants born to women with HCV infection increased by 68% (from 1 in 536 [0.19%] births to 1 in 308 births).4 Successful treatment of HCV infection in this emerging population therefore needs to be mindful of the specific characteristics of this particular population, one of which is the use of ethinyl estradiol–containing medications as oral contraceptive therapy. Oral contraceptive pills (OCPs) have the potential for drug–drug interactions, particularly when coadministered with inhibitors or inducers of cytochrome 450 (CYP 3A) or uridine 5ʹ-diphospho-glucuronosyltransferases.5–9 Some direct-acting antiviral regimens for HCV infection pose safety or efficacy concerns if coadministered with OCPs or hormone replacement therapies (HRT) that contain ethinyl estradiol.10–12
The fixed-dose combination of elbasvir (EBR) 50 mg/grazoprevir (GZR) 100 mg is approved in the United States, Europe, and other countries worldwide for the treatment of people with HCV genotype (GT) 1 or GT4 infection.13,14 The individual drugs have been shown to be potent in vitro,15–18 and in clinical trials, this combination was highly effective across a wide range of people with HCV infection and various comorbidities.19–24 The clinical pharmacology of EBR/GZR is also well-established, enabling EBR/GZR to be used in a range of individuals with HCV infection who are receiving concomitant medications.13,14 Phase 1 clinical trials have shown no clinically meaningful impact of multiple doses of EBR/GZR on the pharmacokinetics of ethinyl estradiol/levonorgestrel in healthy female adults without HCV infection, suggesting that EBR/GZR can be coadministered to women with HCV infection who are taking OCPs to prevent pregnancy.25 Ethinyl estradiol and levonorgestrel have the potential for drug–drug interactions when coadministered with inhibitors or inducers of CYP3A or uridine 5′-diphospho-glucuronosyltransferases. EBR is a substrate of CYP3A/P-glycoprotein (P-gp) and an inhibitor of breast cancer resistance protein, and GZR is a substrate of CYP3A/P-gp and organic anion transporter polypeptide 1B1/1B3 and is also a weak CYP3A inhibitor and a breast cancer resistance protein inhibitor. Based on these metabolic pathways, neither ethinyl estradiol nor levonorgestrel is expected to alter either EBR or GZR pharmacokinetics.25 In order to fully characterize the clinical safety and efficacy profile of EBR/GZR in women with HCV infection who are taking OCPs or HRT, a post hoc, retrospective analysis was conducted to examine the impact of concomitant OCP or HRT in women with HCV GT1 or GT4 infection receiving EBR/GZR in phase 2/3 clinical trials.
Methods
This was an integrated retrospective analysis of data from 12 international phase 2/3 clinical trials. The original studies were carried out in accordance with the Declaration of Helsinki, current guidelines on Good Clinical Practices, and local ethical and legal requirements (Table 1). All participants in these studies provided voluntary written informed consent before trial entry. Previous reports have described the methodology and primary outcomes from these studies (C-SCAPE26 [NCT01932762; Protocol PN047-03]; C-SURFER21 [NCT02092350/Protocol PN052]; C-EDGE CO-INFECTION22 [NCT02105662/Protocol PN061]; C-EDGE Treatment-Naive19 [NCT02105467/Protocol PN060]; C-EDGE Treatment-Experienced20 [NCT02105701/Protocol PN068]; C-WORTHY27,28 [NCT01717326/Protocol PN035]; C-EDGE CO-STAR24 [NCT02105688/Protocol PN062]; C-EDGE Head-2-Head29 [NCT02358044/Protocol PN077]; Japan phase 2/3 study30 [NCT02203149/Protocol PN058]; C-EDGE-IBLD23 [NCT02252016/Protocol PN065]; C-CORAL31 [NCT02251990/Protocol PN067]; C-SALT32 [NCT02115321/Protocol PN059].
