Back to Journals » Infection and Drug Resistance » Volume 13
Eight-Year Surveillance of Uropathogenic Escherichia coli in Southwest China
Authors Sun J , Du L, Yan L, Dai W, Wang Z, Xu X
Received 21 February 2020
Accepted for publication 7 April 2020
Published 28 April 2020 Volume 2020:13 Pages 1197—1202
DOI https://doi.org/10.2147/IDR.S250775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jide Sun,1 Li Du,2 Li Yan,1 Wei Dai,1 Zhu Wang,1 Xiuyu Xu1
1Department of Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2College of Basic Science, Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Li Yan Tel +86 23 89012742
Fax +86 23 89012513
Email [email protected]
Purpose: To assess antimicrobial resistance profiles change in uropathogenic Escherichia coli (UPEC) during an 8-year period, especially extended-spectrum β-lactamase (ESBL)-producing and carbapenem-resistant isolates.
Materials and Methods: A retrospective observational study of urinary tract infections (UTIs) was performed in a territory hospital between 2012 and 2019. Isolates were identified using matrix-assisted laser desorption/ionization time of flight mass spectrometry or the VITEK 2 Compact system. The antimicrobial susceptibility testing was performed using the VITEK 2 Compact system and the modified Kirby–Bauer disc diffusion method.
Results: Of the 7713 non-repetitive UPEC isolates, 7075 (91.7%) were from inpatients and 638 (8.3%) were from outpatients. The prevalence of ESBL declined from 62.5% to 49.7% (P = 0.003). Except for cefoxitin, the resistance rates of ESBL-producing isolates were mostly higher than that of non-ESBL-producing isolates (P < 0.001). The resistance rates of ampicillin (P = 0.013), ampicillin/sulbactam (P = 0.013), ceftriaxone (P < 0.001), gentamycin (P = 0.001), tobramycin (P = 0.011), and trimethoprim/sulfamethoxazole (P = 0.028) declined slightly, while the resistance rate of imipenem increased slightly (P = 0.001). The prevalence of carbapenem-resistant Escherichia coli was < 2.0%.
Conclusion: ESBL-producing Escherichia coli is still the main drug-resistant bacteria causing UTIs. We should pay attention to antimicrobial resistance in high-risk inpatient areas and take effective measures to prevent and control nosocomial infections.
Keywords: UTIs, ESBL, E. coli, CRE, antimicrobial resistance, trends
Introduction
Urinary tract infection (UTI) is one of the most common clinical infectious diseases. In China, UTI ranks second in nosocomial infections, after respiratory tract infection.1–3 UTIs are usually caused by bacteria originating from the digestive tract, and Escherichia coli (E. coli) is the primary pathogen.4 Quantitative urine culture remains the gold standard for diagnosing UTIs in symptomatic patients;5,6 However, oral antibiotics are usually prescribed empirically before the results of urine culture and antimicrobial susceptibility testing are available in healthy, non-pregnant, reproductive-age women presenting with symptomatic acute uncomplicated cystitis.
Empirical therapy should be based on local antimicrobial surveillance data from previous years. Recently, as the widespread use of broad-spectrum antibiotics, some changes have taken place in the antimicrobial resistance (AMR) profiles of uropathogens, including uropathogenic Escherichia coli (UPEC), which brings difficulties for clinical treatment. In England, the nonsusceptibility to third-generation cephalosporins for UPEC from hospital specimens from 6.3% in 2010 to 7.4% in 2013.7 In Canadian, multidrug-resistant (MDR) phenotypes of UPEC from outpatients increased from 9.7% in 2007 to 16.5% in 2016.8
We present AMR data from routine laboratory-based surveillance of UPEC isolates in Southwest China for the period 2012–2019. We aimed to describe first- and second-line antimicrobial agents resistance levels and assess the changing antibiotic sensitivity profiles among E. coli isolated from urine, especially extended-spectrum β-lactamase (ESBL)-producing and carbapenem-resistant Escherichia coli (CREC) isolates.
