Back to Journals » Infection and Drug Resistance » Volume 15
Egyptian Consensus on the Role of Lung Ultrasonography During the Coronavirus Disease 2019 Pandemic
Authors Zaky S , Fathelbab HK, Elbadry M, El-Raey F, Abd-Elsalam SM, Makhlouf HA, Makhlouf NA , Metwally MA , Ali-Eldin F, Hasan AA, Alboraie M , Yousef AM, Shata HM, Eid A, Asem N , Khalaf A, Elnady MA, Elbahnasawy M , Abdelaziz A, Shaltout SW , Elshemy E, Wahdan A, Hegazi MS, Abdel Baki A , Hassany M
Received 24 December 2021
Accepted for publication 28 March 2022
Published 20 April 2022 Volume 2022:15 Pages 1995—2013
DOI https://doi.org/10.2147/IDR.S353283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Samy Zaky,1,* Hanaa K Fathelbab,2 Mohamed Elbadry,3,* Fathiya El-Raey,4,* Sherief M Abd-Elsalam,5 Hoda A Makhlouf,6 Nahed A Makhlouf,7 Mohamed A Metwally,8 Fatma Ali-Eldin,9 Ali Abdelazeem Hasan,6 Mohamed Alboraie,10 Ahmed M Yousef,11 Hanan M Shata,12 Alshaimaa Eid,1,* Noha Asem,13 Asmaa Khalaf,14 Mohamed A Elnady,15 Mohamed Elbahnasawy,16 Ahmed Abdelaziz,4 Shaker W Shaltout,17 Eman E Elshemy,1,* Atef Wahdan,18 Mohamed S Hegazi,4 Amin Abdel Baki,19,* Mohamed Hassany19 On behalf of Ministry of Health and Population COVID-19 board and Egyptian Society of fever (ESF) and UCHID-COVID-19 special interest group
1Department of Hepatogastroenterology and Infectious Diseases; Al-Azhar University, Cairo, Egypt; 2Department of Endemic diseases; Minia University, Minia, Egypt; 3Department of Endemic Medicine, Helwan University, Cairo, Egypt; 4Department of Hepatogastroenterology and Infectious Diseases Al-Azhar University, Damietta, Egypt; 5Department of Tropical Medicine, Tanta University, Tanta, Egypt; 6Department of Chest, Assiut University, Assiut, Egypt; 7Department of Tropical Medicine and Gastroenterology, Assiut University, Assiut, Egypt; 8Department of Hepatology, Gastroenterology, and Infectious Diseases, Benha University, Benha, Egypt; 9Department of Tropical medicine; Ain Shams University, Cairo, Egypt; 10Department of Internal Medicine; Al-Azhar University, Cairo, Egypt; 11Department of Community and Industrial Medicine, Damietta, Al-Azhar University, Damietta, Egypt; 12Department of Chest Medicine; Mansoura University, Mansoura, Egypt; 13Department of Public Health and Community Medicine, Cairo University and Ministry of Health and Population, Cairo, Egypt; 14Department of Radiology, Minia University, Minia, Egypt; 15Department of Pulmonary Medicine, Faculty of Medicine, Cairo University, Cairo, Egypt; 16Department of Emergency Medicine and Traumatology, Tanta University, Tanta, Egypt; 17Department of Tropical Medicine, Port Said University, Port Said, Egypt; 18Department of Chest Diseases, Damietta, Al-Azhar University, Damietta, Egypt; 19Department Hepatology, Gastroenterology and Infectious diseases National Hepatology and Tropical Medicine Research Institute NHTMRI, Cairo, Egypt
*These authors contributed equally to this work
Correspondence: Sherief M Abd-Elsalam, Department of Tropical Medicine, Tanta University, Tanta, Egypt, Tel +201063319696, Email [email protected]
Background & Aims: Coronavirus disease 2019 (COVID-19) is a global health problem, presenting with symptoms ranging from mild nonspecific symptoms to serious pneumonia. Early screening techniques are essential in the diagnosis and assessment of disease progression. This consensus was designed to clarify the role of lung ultrasonography versus other imaging modalities in the COVID-19 pandemic.
Methods: A multidisciplinary team consisting of experts from different specialties (ie, pulmonary diseases, infectious diseases, intensive care unit and emergency medicine, radiology, and public health) who deal with patients with COVID-19 from different geographical areas was classified into task groups to review the literatures from different databases and generate 10 statements. The final consensus statements were based on expert physically panelists’ discussion held in Cairo July 2021 followed by electric voting for each statement.
Results: The statements were electronically voted to be either “agree,” “not agree,” or “neutral.” For a statement to be accepted to the consensus, it should have 80% agreement.
Conclusion: Lung ultrasonography is a rapid and useful tool, which can be performed at bedside and overcomes computed tomography limitations, for screening and monitoring patients with COVID-19 with an accepted accuracy rate.
Keywords: lung ultrasound, consensus, coronavirus disease 2019, Egypt
Introduction
The coronavirus disease 2019 (COVID-19) is a pandemic caused by a novel coronavirus species named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Its clinical manifestations range from nonspecific symptoms, such as fever, nausea, and dry cough, to serious pneumonia.1 Available evidence has recommended early therapy given its association with better and faster recovery.2 This highlights the importance of early screening techniques hindering in the progression of the disease.