Participants
Female participants with HCV GT1 or GT4 infection from these 12 studies were included in the present analysis (Table 1). All original studies had generally similar inclusion and exclusion criteria. In brief, participants were aged >18 years with chronic HCV infection and HCV RNA >10,000 IU/mL at baseline. Participants were treatment-naive or had previously failed interferon-based HCV therapy, were HCV monoinfected or HCV/HIV coinfected, and were either noncirrhotic or had compensated Child-Pugh A cirrhosis. In these studies, cirrhosis was defined as liver biopsy consistent with METAVIR fibrosis score of F4; FibroScan® >12.5 kPa within 12 months of study entry; or aspartate aminotransferase (AST)-to-platelet ratio >2.0 and FibroTest >0.75 within 12 months of study entry. Other comorbidities present in this population were chronic kidney disease stage 4 or 5 (including those on hemodialysis),21 inherited blood disorders,23 and those receiving opioid agonist therapy.24 Individuals with decompensated liver disease (as indicated by a presence or history of ascites, esophageal or gastric variceal bleeding, hepatic encephalopathy, or other signs of advanced liver disease), or evidence of hepatocellular carcinoma were excluded from the original studies.
 |
Table 1 Original treatment studies |
Treatment
To be included in this analysis, participants were required to have received EBR 50 mg/GZR 100 mg once daily, administered either as a coformulated fixed-dose combination tablet or as separate entities, for 12 weeks. In the United States and Europe, EBR/GZR plus ribavirin for 16 weeks is approved for the treatment of certain HCV-infected patient subgroups; however, the 12-week regimen of EBR/GZR (no ribavirin) represents the most commonly utilized regimen in current clinical practice. The present analysis will therefore focus solely on individuals who received the 12-week regimen in the phase 2/3 clinical trials.
Outcomes
The primary end point of all original studies was sustained virologic response at 12 weeks after completion of therapy (SVR12). Plasma HCV RNA levels were measured using the Cobas® AmpliPrep/Cobas® TaqMan® HCV test (version 2.0, Roche Molecular Diagnostics, Branchburg, NJ, USA) with a lower limit of quantitation of 25 IU/mL in the phase 2 studies and 15 IU/mL in the phase 3 studies. In all studies, relapse was defined as detectable HCV RNA following the end of therapy after undetectable HCV RNA at the end of therapy.
Analyses
This was a retrospective analysis of data from phase 2/3 clinical trials. For the purposes of this analysis, female participants were stratified according to whether they were receiving OCP (yes, no) or HRT (yes, no) for ≥7 days during treatment with EBR/GZR in the original treatment study. Efficacy and safety comparisons were made between participants who were and those who were not receiving OCP/HRT. Efficacy analyses included all eligible participants who received ≥1 dose of study (the full analysis set). A supportive analysis is also described that was based on the modified full analysis set population, which excluded participants who discontinued from the trial for reasons unrelated to the study drug. Safety analyses included all participants who received ≥1 dose of study medication.
Results
A total of 1,022 women with HCV GT1 or GT4 infection who received EBR/GZR for 12 weeks were included in the full analysis set, including 81 participants receiving OCP/HRT (75 [92.6%] of whom were on OCP/HRT for the complete 12 weeks of treatment), and 941 women not receiving OCP/HRT. Concomitant therapies used by the 81 women receiving OCP/HRT are listed in Table S1.There were a total of 100 reports of OCP/HRT use among the 81 women who received OCP/HRT (who included those who may have stopped and restarted therapy and therefore had >1 record of OCP/HRT use). The most common reasons for use of OCP/HRT medications were contraception (n=45), hormone replacement therapy (n=21), vaginitis/vaginal atrophy/vaginal dryness (n=7), and uterine bleeding (n=5). (Table S2). Most of the women receiving OCP/HRT were originally treated in the C-EDGE Treatment-Naive19 (n=21, 25.9%), C-CORAL31 (n=13, 16%), CO-STAR24 (n=11, 13.6%), and the Japanese phase 2/3 studies30 (n=9, 11%) (Table 1). Most participants receiving OCP/HRT had HCV GT1a (n=38, 46.9%) or GT1b (n=39, 48.1%) infection, were treatment-naive (n=64, 79%), noncirrhotic (n=74, 91.4%), and were aged >35 years (n=58, 71.6%). Women not receiving OCP/HRT were more likely to have HCV GT1b infection than those receiving OCP/HRT (70.0% vs 48.1%, respectively) and aged >35 years (89.7% vs 71.6%, respectively) (Table 2). Participants receiving opioid agonist therapy enrolled from the CO-STAR study24 represented 13.6% (11/81) of women receiving OCP/HRT and 6.1% (57/941) of women not receiving OCP/HRT.