Materials and Methods
Study Design
This retrospective study was conducted at the First Affiliated Hospital of Chongqing Medical University, a large comprehensive tertiary-care center in southwest China, with 3200 beds. The microbiological laboratory receives about 12,000 urine culture specimens per year. Urine specimens were cultured on blood agar plates and MacConkey agar plates (ThermoFisher Scientific, Shanghai, China), and Isolates were identified using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) (VITEK®MS, bioMérieux, Marcy l’Etoile, France) or the VITEK 2 Compact system (bioMérieux, Marcy l’Etoile, France). The antimicrobial susceptibility testing (AST) was performed using the VITEK 2 Compact system and Modified Kirby–Bauer disc diffusion method. The clinical and microbiological information of isolates were separately collected from the electronic medical record system (EMRS) and the laboratory information system (LIS).
Inclusion Criteria
Urine specimens with suspected (104 ~105 CFU/mL) or significant (>105 CFU/mL) growth of E. coli from January 2012 to December 2019; patients with at least three of the following symptoms: urgency, frequency, dysuria, hematuria, bladder or perineal discomfort, ipsilateral or bilateral low back pain, significant tenderness or throbbing pain in the costalspinal angle; routine urine test indicated that leukocyte, urinary protein or nitrite were positive.
Exclusion Criteria
Urine specimens with negative culture, no suspected or significant growth, or growth other than E. coli; duplicate specimens from the same patient within 3 days for the same specimen type.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was performed using the Vitek Compact 2 system and disc diffusion. The antibiotic discs (Oxoid, Hampshire, UK) used for Enterobacteriaceae included Ampicillin (10 μg), Aztreonam (30 μg), Cefoperazone/Sulbactam (75/30 μg), Ciprofloxacin (5 μg), Gentamicin (10 μg), Meropenem (10 μg). Susceptibility interpretations were based on clinical breakpoints recommended by the Clinical Laboratory Standards Institute (CLSI).9 ESBL production in E. coli was screened using the Vitek Compact 2 system and confirmatory test followed CLSI guidelines.
Statistical Analysis
Raw susceptibility test results of UPEC isolates were processed by Whonet 5.6 software (WHO, Geneva, Switzerland). Simple linear regression was used to assess the statistical significance of AMR trends over the study period. Chi-square test or Fisher’s exact test was employed to compare resistance rates between groups. All statistical analyses were performed using GraphPad Prism 6 software (GraphPad, San Diego, CA, USA), and a two-tailed P-value of less than 0.05 was considered statistically significant.
Results
Of the total 7713 non-repetitive UPEC isolates, 7075 (91.7%) were from inpatients, 638 (8.3%) were from outpatients; males and females accounted for 26.6% and 73.4%, respectively. The ward distribution of inpatients was: 1882 of urology, 641 of gynecology, 638 of neurology, 628 of endocrinology, and 579 of intensive care unit (ICU), accounting for 24.4%, 8.3%, 8.3%, 8.1%, and 7.5%, respectively.
Overall from 2012 to 2019, UPEC isolates exhibited low resistance to carbapenems, nitrofurantoin, amikacin, cefoperazone/sulbactam, and piperacillin/tazobactam, with resistance rates <6.5%; they demonstrated high resistance to ampicillin, ampicillin/sulbactam, ceftriaxone, quinolones, and trimethoprim/sulfamethoxazole, with resistance rates ranged from 42.8% to 90.6%. Imipenem-resistant UPEC isolates showed an increasing trend (P = 0.001), while decreasing resistance trends were observed for ampicillin (P = 0.013), ampicillin/sulbactam (P = 0.013), ceftriaxone (P < 0.001), gentamicin (P = 0.001), tobramycin (P = 0.011), trimethoprim/sulfamethoxazole (P = 0.028) (Table 1).
 |
Table 1 Trends of Antimicrobial Resistance Rate (%) in UPEC Isolates |
The prevalence of ESBL-producing isolates decreased from the highest 62.5% in 2013 to 49.7% in 2019 (P = 0.003) (Table 1). Except for cefoxitin, the resistance rates of ESBL-producing isolates were mostly higher than that of non-ESBL-producing isolates (P < 0.001), exceeding 80% to ampicillin, ceftriaxone and ciprofloxacin (Table 2).