Unfortunately, real-time polymerase chain reaction (RT-PCR) has some limitations. First, it produces false-negative results when the viral load in the specimen is low, resulting in a low detection. Second, it cannot reflect disease incidence or forecast disease progression. Third, there is a reagent stock shortage, and new reagents require intensive research and development. Fourth, it is a time-consuming procedure. Because of these limitations, researchers suggest using computed tomography (CT) as the primary method for diagnosing COVID-19. Furthermore, patients clinically suspected to have COVID-19 with positive CT results but unfavorable PCR results, the patient should be isolated and managed as a positive case as soon as possible.3 However, the number of cases may exceed the capability of the radiological department. Moreover, patients may be too ill to be moved, or the lack of staff and personal protective equipment (PPE) may preclude this possibility. Furthermore, equipment washing may cause delays in releasing test results. Finally, in resource-constrained environments, traditional radiology might not be available. Lung ultrasonography (LUS) is an excellent option during epidemics due to the lack of ionizing radiation contamination, ease of equipment sterilization, and significantly lower cost. Furthermore, low-cost portable ultrasound machines have recently been developed. These instruments could significantly reduce the costs of LUS implementations.4
LUS may now be conducted immediately at the bedside by a single operator, eliminating the possibility of cross-contamination, limiting healthcare workers’ susceptibility to extreme acute SARS-CoV-2 infection, and minimizing PPE shortages in several healthcare settings.5 Furthermore, the absence of radiation in LUS is crucial in patients who are more vulnerable to radiation toxicity, such as pregnant women, in which LUS is a reliable instrument for tracking COVID-19 progression.6 Considering that, CT is not widely available in several developing countries, for confirming suspected COVID-19 cases, a comparatively inexpensive initial diagnostic and screening method is needed. Such a technique may be ultrasonography given its feasibility and portability and reliable sensitivity. However, ultrasonography is recommended to be used as an integrated diagnostic instrument.7 Furthermore, in health care settings with inadequate resources, LUS with oxygen saturation monitoring can be is an effective method for determining the priority at which COVID-19 patients.8 This context calls for better understanding on the features of LUS, such as its efficacy in both diagnosing and tracking the progression of lung diseases, including viral pneumonia. This consensus was designed to clarify the role of lung ultrasonography versus other imaging modalities in the COVID-19 pandemic.
Methods
For consensus development, We used Nominal Group Process and Consensus Development Panel. The chairperson and two coordinators of the consensus are members of the Egyptian scientific committee for management of COVID-19. They identified the research questions and the tasks of the consensus. They selected a multidisciplinary group consisting of experts in research and management of COVID-19 in Egypt. Selection of the expert group was based upon their practical input in diagnosis and treatment of COVID-19, their publication in the field of the consensus, their input in epidemiological research and their representation for the specialties included in management, namely tropical medicine, infectious diseases, pulmonology, emergency medicine, radiology, and epidemiology. The expert also represented most of Egyptian Universities and different geographical areas in Egypt.
Four tasks were identified:
- Importance of imaging study in managing COVID-19.
- Comparing the role of LUS in the diagnosis and follow-up of COVID-19 compared with that of other imaging modalities.
- LUS criteria for the diagnosis of COVID-19.
- Safety and infection control regulations for the use of LUS.
The group has been classified into task groups. Each group was responsible for reviewing the literature from different databases, including PubMed, Cochrane Library, and Scopus, to better understand and abstract the responses to the task.
The statements generated by each group were recirculated anonymously by the chairperson among the entire group using the WhatsApp application. Each member reviewed the statements and provide their comments. After obtaining feedback the statements were reviewed again by the task groups after getting the feedback who then made changes.
A multidisciplinary expert group consisting of 32 delegate members held a face to face meeting for 6 h on July 16, 2021. Each statement was presented by one of the task groups, including the statement’s evidence. The presenter and facilitator responded to the group questions. After discussing each statement, an anonymous electronic voting was run. Each statement was voted as either “agree,” “disagree,” or “neutral.”
The statement should have a predefined score of 80% agreement to be accepted and reported in this manuscript.
Summary of the Statements and Their Percentage of Agreement (Table 1)
Statement 1
Objective staging of the disease severity and evaluation of the course of infection help make proper treatment choices and improve prognosis. Consequently, this would reflect the reduction in the chain of transmission and the overall morbidity and mortality from COVID-19.
 |
Table 1 Summary of the Statements and Their Percentage of Agreement |
Rationale
COVID-19 was classified as a class B infectious disease by the Centers for Disease Control and Prevention (CDC) on January 20, 2020.9 Even with substantial suppression measures in place, there may be shortage of beds, physicians, oxygen, and PPE. Because of measures, such as school lockdowns and distance restrictions, poverty and undernutrition may increase, where as educational achievement and recent gains in access to health care may decrease.10
The most essential steps in controlling COVID-19 are rapid case detection, severity assessment, and contact tracing because they allow close contacts or even suspected persons to be identified, isolated, and tested for the disease.11
The uncertainty in the clinical symptoms of COVID-19 is a challenge, and atypical patients must be carefully diagnosed since they may serve as carriers of the disease in the community. In addition to clinical criteria (pneumonia with fever and cough without or with indications of hypoxia), COVID-19 case definition considers radiological criteria (because “some cases may have no clinical signs or symptoms; however, chest CT or LUS shows subclinical lung lesions”).12
Staging is a method for determining the severity of a disease based purely on predetermined diagnostic criteria to provide information regarding the illness severity during hospitalization.13 With such information, we can establish a diagnosis, recommend a treatment plan, and predict the outcomes.14 Otherwise, the more delays in diagnosis; the higher the death rates.15
In developing countries, such as Africa, COVID-19 management options may be restricted given that the epidemic’s peak capacity may be several fold larger than the baseline capacity.16
LUS is a safe and simple method for monitoring pneumonia and other respiratory diseases.17
Statement 2
Indication for Lung Imaging
- Given that respiratory dysfunction is the main cause of morbidity and mortality in patients with COVID-19, lung imaging is considered a key tool for assessing the disease.
- Lung imaging is indicated for the medical triage of patients suspected to have COVID-19, who present with moderate to severe disease. It also helps to determine the proper site for patient’s care: home, hospital ward, or intensive care unit.
Imaging may also be advised to assess disease progression during the follow-up of confirmed COVID-19 cases.
Rationale
Lung imaging is the cornerstone for the diagnosis and severity classification of SARS-CoV-2 infection according to the National Institute of Health guidelines.18 It is essential in diagnosing moderate and severe disease.
The observed abnormalities on chest X-rays vary; however, bilateral multifocal opacities are the most common. The observed abnormalities in chest CT also vary; however, bilateral peripheral ground-glass opacities (GGOs) are the most common findings, with consolidation areas occurring later in the clinical course of COVID-19. Imaging results may be normal early in infection and can be abnormal in the absence of symptoms.19
A meta-analysis of 28 studies involving 2655 patients has reported that chest CT is helpful for not only the diagnosis of the disease but also the assessment of disease progression based on follow-up chest CT scans.20
Latrice et al reported that chest X-ray could be used as the first-line imaging modality in areas with high levels of contagion and in the serial evaluation of hospitalized and critically ill patients. In contrast, high-resolution CT (HRCT) showed a low specificity in areas with a low prevalence of disease and should be considered the modality of choice in determining differential diagnoses with other infectious and noninfectious lung diseases and in managing patients with preexisting lung disease.21
Yassa et al recommended LUS for asymptomatic pregnant patients with positive PCR for whom CT is not planned and those with initial negative CT findings for follow-up. Also, in those with mild symptoms who do not give consent for chest CT. In addition, abnormal LUS imaging in a symptomatic patient with “mild” clinical severity can be suggestive of a probable bad prognosis, particularly in pregnant women.22
Statement 3
Chest CT is more sensitive and effective in triaging or following up patients with COVID-19 than chest X-ray.