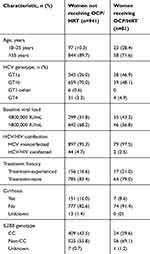 |
Table 2 Participant demographics |
Virologic response
In the full analysis set, sustained virologic response (SVR) rates were similar in women receiving OCP/HRT and those not receiving OCP/HRT (95.1% [77/81] vs 96.3% [906/941]) (Table 3). A total of 4 women receiving OCP/HRT (relapse, n=2; nonvirologic failure, n=2) and 35 women not receiving OCP/HRT (relapse, n=21; reinfection, n=2; nonvirologic failure, n=12) failed to achieve SVR12. When participants with nonvirologic failure who discontinued treatment for reasons unrelated to study medication were excluded from the modified full analysis set, SVR12 rates were 97.5% (77/79) and 97.5% (906/929) in women receiving and those not receiving OCP/HRT, respectively.
 |
Table 3 Virologic outcomes |
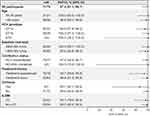
Subgroup analysis indicates that SVR12 rates remained high across all subgroups within the population of women receiving OCP/HRT (Figure 1). Rates of SVR12 were high in participants with HCV GT1a, GT1b, and GT4 infection (94.6%, 100%, and 100%, respectively) and in both treatment-naive, and treatment-experienced participants (98.4% and 93.7%, respectively). All women aged 18–35 years achieved SVR (100%, 21/21) compared with 96.6% of those aged >35 years (56/58). Among the 8 participants with cirrhosis receiving OCP/HRT in this analysis, 7 achieved SVR (85.7%) and 1 relapsed. SVR rates also remained >90% regardless of baseline viral load, HIV coinfection status, or IL28B genotype.
Two participants receiving OCP/HRT experienced virologic failure. A 46-year-old woman with β-thalassemia and HCV GT1a infection with cirrhosis who was receiving estradiol/norethindrone relapsed at follow-up week 12 after having undetectable HCV RNA at the end of treatment. She had no baseline nonstructural protein 5A (NS5A) resistance-associated substitutions (RASs) and had treatment-emergent Q30H and Y93H RASs at the time of failure. She was a previous null responder to peginterferon/ribavirin, had an elevated baseline international normalized ratio of 2.7 (normal range, 0.9–1.1), and had a baseline HCV RNA of 4,203,381 IU/mL. She was randomized to deferred treatment, and per protocol received EBR/GZR for 12 weeks after an initial placebo treatment period. She reported no interruption to study medication. Her concomitant medications included deferasirox, fluticasone, fentanyl, levothyroxine, and ciprofloxacin. A 59-year-old treatment-naive, noncirrhotic woman with HCV GT1a infection receiving estradiol experienced relapse at follow-up week 8 after achieving undetectable HCV RNA at follow-up week 4. Her baseline HCV RNA was 1,939,436 IU/mL, and she had no baseline NS5A RASs. She had received EBR/GZR for 12 weeks and reported no interruption to study medication. At virologic failure, she had a Y93 RAS. Her concomitant medications included: enalapril, atenolol, pentoxifylline, ketorolac, omeprazole, fluticasone, insulin, etodolac, and albuterol.