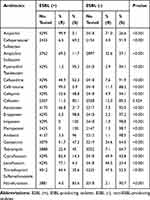 |
Table 2 The Comparison of Antimicrobial Susceptibility Profile Between ESBL-Producing and Non-ESBL-Producing Isolates |
During the 8-year period, a total of 94 non-repetitive CREC strains (defined as resistance to any of the carbapenems) were isolated from patients with UTIs, and the top four ward sources were urology, ICU, geriatrics, neurology, accounting for 29.8%, 13.8%, 8.5%, 8.5%, respectively. CREC isolates presented high susceptibility only to amikacin (88.2%) and nitrofurantoin (71.9%), while resistance to cefoperazone/sulbactam, ampicillin/sulbactam, third- and fourth-generation cephalosporins exceeded 80%, and quinolones even reached about 95% (Table 3).
 |
Table 3 Antimicrobial Susceptibility Profiles of 94 Uropathogenic CREC Isolates |
Discussion
UTI is a common infection both in community and hospital settings. E. coli is a major pathogen causing UTIs. In recent years, the prevalence of ESBL-producing E. coli stays at a high level, and CREC is also increasing year by year. AMR of Enterobacteriaceae bacteria has become more and more serious. This retrospective study focuses on changes in AMR of UPEC to different classes of antimicrobials, especially ESBL-producing and CREC isolates.
Many risk factors associated with UTIs have been reported, including bradyuria due to anatomical abnormality and dysfunction of urinary system, invasive operation such as urinary tract catheterization and urinary endoscopy, chronic basic diseases such as diabetes, acute or chronic kidney diseases, long-term bedridden, elderly and women10,11 In our study, inpatients with infections caused by UPEC isolates mainly were from urology (24.4%), gynecology (8.3%), neurology (8.3%), endocrinology (8.1%) and ICU (7.5%), and females were more than males (73.4% vs 26.6%). The distributions of wards and sex are basically consistent with that the literature reported.
Nitrofurantoin, fosfomycin, trimethoprim/sulfamethoxazole (TMP/SMX), levofloxacin, and β-lactams are common drugs used to treat UTIs.12 In the current study, cefoperazone/sulbactam, piperacillin/tazobactam, amikacin, nitrofurantoin and carbapenems were the most active agents against UPEC isolates (resistance rate <10%); ampicillin, ampicillin/sulbactam, ceftriaxone and quinolones, trimethoprim/sulfamethoxazole were less active (resistance rate ≥50%). Although quinolones have a relatively high resistance rate, they are still a choice for clinical treatment of UTIs. According to pharmacokinetics/pharmacodynamics (PK/PD), quinolones have a much higher level in urine than in blood. However, the current breakpoints recommended by CLSI M100 standard for antimicrobial susceptibility testing are based on drug concentration levels in blood. Armstrong et al found that when the minimum inhibitory concentration (MIC) of Enterobacteriaceae to levofloxacin was 4 μg/mL, the clinical cure rate could reach 100%; when MIC was 32 μg/mL, the clinical cure rate was also above 80%.13 During the 8-year period, the susceptibilities have changed to ampicillin, ampicillin/sulbactam, trimethoprim/sulfamethoxazole, ceftriaxone, gentamicin, imipenem, tobramycin in UPEC isolates; except for imipenem with declining susceptibility, other six antibiotics presented increasing susceptibility trends, which is roughly the same as the multi-center AMR surveillance data in China,14,15 but different from data of other countries.16–18 The diversity may be related to region, hospital scale, disease type, hospital medication habits such as antibiotics rotation and defined daily doses (DDDs), etc. Since high nephrotoxicity, the DDDs of aminoglycosides are low, which perhaps explained the increasing susceptibility. As the first-line drug for the simple UTIs treatment, trimethoprim/sulfamethoxazole has no obvious advantage for the therapy of serious complicated UTIs due to the only oral agents in our hospital, and it is rarely used clinically.