- The typical HRCT pattern consists of multiple GGOs mainly in the peripheral lung regions and basal distribution.
- GGOs may be combined with other features, such as pulmonary consolidation, crazy paving, halo signs, basal reticulations, and vascular enlargement.
Rationale
CT findings differ according to the stage and severity of the disease and associated comorbidities. The sensitivity of CT depends on the duration of symptoms. Negative CT findings were reported in 56% of cases scanned within 2 days after the onset of symptoms, whereas negative scans were reported in 9% of cases who underwent CT within 3–5 days and 4% of those scanned 6–12 days after symptom onset.23,24
CT has a higher sensitivity than RT-PCR. Accordingly, CT has a sensitivity of 97.2%, whereas initial rRT-PCR has a sensitivity of 83.3%. Microbiological tests, such as rRT-PCR, may not be available in emergency situations, and their results take 4–5 days. Moreover, rRT-PCR can provide false-negative results when the viral load is insufficient,25 with other reasons including improper sampling, the timing of sampling, or laboratory issues.26
In the early stages of the disease, the typical HRCT pattern consists of single or multiple GGOs, mainly distributed in the subpleural and basal regions, which continue to expand as the disease progresses. In the later stages of COVID-19, GGO is often combined with other imaging features, such as pulmonary consolidation, crazy paving, halo signs, basal reticulations, and vascular enlargement. Thus, during pandemics, similar to the current situation, chest CT can be used as a tool for screening symptomatic patients as it is cheap, readily available, given its lower cost, ready availability, and rapid results.27–32
Statement 4
The limitations of chest CT during the COVID-19 pandemic in developing countries include burden, cost, unavailability, infection, repetition, and exposure to ionizing radiation.
Rationale
The number of cases may exceed the capability of the radiological department. Moreover, patients may be too ill to be moved, or the lack of staff and PPE may preclude this possibility. Furthermore, equipment disinfection may delay the speed of tests. Finally, in resource-constrained environments, traditional radiology may not be available at all. Thus, the use of CT has been widely used as an initial screening and diagnostic technique for viral pneumonia (including COVID-19). Normal radiology imaging can have certain shortcomings in a pandemic.3
In the early stages of COVID-19, lung involvement may precede clinical manifestations and even positive PCR swab test. Thus, early chest CT is recommended for screening suspected patients.33 The high contagiousness of SARS-CoV-2 and the difficulties of moving sick patients (in ICU), in addition to radiation hazards and its unavailability in primary healthcare centers, are obstacles limiting the use of CT and necessitate the use of a more practical alternative.34
The need for CT increases the burden in many healthcare centers worldwide, including chest and/or fever hospitals. Accordingly, Egypt can be considered one of the lower-middle-income countries with limited resources.35
Statement 5
LUS allows for a rapid bedside testing that does not involve exposure to ionizing radiation, has a lower cost, and can be repeated (whenever indicated) without significant risks to the patient.
Rationale
LUS had an accuracy similar to that of chest CT in detecting lung abnormalities in patients with COVID-19. It is a highly sensitive and specific approach that can be used instead of CT, with the additional value of being rapid, easy to use, readily available. Moreover, it can be used at the bedside and can be repeated without exposure to radiation hazards.35 Hense, LUS can be used in triaging symptomatic cases, assessing lung damage severity, and evaluating disease progression.36 LUS is an excellent option during epidemics due to the lack of ionizing radiation contamination, ease of equipment sterilization, and significantly lower cost. Furthermore, low-cost portable ultrasound machines have recently been developed, which could significantly reduce the costs of LUS implementations, in terms of both buying and shipping the equipment.4
LUS may now be immediately performed at the bedside by a single operator, eliminating the chance of cross-contamination, limiting health care workers’ susceptibility to extreme acute SARS-CoV-2 infection, and alleviating PPE shortages in several health care settings.5 Furthermore, the absence of radiation in LUS is crucial for patients who are more vulnerable to radiation toxicity, such as pregnant women, in whom LUS is a reliable instrument for tracking COVID-19 disease progression.6
Statement 6
a: A normally aerated lung is characterized by the presence of the following:
- Bright, thin, smooth pleural line between two ribs.
- Lines that are repeated horizontal artifacts parallel to the pleural line.
- Normal lung sliding.
Rationale
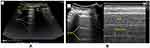
In the normal aerated lung, more than 99.9% of ultrasound beams at the visceral pleura–lung air boundary. This boundary appears as a white, bright, thin, smooth band (pleural line) measuring up to 2 mm, seen 0.5 cm deeper between upper and lower ribs.8,37 The appearance of two ribs and a pleural line in between them is called the bat sign. Beyond this pleural line, reverberation artifacts produced by the bouncing of echo38 between the pleural line and probe can be observed. These motionless, regularly spaced (equal to the distance between the skin and the pleural line) and gradually fading horizontal white lines, which resemble the pleural line, are called A-lines (Figure 1A) (A profile).39
Movement of the visceral pleura over the parietal pleura produces lung sliding, and this white band appears dynamic. Lung sliding is the depiction of a regular rhythmic movement synchronized with respiration occurring between the parietal and visceral pleurae that are either in direct apposition or separated by a thin layer of intrapleural fluid. Lung sliding indicates ventilation in the inspected area. In time motion mode (M mode), the structures until the parietal pleura appear as horizontal lines, and this sandy pattern represents lung sliding. This finding is called the seashore sign (Figure 1B). The presence of the pleural line, lung sliding, A-lines in 2D, and seashore sign in the M mode means an aerated lung.40–43
b: A poorly aerated lung is characterized by multiple B-lines, irregular pleural lines with or without subpleural consolidative patterns on LUS.
- Moderate loss of lung aeration (interstitial syndrome): multiple (>3) spaced B-lines. This corresponds to a “ground-glass area” on lung HRCT.
- Severe aeration loss (diffuse alveolar edema): diffuse coalescent B-lines occupying most of the intercostal space.