Adverse events
Adverse events (AEs) were reported by 80.2% (65/81) of women receiving OCP/HRT and by 65.7% (618/941) of those not receiving OCP/HRT (Table 4). Similarly, treatment-related AEs were reported by 40.7% (33/81) and 30.1% (283/941) of women receiving and those not receiving OCP/HRT, respectively, while the respective rates of serious AEs were 2.8% (26/941) and 6.2% (5/81) (Table S3). Five serious AEs in 3 participants were considered related to treatment with EBR/GZR: elevated alanine aminotransferase (ALT) and AST (n=1 each; same participant), gastritis erosive and hypophosphatemia (n=1 each; same participant), and atrial fibrillation (n=1). None of the participants was receiving concomitant OCP/HRT. Five serious AEs were reported by 5 participants who were receiving concomitant OCP/HRT (n=1 each): hemorrhagic erosive gastritis, muscular weakness, bipolar disorder, schizophrenia, and uterine hemorrhage; none were considered related to study drug.
 |
Table 4 Adverse events |
On-treatment changes in liver transaminase levels, bilirubin, and hemoglobin were generally similar in women not receiving and those receiving OCP/HRT. During the phase 2/3 trials of EBR/GZR, elevations of ALT and/or AST levels were observed late in the course of therapy (ie, after treatment week 4) among a proportion of participants.33 In the current analysis, ALT/AST elevations >500 IU/L or ALT/AST elevations >100 IU/L and >3× baseline were reported in 18 (1.9%) women not receiving OCP/HRT and 2 (2.4%) women receiving OCP/HRT. Of those participants receiving OCP/HRT, the first ALT elevation was reported by a 56-year-old noncirrhotic white woman with HCV GT4 infection receiving promestriene. At baseline her ALT was 62 IU/L and her clinical course remained uneventful until treatment week 8, when her ALT levels increased to >500 IU/L. She had no abdominal symptoms, fever, or rash, and her direct bilirubin (0.16 mg/dL), total bilirubin (0.39 mg/dL), and international normalized ratio (INR) (1.1) were normal. Treatment with EBR/GZR was discontinued per protocol (ALT or AST >500 IU/L). Her ALT event was confounded by the ingestion of alcohol the day before the abnormal ALT test and the self-administration of several doses of etifoxine for anxiety, a drug with known hepatotoxic potential. She underwent extensive evaluation, all of which did not reveal an infectious or obstructive etiology. The participant discontinued medication on day 59, and the elevation resolved within 4 weeks. She achieved SVR12. Other concomitant medications taken during treatment were desloratadine, acyclovir, aspirin, ibuprofen, and pinaverium. The second ALT elevation was reported by a 33-year old noncirrhotic white woman with HCV GT1b infection and β-thalassemia receiving estradiol valerate (+) norgestrel. Her baseline ALT was 54 IU/L, but at treatment week 8 she had a transient elevation of ALT to 331 IU/L that resolved by the next laboratory evaluation at treatment week 2 (ALT=34 IU/L). Both her total bilirubin and INR values were consistent with day 1 values. Her ALT elevation resolved by day 73 with continued treatment. The participant achieved SVR12. Other concomitant medications taken during treatment were deferoxamine, esomeprazole, amoxicillin, and levothyroxine.
Discussion
Data from this retrospective, post hoc analysis indicate that the safety and efficacy profile of EBR/GZR administered for 12 weeks is generally unaffected by concomitant OCP/HRT. SVR12 rates were >95% regardless of OCP/HRT administration. Excluding participants who did not have a virologic outcome, SVR12 was achieved by 97.5% of those receiving and those not receiving OCP/HRT. The safety profile of EBR/GZR in women receiving OCP/HRT was also generally similar to that in women not receiving OCP/HRT. Drug-related serious AEs were reported only by women not receiving OCP/HRT.
Young adults represent a rapidly growing segment of the population with new HCV infection, driven largely by the increasing parenteral use of recreational drugs. Treatments for HCV infection therefore require continual re-evaluation to ensure that they meet the requirements of this changing epidemiology. While younger people do not have the same comorbidities and hence do not have the same requirements for medication that are seen in older individuals, evaluation of drug–drug interactions remains an important consideration in establishing the clinical profile of HCV therapies. Ethinyl estradiol–containing products, used as OCPs and also as HRT, are a common therapy across a broad range of women with HCV infection.