Patients with long-term hospitalization or invasive procedures, and unreasonable use of antibiotics, raise the risk of infection and colonization of ESBL-producing isolates.19 Increasing ESBL-producers greatly limit therapeutic options for related infections.20,21 In the present study, we observed a decreasing trend of ESBL prevalence, which may be attributed to the restricted use of third-generation cephalosporins; even so, the overall prevalence rate was still up to around 50%. The ESBL plasmid-bearing strains are often multi-drug resistant due to the combined carrying of resistance genes to aminoglycosides, quinolones, sulfonamides, and other antibiotics, which can reproduce and proliferate among the homologous and heterologous bacteria.22,23
For severe infections caused by ESBL-producing isolates, carbapenems are often the preferred empirical therapeutic choice. Unfortunately, carbapenem-resistant Enterobacteriaceae (CRE) is globally increasing year by year and poses an urgent public health threat. Between 2012 and 2019, imipenem-resistant UPEC isolates demonstrated an upward tendency in our study, and ertapenem-resistant strains ranged from 0.5% to 1.7%. The climbing CREC prevalence may be attributed to the increased empirical treatment of carbapenems. Three major mechanisms are involved in Enterobacteriaceae resistance to carbapenems: production of carbapenemases, production of efflux pumps and porin mutations or loss.24 CRE is usually resistant to all β-lactams and most other antibiotics, leading to a limited clinical choice of antimicrobial agents, sometimes only tigecycline and colistin are available.25 However, the level of tigecycline in the urinary tract is not high, and the treatment should be cautious.
There were some limitations in our study. First, since it was a retrospective study and the majority of isolates were not collected, no molecular diagnostic approaches were conducted in this paper. Second, the patient-mix was not optimal, which may lead to sub-optimal results. Finally, our study was done in a single tertiary-care teaching hospital with limited data; therefore, the results could not reflect the local AMR precisely.
Conclusion
E. coli is the most common pathogenic bacteria causing UTIs, and ESBL-producing isolates were still the major drug-resistant bacteria in our settings. Carbapenem-resistant UPEC isolates were gradually increasing, which needs to be paid enough attention. Nitrofurantoin, amikacin, piperacillin/tazobactam, cefoperazone/sulbactam, and carbapenems had high susceptibility, and were the preferable choice for the empirical treatment of UTIs. Of course, some factors, such as the cost and safety of antibiotics in the target population, should also be considered when changing therapy. In a word, local timely resistance surveillance data, proper guidelines, and management of antibiotic usage can help to prevent and control antimicrobial resistance.
Ethics Approval
The study was approved by the Ethics Committee of Chongqing Medical University. Due to its retrospective design and anonymous information, the ethics committee waived the requirement for informed consent from patients.
Acknowledgments
The authors are very grateful to all medical staff and patients for participating in the First Affiliated Hospital of Chongqing Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. China Antimicrobial Surveillance Network. Available from: www.chinets.com.
2. Hu F, Guo Y, Zhu D, et al. CHINET surveillance of bacterial resistance in China: 2018 report [in Chinese]. Chin J Infect Chemother. 2020;20(1):1–10.
3. Hu F, Guo Y, Zhu D, et al. Antimicrobial resistance profile of clinical isolates in hospitals across China: report from the CHINET surveillance program, 2017 [in Chinese]. Chin J Infect Chemother. 2018;18(3):241–251.
4. Zhang H, Johnson A, Zhang G, et al. Susceptibilities of gram-negative bacilli from hospital- and community-acquired intra-abdominal and urinary tract infections: a 2016–2017 update of the Chinese SMART study. Infect Drug Resist. 2019;12:905–914. doi:10.2147/IDR.S203572
5. Bader MS, Loeb M, Brooks AA. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad Med. 2017;129:242–258. doi:10.1080/00325481.2017.1246055
6. Yarborough ML. Impact of reflex algorithms on urine culture utilization. Clin Microbiol Newsl. 2018;40:19–24. doi:10.1016/j.clinmicnews.2018.01.003
7. Ironmonger D, Edeghere O, Bains A, et al. Surveillance of antibiotic susceptibility of urinary tract pathogens for a population of 5.6 million over 4 years.. J Antimicrob Chemother. 2016;16(1):1744–1750. doi:10.1093/jac/dkv043
8. Karlowsky JA, Lagacé-Wiens PRS, Adam HJ, et al. In vitro susceptibility of urinary Escherichia coli isolates to first- and second-line empirically prescribed oral antimicrobials: CANWARD surveillance study results for Canadian outpatients, 2007–2016. Int J Antimicrob Agents. 2019;54(1):62–68. doi:10.1016/j.ijantimicag.2019.04.012
9. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Ninth Edition M100-S29. Wayne, PA, USA: CLSI; 2019.