- Complete aeration loss (lung consolidation [C]): the presence of a tissue pattern characterized by dynamic air bronchograms.
Rationale
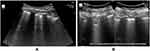
SARS-CoV-2 infection initially presents as first proceeds with an interstitial inflammatory infiltrate, resulting in alveolo-interstitial pneumonia and endothelial damage.44 In the early stages of COVID-19, changes in the lungs are localized and mainly found in the subpleural area of one or both lungs.45 LUS showed irregular thickening of the pleural lines, which progressed to the formation of B-lines and loss of A-lines (Figure 2).38,46 Later, the pathology involves multiple lobes, leading to more air loss and consolidations of some lesions surrounded by several B-lines (Figure 2B).45 Fluid accumulations in the subpleural interstitium and alveolar spaces change the air–fluid ratio and form this characteristic artifact (ie, B-lines). The B-lines are defined as discrete laser-like vertical hyperechoic reverberation artifacts starting from the pleural line and extend to the bottom of the screen without fading and moving synchronously with lung sliding.47 A positive region “B-pattern” is defined as the presence of three or more B-lines in a longitudinal plane between two ribs.8,47–50 Multiple B-lines are correlated with the ground-glass areas on lung CT of confirmed COVID-19 cases.5 Multiple B-lines range from focal to multifocal, spaced, or continuously fused (confluent) B-lines (white-lung sign) with the disappearance of A-lines according to disease stage.51
Over time, subpleural consolidations start to appear and spread bilaterally on both the posterior lower lobes on LUS. When a complete consolidation of the lung occurs, the echo structure of the lung itself becomes visible with characteristic air bronchogram, representing the air inside alveoli or bronchi surrounded by inflammation or pus; and the pleural lines are completely obscured.51–54
The characteristic ultrasonographic findings of COVID-19 lung involvement includes thickening irregularity of the pleural line; in various B-lines patterns (ie, focal, multifocal, and confluent); and consolidations with occasional mobile air bronchograms.37,38,52,53,55–60 In COVID −19-induced acute respiratory distress syndrome (ARDS), LUS shows a white area in which neither A-lines nor separated B-lines are visible. This presentation is called a “white lung”.38
However, B-lines are non-specific artifacts associated with increased extravascular lung water or partial loss of lung aeration,46 and they can be detected in a variety of pulmonary diseases, including interstitial lung disease, heart failure, acute respiratory distress syndrome, etc. However, LUS manifestations in COVID-19 patients shared not only the features of an increase in B-lines but also consolidations, irregular or blurred pleural line.61
Statement 7
During the COVID-19 pandemic, the presence of multiple (≥3) B-lines with or without subpleural consolidations distributed bilaterally, peripherally, or basally in patches with spared areas on LUS is highly suggestive of COVID-19 pneumonia in clinically suspected patients.
Rationale
Chest ultrasonography showed predominant affection of the posterior and inferior areas of the lung (lower lobes).55 Simultaneous affection of the upper lobe was associated with a more severe clinical course.62 Bilateral, peripheral, and multilobar lung lesions have been frequently found in patients with COVID-19 on chest US and confirmed by CT.26,63,64 Moreover, 94% of patients with confirmed COVID-19 demonstrated pathological B-lines (Figure 2), with 82% of them showing bilateral distribution in. The multifocal appearance of B-lines was predominantly found in 59% of patients. Pleural irregularities occurred mostly occurred bilaterally (89%). Pulmonary consolidations, which are characterized by a rather focal and mostly subpleural appearance presenting frequently in not only the basal but also apical parts of the lung were found in 77% of COVID-19 cases. Abnormal lung sliding was observed in only 17% of patients, especially those with multifocal consolidations.54,62,65 Patchy distribution (Figure 3) of spaced or confluent B-lines and small white-lung regions were observed in patients with early COVID-19. As the disease progresses, patchy small subpleural consolidations appear with associated areas of white lung.2 The LUS findings of 197 patients (81.1%) were completely coincident with CT findings with a Kappa agreement value of 0.77, and this offered a diagnostic sensitivity of 74%, a diagnostic specificity of 97.9%, positive predictive value of 90.2%, and negative predictive value of 93.6% for LUS in triaging patients with COVID-19.8 The World Health Organization defines cases having the following LUS features as those with probable COVID-19: thickened pleural lines, B-lines (multifocal, discrete, or confluent), and consolidative patterns with or without air bronchograms.22,65
Unlike to COVID-19 pneumonia, ultrasound images of community-acquired pneumonia often show large and circumscribed consolidation accompanied by bronchial gas phase or liquid phase and pleural effusion. Consolidations were more extensive in bacterial pneumonia. Lung tissue was completely degassed and exhibited a solid tissue echo “a pattern showing” “hepatic-like changes”.66
Statement 8
The LUS score based on the examination of 12 standard regions can be used to assess lung aeration changes and follow up disease progression.
Rationale
LUS score can measure the loss of lung aeration produced by diverse pathological situations.67,68 Some studies have proven that LUS could predict the outcomes in patients with SARS-CoV-2 infection. LUS has been strongly correlated with lung involvement and provides risk stratification, including prediction of mechanical ventilation requirement and mortality risk.69,70 To assess the disease severity, different protocols for the LUS scoring system (Table 2) had been developed. The emergency department LUS protocol for patients with COVID-19 using bedside LUS, can grade disease severity and help clinicians make appropriate decisions and follow up patients with COVID-19. Patients can be examined in the sitting position. When such a position was not possible due to worsening clinical condition or lack of compliance, the examination could be performed in the supine, semi-recumbent, or lateral decubitus positions on both sides.71
 |
Table 2 Different Protocols for the Lung Ultrasound Scoring System |
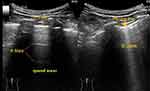
The LUS protocol involves the examination of 12 lung regions, with the entire procedure taking approximately 5 min when performed by an experienced physician.72 On each side, six lung regions of interest (Figure 4), delineated by a parasternal line, anterior axillary line, posterior axillary line, and paravertebral line, are examined.71 Each region is scored from 0 to 3 according to four ultrasound aeration patterns (Figure 5): 0 = normal aeration, 1 = interstitial syndrome, 2 = alveolar edema, and 3 = consolidation.70–72 The final LUS score is the sum of the points across in all 12 regions and ranges from 0 to 36.74,75
We seek from this statement to help physicians in ICU and ER, not only sonographers, to practice lung sonography in a simple, fast, and applicable technique. This technique includes well-known 12 lung zones and well-known anatomical lines as if they are employing the ultrasound probe as their stethoscope. The LUS score (0–3) does not only aid in the diagnosis of COVID-19 pneumonia but also helps in triaging COVID patients in ER, predicting outcomes and the need for invasive mechanical ventilation, and follow-up of these patients.76 Volpicelli et al conducted an international multisystem study on 1462 patients. In this study, all intercostal spaces were evaluated in 5 scans (see Table 2), additional scans were done to evaluate focal abnormalities, many video clips were captured to evaluate series of intercostal spaces per area, both the ultrasound convex and linear probes were used, intercostal spaces were evaluated in longitudinal and oblique views with additional tilting to optimize visualization of pleura. However, this study helps only in the early diagnosis of COVID-19, lacking the advantage of follow-up and predicting outcomes of COVID-19 patients.77
Statement 9
Bedside LUS, by a well-trained physician or expert, is a useful screening and monitoring tool for suspected or confirmed COVID-19 cases, with an acceptable accuracy rate.