The potential of EBR or GZR to impact the pharmacokinetics of an oral contraceptive in healthy, postmenopausal or oophorectomized women was recently examined in 2 drug–drug interaction studies.25 In these one-way studies, EBR did not substantially alter the plasma exposure of ethinyl estradiol or levonorgestrel. Coadministration of GZR also did not substantially alter the plasma exposure of ethinyl estradiol; however, the plasma area under the curve from zero to infinity (AUC0-∞) of levonorgestrel was increased by 23% in the presence of GZR.25 These data indicate that EBR does not substantially alter the plasma exposure of ethinyl estradiol/levonorgestrel. The small increase in levonorgestrel AUC0-∞ seen in the presence of GZR is consistent with GZR being a weak CYP3A inhibitor and is not considered clinically significant.13,14
Careful consideration of OCP/HRT use in women who are taking direct-acting antiviral agents is warranted. In contrast to the phase 1b study by Marshall et al, a 2- to 3-fold increase in norelgestromin and norgestrel concentrations is reported when ethinyl estradiol/norgestimate is coadministered with ombitasvir/paritaprevir/ritonavir/dasabuvir tablets.10 In addition, during clinical trials with the ombitasvir/paritaprevir/ritonavir/dasabuvir combination regimen, ALT elevations were significantly more frequent in women who were using ethinyl estradiol–containing medications such as OCPs, contraceptive patches, or contraceptive vaginal rings compared with those not receiving these ethinyl estradiol–containing products.10 Based on these findings, ethinyl estradiol–containing medications are contraindicated in women receiving the ombitasvir/paritaprevir/ritonavir/dasabuvir combination therapy.10 Similarly, coadministration of ethinyl estradiol in individuals with HCV infection receiving glecaprevir/pibrentasvir has also been reported to increase the risk of ALT elevations and is not recommended.11 Elevated serum concentrations of GZR have also been associated with increased serum transaminase elevations. However, in the present analysis, on-treatment changes in liver transaminase levels, bilirubin, and hemoglobin were generally similar in the participants receiving OCP/HRT and those not receiving OCP/HRT, consistent with no elevation in serum concentrations of GZR in the presence of OCP/HRT therapies.
Limitations of this analysis include the post hoc, retrospective nature of the study. The populations of women receiving and those not receiving OCP/HRT were not randomized in this analysis, and thus differences in these populations exist, such as the proportions of patients with GT1b infection (48.1% vs 70.7%) and of those aged 18–35 years (28.4% vs 9.5%). Analysis of specific participant subgroups, such as those with HIV coinfection, cirrhosis, or HCV GT4 infection, are also limited by the relatively small number of women receiving OCP/HRT in these subgroups. The analysis is also limited owing to the heterogeneous nature of OCP and HRT medications. For the purposes of this analysis, all OCP/HRT medications were grouped into a single population to increase the population sample size under investigation; however, it should be noted that because of the heterogeneous nature of the medications, care should be exercised when drawing conclusions based on any individual OCP or HRT medication. Our data also cannot exclude the possibility of minor drug-drug interactions between EBR/GZR and components of OCP/HRT therapy other than ethinyl estradiol/levonorgestrel.
In conclusion, the efficacy and safety of EBR/GZR administered for 12 weeks was similar in female participants receiving concomitant OCP/HRT compared with those not on concomitant OCP/HRT. Rates of SVR12 were high regardless of treatment history, presence of cirrhosis, baseline viral load, or age. Similarly, rates of AEs and drug-related AEs were generally similar in women receiving OCP/HRT and those not receiving OCP/HRT. These data support observations from a phase 1 drug-interaction study25 indicating that EBR/GZR can be safely used for the treatment of HCV GT1 or GT4 infection in women receiving concomitant OCP/HRT.
Data sharing statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to [email protected].
Acknowledgment
We extend our gratitude to the participants, their families, investigators, and site personnel who participated in this study. Medical writing and editorial assistance was provided by Tim Ibbotson, PhD, of ApotheCom, Yardley, PA, and funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). The trials included in this integrated analysis were sponsored and funded in full by MSD. MSD contributed to the trial design, study execution and management, data collection, and statistical analyses of the original treatment studies, and also conducted the integrated analysis described in this report. Portions of the data described in the manuscript were previously presented at The International Liver Congress; April 11–15, 2018; Paris, France [abstract #801].