10. Elnasasra A, Alnsasra H, Smolyakov R, et al. Ethnic diversity and increasing resistance patterns of hospitalized community-acquired urinary tract infections in southern Israel: a prospective study. Isr Med Assoc J. 2017;19(9):538–542.
11. Li X, Chen Y, Gao W, et al. A 6-year study of complicated urinary tract infections in southern China: prevalence antibiotic resistance clinical and economic outcomes. Ther Clin Risk Manag. 2017;13(1):1479–1487. doi:10.2147/TCRM.S143358
12. Lai B, Zhang B, Li Y, et al. In vitro susceptibility of Escherichia coli strains isolated from urine samples obtained in mainland china to fosfomycin trometamol and other antibiotics a 9-year surveillance study (2004–2012). BMC Inf Dis. 2014;14:66. doi:10.1186/1471-2334-14-66
13. Armstrong ES, Mikulca JA, Cloutier DJ, et al. Outcomes of high-dose levofloxacin therapy remain bound to the levofloxacin minimum inhibitory concentration in complicated urinary tract infections. BMC Infect Dis. 2016;16(1):1–11. doi:10.1186/s12879-016-2057-2
14. Yang Q, Zhang H, Wang Y, et al. Antimicrobial susceptibilities of aerobic and facultative gram-negative bacilli isolated from Chinese patients with urinary tract infections between 2010 and 2014. BMC Infect Dis. 2017;17:192. doi:10.1186/s12879-017-2296-x
15. Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22(Suppl1):S9–S14. doi:10.1016/j.cmi.2016.01.001
16. Prasada S, Bhat A, Bhat S, et al. Changing antibiotic susceptibility pattern in uropathogenic Escherichia coli over a period of 5 years in a tertiary care center. Infect Drug Resist. 2019;12:1439–1443. doi:10.2147/IDR.S201849
17. Veeraraghavan B, Jesudason MR, Prakasah JAJ, et al. Antimicrobial susceptibility profiles of gram-negative bacteria causing infections collected across India during 2014–2016: study for monitoring antimicrobial resistance trend report. Indian J Med Microbiol. 2018;36(1):32–36. doi:10.4103/ijmm.IJMM_17_415
18. van Driel AA, Notermans DW, Meima A, et al. Antibiotic resistance of Escherichia coli isolated from uncomplicated UTI in general practice patients over a 10-year period. Eur J Clin Microbiol Infect Dis. 2019;38:2151–2158. doi:10.1007/s10096-019-03655-3
19. Gupta K, Grigoryan L, Trautner B. Urinary tract infection. Ann Intern Med. 2017;167(7):ITC49–ITC64.
20. García-Tello A, Gimbernat H, Redondo C, et al. Extended-spectrum beta-lactamases in urinary tract infections caused by Enterobacteria: understanding and guidelines for action. Actas Urol Esp. 2014;38(10):678–684. doi:10.1016/j.acuro.2014.05.004
21. Shakya P, Shrestha D, Maharjan E, et al. ESBL production among E. coli and Klebsiella spp. causing urinary tract infection: a hospital based study. Open Microbiol J. 2017;11:23–30. doi:10.2174/1874285801711010023
22. De Souza GM, Neto ERDS, da Silva AM, et al. Comparative study of genetic diversity, virulence genotype, biofilm formation and antimicrobial resistance of Uropathogenic Escherichia coli (UPEC) isolated from nosocomial and community acquired urinary tract infections. Infect Drug Resist. 2019;12:3595–3606. doi:10.2147/IDR.S228612
23. Bennett PM. Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol. 2008;153(1):S347–S357. doi:10.1038/sj.bjp.0707607
24. Haidar G, Clancy CJ, Chen L, et al. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(9):e00642–e00717. doi:10.1128/AAC.00642-17
25. Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi:10.1128/CMR.05035-11
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