Rationale
LUS has an established accuracy for diagnosing lung diseases. It can be used for daily assessment and monitoring of patients with COVID-19. Growing literature and international networks have encouraged using LUS to diagnose COVID-19 pneumonia.78 LUS results are similar to those obtained by HRCT and outperform the standard chest X-ray results for assessing pneumonia and ARDS, with additional benefits of ease of use in point of care and acceptable accuracy.79
Thus, LUS may assist in tracking the clinical disease course and treatment outcomes and altering ventilator settings in severely ill patients or those undergoing mechanical ventilation.7,80,81 LUS has shown high sensitivity to pulmonary lesions and can overcome the time gap for transferring patients to the CT unit.82 Moreover, it can show the resolution of lung pathology after 96 h of admission.79
In the United States, point of care ultrasonography training is highly recommended in programs for internal medicine residency evaluated the efficacy of a 2-week ultrasound elective training for internal medicine residents including acquisition and retention of knowledge and performing thoracic ultrasonography.83 They concluded that the interactive ultrasound elective training for 2 weeks demonstrated efficacy in knowledge acquisition and retention related to ultrasound.84,79
Statement 10
Infection Control is Paramount
-National and local guidelines on PPE usage should be followed.
-The probe should be disinfected before and after patient examination to avoid nosocomial infections and cross-contamination.
Rationale
SARS-CoV-2 is transmitted mainly through close contact and respiratory droplets in the ultrasound room as LUS is not an aerosol-generating procedure. However, it is different in intensive care units where aerosol-generating procedures may be performed, and airborne transmission can occur.85 Adherence to infection prevention and control measures is essential to protect patients and healthcare workers. We will discuss the following three points:
- PPE
- Cleaning and disinfection of the ultrasound probe
- Environmental cleaning and disinfection
PPE
- The ultrasound procedure requires close contact with suspected or infected patient. LUS is not an aerosol-producing procedure. Therefore, the following PPE should be worn: surgical mask, eye protection, gown, and gloves.86
- The number of persons in the ultrasound room should be limited when examining a patient with suspected or confirmed COVID-19.87
- All persons in the room not in close contact with the patient should wear a surgical mask and eye protection.
- During environmental cleaning, workers should wear a surgical mask and goggles.
- Adherence to the Five Moments for Hand Hygiene using alcohol-based hand soaps is crucial, especially after removing gloves.88
- Proper disposal of PPE after removal is a must.
Environmental Surface Cleaning and Disinfection
- The use of furniture, which should be made of easily cleaned and disinfected materials, should be limited as much as possible. All unnecessary decorations and accessories should be removed to facilitate the process of cleaning and disinfection.
- The ultrasound room should be thoroughly cleaned using detergent and water daily and when needed.
- Surfaces should be disinfected, especially frequently touched surfaces, using hospital-approved disinfectants.89
- All surfaces should be disinfected between patients, focusing on the examination bed monitors, computer keyboard, computer mice, gel container, door handles, cabinet knobs, light switches, chairs, and counter tops.90
- Any used linen should be disposed between patients in a contaminated linen bag. Using disposable linens is advisable if available.91
Ultrasound Probe Disinfection
Cleaning and disinfection for external probes:
- Given that LUS is a noncritical procedure, low- or intermediate-level disinfection of the transducer is enough to denature most bacteria, some fungi, and some viruses, such as COVID-19.90
- The transducer should be cleaned, followed by disinfection. Ultrasound gels and any debris should be removed using a paper towel, after which a detergent-impregnated wipe approved for use on medical devices should be used. For disinfection, the probe should be wiped using a low- or intermediate-level disinfectant compatible with the ultrasound probe according to the manufacturer’s instructions.92
- The entire ultrasound machine should be disinfected, particularly the keyboard, screen, and ultrasound probe cord, using an approved ultrasound machine disinfectant wipe or solution.93
- Plastics or disposable covers can be used to decrease contamination of the probe and the entire ultrasound machine, especially the keyboard and probe cord. All disposable covers should be removed. The ultrasound machine and probe should be disinfected between patients.89
Disinfectants Used for Environmental Cleaning
• Ethanol (70–90%).
• Chlorine-based products (eg, hypochlorite) at 0.1% (1000 ppm) for general environmental disinfection or 0.5% (5000 ppm) for large spills of blood and body fluids.
• Hydrogen peroxide (more than 0.5%).
Contact time of a minimum of 1 min is recommended for these disinfectants.94
Disinfectants for Cleaning the Ultrasound Transducer
Chemical “wet” disinfection:
• 2.4–3.2% glutaraldehyde products (eg, Cidex [ASP, Advanced Sterilization Products, Zug, Switzerland], Metricide [Metrex, Orange, CA, USA], and Procide [Metrex, Orange, CA, USA]).
• Non-glutaraldehyde agents (eg, Cidex OPA [o-phthalaldehyde], Cidex PA [hydrogen peroxide], and peroxyacetic acid).
• Approved multi-step disinfectant wipes containing chlorine dioxide, used extensiveily in the United Kingdom and Australia (eg, Tristel Duo [Tristel, Snailwell, Cambridgeshire, UK]).
• 7.5% hydrogen peroxide solution (works by producing destructive hydroxyl free radicals).
• Sodium hypochlorite 0.21% (Antisapril Blu 2% [Monteroni d’Arbia, Italy]).