Disclosure
Dr Hézode has received personal fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, and MSD. Dr Kwo received grants from the Regenstrief Institute and Target Registeries, has received grants and personal fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, MSD, and Janssen, and has received personal fees from Quest, Arrowhead, Surrozen, Ferring, Conatus, Dova and Shinogi. Dr Sperl has received grants from Gilead Sciences and personal fees from Gilead Sciences, MSD, AbbVie, Herbacos Recordati, and Intercept. Drs Hwang, Robertson, and Haber are current employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and stockholders in Merck & Co., Inc., Kenilworth, NJ, USA. Drs Long and Talwani were employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, at the time that the study was conducted. The authors report no other conflicts of interest in this work.
References
1. New hepatitis C infections nearly tripled over five years. Centers for Disease Control and Prevention website. 2017. Available from: https://www.cdc.gov/nchhstp/newsroom/2017/Hepatitis-Surveillance-Press-Release.html.
2. Hepatitis C. Annual epidemiological report for 2015. European Centre for Disease Prevention and Control website. December 6, 2017. Available from: https://ecdc.europa.eu/en/publications-data/hepatitis-c-annual-epidemiological-report-2015.
3. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive-aged women and children in the United States, 2006 to 2014. Ann Intern Med. 2017;166(11):775–782. doi:10.7326/M16-2350
4. Koneru A, Nelson N, Hariri S, et al. Increased hepatitis C Virus (HCV) detection in women of childbearing age and potential risk for vertical transmission - United States and Kentucky, 2011–2014. MMWR Morb Mortal Wkly Rep. 2016;65(28):705–710. doi:10.15585/mmwr.mm6528a2
5. Zhang H, Cui D, Wang B, et al. Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: a new look at an old drug. Clin Pharmacokinet. 2007;46(2):133–157. doi:10.2165/00003088-200746020-00003
6. Benedetti MS. Enzyme induction and inhibition by new antiepileptic drugs: a review of human studies. Fundam Clin Pharmacol. 2000;14(4):301–319.
7. Hall SD, Wang Z, Huang SM, et al. The interaction between St John’s wort and an oral contraceptive. Clin Pharmacol Ther. 2003;74(6):525–535. doi:10.1016/j.clpt.2003.08.009
8. Fattore C, Cipolla G, Gatti G, et al. Induction of ethinylestradiol and levonorgestrel metabolism by oxcarbazepine in healthy women. Epilepsia. 1999;40(6):783–787.
9. Fotherby K. Levonorgestrel. Clinical pharmacokinetics. Clin Pharmacokinet. 1995;28(3):203–215. doi:10.2165/00003088-199528030-00003
10. Viekira Pak [prescribing information]. North Chicago (IL): AbbVie Inc.; 2015.
11. Mavyret [prescribing information]. North Chicago (IL): AbbVie Inc.; 2018.
12. Lin WH, Feng HP, Shadle CR, O’Reilly T, Wagner JA, Butterton JR. Pharmacokinetic and pharmacodynamic interactions between the hepatitis C virus protease inhibitor, boceprevir, and the oral contraceptive ethinyl estradiol/norethindrone. Eur J Clin Pharmacol. 2014;70(9):1107–1113. doi:10.1007/s00228-014-1711-0
13. Zepatier [prescribing information]. Kenilworth (NJ): Merck & Co., Inc.; 2018.
14. Zepatier [summary of product characteristics]. Hoddesdon (Hertfordshire): Merck Sharp & Dohme Ltd.; 2018.
15. Lahser FC, Bystol K, Curry S, et al. The combination of grazoprevir, a hepatitis C virus (HCV) NS3/4A protease inhibitor, and elbasvir, an HCV NS5A inhibitor, demonstrates a high genetic barrier to resistance in HCV genotype 1a replicons. Antimicrob Agents Chemother. 2016;60(5):2954–2964. doi:10.1128/AAC.00051-16
16. Harper S, McCauley JA, Rudd MT, et al. Discovery of MK-5172, a macrocyclic hepatitis C virus NS3/4a protease inhibitor. ACS Med Chem Lett. 2012;3(4):332–336. doi:10.1021/ml300017p
17. Summa V, Ludmerer SW, McCauley JA, et al. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob Agents Chemother. 2012;56(8):4161–4167. doi:10.1128/AAC.00324-12
18. Coburn CA, Meinke PT, Chang W, et al. Discovery of MK-8742: an HCV NS5A inhibitor with broad genotype activity. ChemMedChem. 2013;8(12):1930–1940. doi:10.1002/cmdc.201300343
19. Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic HCV genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med. 2015;163(1):1–13. doi:10.7326/M15-0785
20. Kwo P, Gane E, Peng CY, et al. Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. 2017;152:164–175. doi:10.1053/j.gastro.2016.09.045
21. Roth D, Nelson DR, Bruchfeld A, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–1545. doi:10.1016/S0140-6736(15)00349-9
22. Rockstroh JK, Nelson M, Katlama C, et al. Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015;2(8):e319–e327. doi:10.1016/S2352-3018(15)00108-3
23. Hezode C, Colombo M, Bourliere M, et al. Elbasvir/grazoprevir for patients with hepatitis C virus infection and inherited blood disorders: a phase III study. Hepatology. 2017;66:736–745. doi:10.1002/hep.29139
24. Dore GJ, Altice F, Litwin AH, et al. Elbasvir-grazoprevir to treat hepatitis C virus infection in persons receiving opioid agonist therapy: a randomized trial. Ann Intern Med. 2016;165:625–634. doi:10.7326/M16-0816
25. Marshall WL, Feng HP, Caro L, et al. No clinically meaningful pharmacokinetic interaction between the hepatitis C virus inhibitors elbasvir and grazoprevir and the oral contraceptives ethinyl estradiol and levonorgestrel. Eur J Clin Pharmacol. 2017;73(5):593–600. doi:10.1007/s00228-017-2216-4
26. Brown A, Hezode C, Zuckerman E, et al. Efficacy and safety of 12 weeks of elbasvir ± grazoprevir ± ribavirin in participants with hepatitis C virus genotype 2, 4, 5 or 6 infection: the C-SCAPE study. J Viral Hepat. 2018;25(5):457–464. doi:10.1111/jvh.12801
27. Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385(9973):1087–1097. doi:10.1016/S0140-6736(14)61793-1
28. Lawitz E, Gane E, Pearlman B, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet. 2015;385:1075–1086. doi:10.1016/S0140-6736(14)61795-5
29. Sperl J, Horvath G, Halota W, et al. Efficacy and safety of elbasvir/grazoprevir and sofosbuvir/pegylated interferon/ribavirin: a phase III randomized controlled trial. J Hepatol. 2016;65:1112–1119. doi:10.1016/j.jhep.2016.07.050
30. Kumada H, Suzuki Y, Karino Y, et al. The combination of elbasvir and grazoprevir for the treatment of chronic HCV infection in Japanese patients: a randomized phase II/III study. J Gastroenterol. 2017;52:520–533. doi:10.1007/s00535-016-1285-y
31. Wei L, Zhdanov K, Burnevich E, et al. Efficacy and safety of elbasvir/grazoprevir in treatment-naïve patients with chronic HCV GT 1, GT 4 and GT 6 infection (C-CORAL): a phase III randomized multinational clinical trial. J Hepatol. 2017;66:S529. doi:10.1016/S0168-8278(17)31460-5
32. Jacobson IM, Poordad F, Firpi-Morell R, et al. Elbasvir/grazoprevir in people with hepatitis C genotype 1 infection and child-pugh class B cirrhosis: the C-SALT study. Clin Transl Gastroenterol. 2019;10(4):e00007. doi:10.14309/ctg.0000000000000007
33. Dusheiko GM, Manns MP, Vierling JM, Reddy KR, Sulkowski MS, Kwo PY. Safety and tolerability of grazoprevir/elbasvir in patients with chronic hepatitis C: integrated analysis of phase 2–3 trials. Hepatology. 2015;62:562A.
Supplementary materials
 |
Table S1 OCP and HRT therapies coadministered with EBR/GZR in contributing phase 2/3 studies |
 |
Table S2 Reasons for using OCP/HRT medications |
 |
Table S3 Serious adverse events |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.