Limitation
One of the limitations of this consensus, is the absence of special statements for the use of LUS in pregnant women or child/newborns which may need special consensus. However, Yassa et al reported that the use of LUS in pregnant women while awaiting the RT-PCR results may have an important role specially in low-resource setting, In pregnant women LUS was reported to be more predictive in detecting COVID −19 Infection than the use of symptoms only.22
Recommendation
For symptomatic patients with SARS-CoV-2 infection, initial evaluation may include chest X-ray, ultrasound, or CT, if indicated. Point of care LUS training courses are highly recommended for junior physicians in triage or designated hospitals.
Acknowledgments
We want to deeply thank and appreciate Ministry of Health and Population COVID-19 board, Egyptian Society of fever (ESF) and UCHID-COVID-19 special interest group: Gamal Esmat; Hossam Hosny; Hanaa Omer; Ehab Kamal; Hatem Elalfy; Ahmed Cordie; Ahmed A. Gomaa; Ahmed S. Abdalmohsen; Akram Abdelbary; Khaled Teima; Ramadan Zaky; Ahmed Mahdy; Ahmed Maher2; Basem Eisa; Dina Attia; Gamal Rabie; Fatema Abdelsalam; Hamdy Ibrahiem; Mahmoud Khalil; Maysaa A. Saeed; Mohamed El Kassas; Mohamed Hamdy; Nemat Abdelmagiud; Usama Hantiur, Rania Samy; Ibrahim F. Mahmoud; Reham Soliman; El-Sayed Emam; Sherief Musa; Yasser Mahrous; Gamal Shiha; Gehan Elassal.
With affiliations from these organizations: Endemic Medicine and Hepatology Department, Faculty of Medicine, Cairo University, Cairo, Egypt; Division of Tropical Medicine Medical Research, National Research Centre, Giza, Egypt; Department of endemic medicine, Mansoura university; Department of Tropical Medicine, Fayoum University, Egypt; Critical Care Medicine, Cairo University, Cairo, Egypt; Ministry of Health and Population, Egypt; Department of Zoonotic diseases, National Research Center, Egypt; Department of Tropical Medicine, Bani Sweif University, Egypt; Tropical medicine and infectious Diseases department, Faculty of Medicine, Zagazig University, Cairo, Egypt; Department of anesthesiology and intensive care, Al-Azhar university, Egypt; Hepatology and Gastroenterology Unit, Internal Medicine Department, Faculty of Medicine, Mansoura University, Egypt; Chest diseases Department, Faculty of Medicine, Cairo University, Cairo, Egypt.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854–887. doi:10.1007/s00134-020-06022-5
2. Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020;39(7):1459–1462. doi:10.1002/jum.15284
3. Wan Y, Shang J, Graham R, Baric RS, Li F, Gallagher T. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127–20. doi:10.1128/JVI.00127-20
4. Convissar DL, Gibson LE, Berra L, Bittner EA, Chang MG. Application of lung ultrasound during the COVID-19 pandemic: a narrative review. Anesth Analg. 2020;131(2):345–350. doi:10.1213/ANE.0000000000004929
5. Yasukawa K, Minami T. Point-of-care lung ultrasound findings in patients with COVID-19 Pneumonia. Am J Trop Med Hyg. 2020;102(6):1198–1202. doi:10.4269/ajtmh.20-0280
6. Buonsenso D, Moro F, Inchingolo R, et al. Effectiveness of rapid lung ultrasound training program for gynecologists and obstetricians managing pregnant women with suspected COVID‐19. Ultrasound Obstet Gynecol. 2020;56(1):110–111. doi:10.1002/uog.22066
7. Alrifai A, El-Raey FM, Yousef AM, Zaky S. Ultrasound in suspected pneumonic COVID-19: our experience. Int J Med Art. 2020;2(4):682–689. doi:10.21608/IJMA.2020.43493.1176
8. Zaky S, Metwally MA, El Badry M, et al. Utility of lung ultrasound in Decision-making to prioritize hospital admission for COVID-19 patients: a developing country perspective. Curr Med Imag. 2021;17(12):1473–1480. doi:10.2174/1573405617666210506164243
9. Qiu Y, Chen X, Shi W. Impacts of social and economic factors on the transmission of coronavirus disease 2019 (COVID-19) in China. J Popul Econ. 2020;33:1127–1172. doi:10.1007/s00148-020-00778-2
10. Chowdhury R, Heng K, Shawon MS, et al. Dynamic interventions to control COVID-19 pandemic: a multivariate prediction modelling study comparing 16 worldwide countries. Eur J Epidemiol. 2020;35(5):389–399. doi:10.1007/s10654-020-00649-w
11. Dahab M, Van Zandvoort K, Flasche S, et al.COVID-19 control in low-income settings and displaced populations: what can realistically be done? Confl Health. 2020;14(1):1–6. doi:10.1186/s13031-020-00296-8
12. Chen D, Tang F, Lu S, Song Q. Toward a clinically based classification of disease severity for paediatric COVID-19–Authors’ reply. Lancet Infect Dis. 2021;21(1):22–23. doi:10.1016/S1473-3099(20)30397-2
13. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 infectious diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132–73. doi:10.1093/cid/cis346
14. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Failure. 2014;1(1):4–25. doi:10.1002/ehf2.12005
15. Cobre AD, Böger B, Fachi MM, et al. Risk factors associated with delay in diagnosis and mortality in patients with COVID-19 in the city of Rio de Janeiro, Brazil. Cien Saude Colet. 2020;25:4131–4140. doi:10.1590/1413-812320202510.2.26882020
16. Honein MA, Christie A, Rose DA, et al. Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, December 2020. Morb Mortal Wkly Rep. 2020;69(49):1860. doi:10.15585/mmwr.mm6949e2
17. Buonsenso D, De Rose C. Implementation of lung ultrasound in low-to-middle income countries: a new challenge global health? Authorea. 2021. doi:10.22541/au.161449326.65024375/v1
18. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available from: https://www.covid19treatmentguidelines.nih.gov/.
19. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi:10.1016/S1473-3099(20)30086-4
20. Ishfaq A, Yousaf Farooq SM, Goraya A, et al. Role of high resolution computed tomography chest in the diagnosis and evaluation of COVID −19 patients -A systematic review and meta-analysis. Eur J Radiol Open. 2021;8:100350. doi:10.1016/j.ejro.2021.100350
21. Larici AR, Cicchetti G, Larici AR, et al. Multimodality imaging of COVID-19 pneumonia: from diagnosis to follow-up. A comprehensive review. Eur J Radiol. 2020;131:109217. PMID: 32861174; PMCID: PMC7430292. doi:10.1016/j.ejrad.2020.109217
22. Yassa M, Birol P, Mutlu AM, Tekin AB, Sandal K, Tug N. Lung ultrasound can influence the clinical treatment of pregnant women with COVID-19. J Ultrasound Med. 2021;40(1):191–203. PMID: 32478445; PMCID: PMC7300952. doi:10.1002/jum.15367
23. World Health Organization. WHO COVID-19 case definition. WHO reference number: WHO/2019-nCoV/Surveillance_Case_Definition/2020.1. Available from: https://apps.who.int/iris/handle/10665/333912?show=ful.
24. Bernheim A, Mei X, Huang M, et al. Chest CT findings in Coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463. PMID: 32077789; PMCID: PMC7233369. doi:10.1148/radiol.2020200463
25. Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;297(3):E346. PMID: 33196374. doi:10.1148/radiol.2020209021
26. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296(2):E115–E117. doi:10.1148/radiol.2020200432
27. Pan Y, Guan H, Zhou S. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306–3309.
28. Qian L, Yu J, Shi H. Severe acute respiratory disease in a huanan seafood market worker: images of an early casualty. Radiol Cardiothorac Imag. 2020;2(1):e200033. doi:10.1148/ryct.2020200033
29. Shi H, Han X, Zheng C. Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology. 2020;295(1):20. doi:10.1148/radio
30. Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):18. doi:10.1148/radiol.2020200236
31. Xu X, Yu C, Zhang L, Luo L, Liu J. Imaging features of 2019 novel coronavirus pneumonia. Eur J Nucl Med Mol Imag. 2020;47(5):1022–1023. doi:10.1007/s00259-020-04720-2
32. Kong W, Agarwal PP. Chest imaging appearance of COVID-19 infection. Radiol Cardioth Imag. 2020;2(1):e200028. doi:10.1148/ryct.2020200028
33. Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1):4. doi:10.1186/s40779-020-0233-6
34. Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295:200370.
35. World bank. Data of Egypt, Arab Rep., lower middle income. Available from: https://data.worldbank.org/?locations=EG-XN.
36. Dudea SM. Ultrasonography and SARS-CoV 2 infection: a review of what we know and do not yet know. Med Ultrason. 2020;22(2):129–132. doi:10.11152/mu-2612
37. Koegelenberg CF, Von Groote-bidlingmaier F, Bolliger CT. Transthoracic ultrasonography for the respiratory physician. Respiration. 2012;84(4):337–350. doi:10.1159/000339997
38. Miller A. Practical approach to lung ultrasound. BJA Educ. 2016;16:39–45. doi:10.1093/bjaceaccp/mkv012
39. Lichtenstein DA. The A-profile (normal lung surface): 2) lung sliding. Lung Ultrasound in the Critically Ill. Springer, Cham. 2016. doi:10.1007/978-3-319-15371-1_10
40. Bouhemad B, Zhang M, Lu Q, Rouby JJ. Clinical review: bedside lung ultrasound in critical care practice. Crit Care. 2007;11:205. doi:10.1186/cc5668
41. Aldrich JE. Basic physics of ultrasound imaging. Crit Care Med. 2007;35(Suppl):S131–7. doi:10.1097/01.CCM.0000260624.99430.22
42. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117–125. doi:10.1378/chest.07-2800
43. Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A. A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest. 2009;136:1014–1020. doi:10.1378/chest.09-0001
44. Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. doi:10.1186/s13054-020-03062-7
45. Zanforlin A, Tursi F, Marchetti G, et al. Clinical use and barriers of thoracic ultrasound: a survey of Italian pulmonologists. Eur Respirat J. 2018. doi:10.1183/13993003.congress-2018.PA378
46. Volpicelli G, Elbarbary M, Blaivas M, et al. International liaison committee on lung ultrasound (ILC-LUS) for International consensus conference on lung ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi:10.1007/s00134-012-2513-4
47. Via G, Storti E, Gulati G, Neri L, Mojoli F, Braschi A. Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol. 2012;78:1282–1296.
48. Mathis G. Thoraxsonography–Part 1: chest wall and pleura. Praxis. 2004;93::615–621. doi:10.1024/0369-8394.93.15.615
49. Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. doi:10.1186/1476-7120-6-16
50. Reissig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med. 2003;22:173–180. doi:10.7863/jum.2003.22.2.173
51. Sultan LR, Sehgal CM. a review of early experience in lung ultrasound in the diagnosis and management of covid-19. Ultrasound Med Biol. 2020;46(9):2530 2545. doi:10.1016/j.ultrasmedbio.2020.05.012
52. Peng QY, Wang XT, Zhang LN, et al. Findings of lung ultrasonography of novel Corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020;46:849–850. doi:10.1007/s00134-020-05996-6
53. Thomas A, Haljan G, Mitra A. Lung ultrasound findings in a 64-year-old woman with COVID-19. Can Med Assoc J. 2020;192:E399. doi:10.1503/cmaj.200414
54. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:7100231.
55. Yi H, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19); February 26, 2020. doi: 10.2139/ssrn.3544750.
56. Buonsenso D, Raffaelli F, Tamburrini E, et al. Clinical role of lung ultrasound for the diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound Obstet Gynecol. 2020;56(1):106–109. doi:10.1002/uog.22055
57. Vetrugno L, Bove T, Orso D, et al. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37(4):625–627. doi:10.1111/echo.14664
58. Kalafat E, Yaprak E, Cinar G, et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. 2020;55(6):835–837. doi:10.1002/uog.22034
59. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi:10.1097/00000542-200401000-00006
60. Buonsenso D, Piano A, Raffaelli F, Bonadia N, de Gaetano DK, Franceschi F. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780. doi:10.26355/eurrev_202003_20549
61. Ji L, Cao C, Gao Y, et al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit Care. 2020;24:700. doi:10.1186/s13054-020-03416-1
62. Shumilov E, Hosseini A, Petzold G, et al. Comparison of chest ultrasound and standard X-ray imaging in COVID-19 patients. Ultrasound Int Open. 2020;6(2):E36–E40. doi:10.1055/a-1217-1603
63. Ng M-Y, Lee EYP, Yang J, et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiology. 2020;2:e200034. doi:10.1148/ryct.2020200034
64. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;295:202–207. doi:10.1148/radiol.2020200230
65. Egyptian ministry of health official report; 2020. Available from: www.mohp.gov.
66. Tan G, Lian X, Zhu Z, et al. Use of lung ultrasound to differentiate coronavirus disease 2019 (COVID-19) pneumonia from community-acquired pneumonia. Ultrasound Med Biol. 2020;46(10):2651–2658. PMID: 32622684; PMCID: PMC7274602. doi:10.1016/j.ultrasmedbio.2020.05.006.
67. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363–1372. doi:10.1016/j.acra.2020.07.002
68. Soummer A, Perbet S, Brisson H, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40(7):2064–2072. doi:10.1097/CCM.0b013e31824e68ae
69. Lichter Y, Topilsky Y, Taieb P, et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020;46(10):1873–1883. doi:10.1007/s00134-020-06212-1
70. Brahier T, Meuwly J-Y, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1408
71. Caltabeloti F, Monsel A, Arbelot C, et al. Auler Jr JOC and Rouby JJ. Early fluid loading in acute respiratory distress syndrome with septic shock deteriorates lung aeration without impairing arterial oxygenation: a lung ultrasound observational study. Crit Care. 2014;18(3):R91. doi:10.1186/cc13859
72. De Alencar JCG, Marchini JFM, Marino LO, et al. Lung ultrasound score predicts outcomes in COVID-19 patients admitted to the emergency department. Ann Intensive Care. 2021;11:6. doi:10.1186/s13613-020-00799-w
73. Bouhemad B, Brisson H, Le-guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J RespirCrit Care Med. 2011;183:341–347. doi:10.1164/rccm.201003-0369OC
74. Volpicelli G, Lamorte A, Villén T. What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med. 2020;46(7):1445–1448. doi:10.1007/s00134-020-06048-9
75. Manivel V, Lesnewski A, Shamim S, Carbonatto G, Govindan T. CLUE: COVID-19 lung ultrasound in emergency department. Emerg Med Australas. 2020;32:694–696. doi:10.1111/1742-6723.13546
76. Mafort TT, Lopes AJ, Costa CH, et al. Changes in lung ultrasound of symptomatic healthcare professionals with COVID -19 pneumonia and their association with clinical findings. J Clin Ultrasound. 2020;48(9):515–521. doi:10.1002/jcu.22905
77. Volpicelli G, Gargani L, Perlini S, et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med. 2021;47(4):444–454. doi:10.1007/s00134-021-06373-7
78. Kruser JM, Schmidt GA, Kory PD. COUNTERPOINT: should the use of diagnostic point-of-care ultrasound in patient care require hospital privileging/credentialing? No. Chest. 2020;157(3):498–500. doi:10.1016/j.chest.2019.10.037
79. Buonsenso D, Piano A, Raffaelli F, Bonadia N, Donati KDG, Franceschi F. novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780.
80. Lin H, Zhang B, Kou H, et al. Application value of lung ultrasound in asymptomatic patients with confirmed COVID-19. Adv ultrasound diagn ther. 2020;4(2):67–72. doi:10.37015/AUDT.2020.200025
81. Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pulmonary diseases. World J Radiol. 2010;2(6):203. doi:10.4329/wjr.v2.i6.203
82. Boero E, Schreiber A, Rovida S, Vetrugno L, Blaivas M. The role of lung ultrasonography in COVID‐19 disease management. J Am Coll Emerg Physicians Open. 2020;1(6):1357–1363. doi:10.1002/emp2.12194
83. Di Pan DO, Salonia J, de Lara FV, Mathew J. Efficacy of a two-week ultrasound elective in knowledge acquisition and retention of thoracic ultrasonography among internal medicine residents. Chest. 2019;156. doi:10.1016/j.chest.2019.08.1453
84. Jonson B. Training for Lung ultrasound score measurement in critically Ill patients. Am J Respir Crit Care Med. 2018;198(3):396–398. doi:10.1164/rccm.201801-0093LE
85. World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution: scientific brief. Geneva; 2020. Available from: https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
86. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance. World Health Organization; April 6, 2020. Available from: https://apps.who.int/iris/handle/10665/331695.
87. World Health Organization. 2020: infection prevention and control during health care when COVID-19 is suspected interim guidance; March 19, 2020.
88. Centers for disease control and Prevention. Hand hygiene recommendations. guidance for healthcare providers about hand hygiene and COVID-19; 2019. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html.
89. Centers for disease control and Prevention. Recommendations for disinfection and sterilization in healthcare facilities: guideline for disinfection and sterilization in healthcare facilities (2008); 2008. Available from: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/recommendations.html.
90. Abramowicz JS, Akiyama I, Evans D, et al.; World Federation for Ultrasound in Medicine and Biology Safety Committee. World federation for ultrasound in medicine and biology position statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID-19. Ultrasound Med Biol. 2020;46(7):1821–1826. doi:10.1016/j.ultrasmedbio.2020.03.033
91. Centers for disease control and Prevention. Background G. laundry and bedding. guidelines for environmental infection control in health-care facilities (2003); 2003. Available from: https://www.cdc.gov/infectioncontrol/guidelines/environmental/background/laundry.html.
92. Basseal JM, Westerway SC, McAuley T. COVID‐19: infection prevention and control guidance for all ultrasound practitioners. Australas J Ultrasound Med. 2020;23(2):90–95. doi:10.1002/ajum.12210
93. ACEP. Guideline on COVID-19: ultrasound machine and transducer cleaning approved march 31, 2020. Available from: https://www.acep.org/globalassets/new-pdfs/guideline-on-covid-19–ultrasound-machine-and-transducer-cleaning_policy_033120.pdf.
94. World Health Organization. Cleaning and disinfection of environmental surfaces in the context of COVID-19: interim guidance 16 May 2020 WHO reference number: WHO/2019-nCoV/disinfection/2020.1; 2020.
95. Ochagavia A, Baigorri F, Mesquida J, et al. Hemodynamic monitoring in the critically patient. Recommendations of the Cardiological Intensive Care and CPR working group of the Spanish society of intensive care and coronary units. Medicina Intensiva. 2014;38:154–169. doi:10.1016/j.medin.2013.10.006
96. Buonsenso D, Pata D, Chiaretti A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med. 2020;8(5):e27. doi:10.1016/S2213-2600(20)30120-X
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.