Back to Journals » OncoTargets and Therapy » Volume 14
EGFR (p. G719A+L747V)/EML4-ALK Co-alterations in Lung Adenocarcinoma with Leptomeningeal Metastasis Responding to Afatinib Treatment: A Case Report
Authors Lu Z , Wang X , Luo Y, Wei J, Zeng Z , Xiong Q, Cai J, Liu A
Received 29 November 2020
Accepted for publication 6 April 2021
Published 23 April 2021 Volume 2021:14 Pages 2823—2828
DOI https://doi.org/10.2147/OTT.S294635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Takuya Aoki
Zhiqin Lu,1 Xia Wang,1,2 Yuxi Luo,1 Jianping Wei,1 Zhimin Zeng,1,2 Qiang Xiong,1,2 Jing Cai,1,2 Anwen Liu1,2
1Department of Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China; 2Jiangxi Key Laboratory of Clinical Translational Cancer Research, Nanchang, Jiangxi Province, People’s Republic of China
Correspondence: Jing Cai; Anwen Liu
Department of Oncology, The Second Affiliated Hospital of Nanchang University, No. 1, Minde Street, Nanchang, Jiangxi Province, 360000, People’s Republic of China
Tel +8615270905381
; Tel +8615270905381
Email [email protected]; [email protected]
Abstract: Leptomeningeal metastasis (LM) is a disastrous complication of advanced lung adenocarcinoma (LAC) associated with poor prognosis and rapid deterioration of performance status. The prevalence of epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) co-alterations in patients with LAC was low. Herein, we report a patient with alterations in both EGFR (p. G719A+L747V) and echinoderm microtubule-associated protein-like ALK (EML4-ALK) fusion and LM who was treated with afatinib. The patient’s clinical symptoms improved, and imaging examination revealed reduced intracranial and extracranial lesions. The progression-free survival (PFS) using afatinib for LM was 25 months, and no severe adverse events occurred.
Keywords: afatinib, leptomeningeal metastasis, EGFR, ALK, co-alterations, lung adenocarcinoma
Introduction
Leptomeningeal metastasis (LM) is a devastating complication of advanced lung adenocarcinoma (LAC) associated with poor prognosis and rapid deterioration of performance status. The incidence of LM is 3–5%, and it occurs in up to 9.4% of non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutation. The median survival time after LM treatment is 3–11 months.1,2
EGFR and anaplastic lymphoma kinase (ALK) gene mutations have been regarded as the most important oncogenic drivers in NSCLC.3 Although early studies reported that these two drivers are mutually exclusive,4 recent studies have shown that EGFR/ALK co-alterations account for a small proportion of NSCLC, ranging from 0.1 to 1.6%. EGFR/ALK co-alterations are more common in women, Asians, nonsmokers, patients with stage IV lung adenocarcinoma and individuals with a higher incidence of central nervous system (CNS) metastasis.5,6 The emergence of more advanced techniques, such as high-sensitivity next-generation sequencing (NGS), revealed that the proportion of patients with EGFR/ALK co-alterations could be much higher than previously expected. To date, there is no consensus on treatment for patients with EGFR/ALK co-alterations.7,8
Afatinib is a second-generation tyrosine kinase inhibitor (TKI)that was approved for metastatic NSCLC with nonresistant EGFR mutations.9 Few studies have shown that afatinib is effective against LM, particularly in patients with NSCLC harboring uncommon EGFR mutations.10,11 Here, we report a patient with advanced LAC harboring alterations in both EGFR (p. G719A+L747V) and echinoderm microtubule-associated protein-like ALK (EML4-ALK) and LM who was treated with afatinib.
Case Report
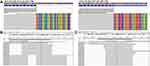
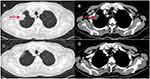
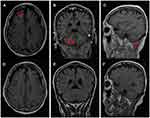
A 72-year-old man presented to our hospital in July 2018. He had no history of smoking and an unremarkable medical history. A thoracic computed tomography (CT) scan revealed a 4.5 cm×5.6 cm solid nodule in the right upper lung (RUL), and brain magnetic resonance imaging (MRI) did not detect brain metastasis. The patient consequently underwent thoracoscopic RUL resection and VATS lymphadenectomy on July 24, 2018. Postoperative pathology examination showed invasive adenocarcinoma in the RUL lesion, with multilineage carcinoma with solid adenocarcinoma containing signet ring cells and no cancer involvement at the bronchial resection margin. Additionally, 2/4/7/9/11 lymph node pathology confirmed no tumor metastasis. The initial pathologic diagnosis was stage IIB lung adenocarcinoma. Immunohistochemistry (IHC) showed ALK-D5F3(-), while NGS–based analysis of surgical specimens revealed an ALK fusion (EML4 exon 13–ALK exon 20, variant allele frequency was 4192, Figure 1A), EGFR 18 exon (c.2156G>C:55241708, p. G719A, abundance 74.8%, Figure 1A) and EGFR exon 19 (c2239T>G:55242469, pL747V, abundance 70.05%, Figure 1A). The patient refused adjuvant treatment. On October 17, 2018, the patient developed dizziness and neck pain. A thoracic CT scan detected a subpleural soft tissue mass of the RUL (Figure 2A and B), and brain MRI detected abnormal enhancement in the brain and leptomeningeal linear enhancement in the cerebellar hemisphere (Figure 3A–C). Lumbar puncture indicated positive cytology of cerebrospinal fluid (CSF) (Figure 4). Except for brain and leptomeningeal metastasis, there was no other site metastasis. A case series by Shin et al6 reported that EGFR-TKIs appeared to yield superior outcomes to ALK-TKIs in patients with NSCLC who harbored EGFR/ALK co-alterations. Considering the rare EGFR mutation, the patient was treated with oral afatinib (40 mg/qd). After 4 weeks, the patient’s dizziness and neck pain were significantly improved, a thoracic CT scan showed diminished subpleural tumor of the right upper lung (Figure 2C and D), and brain MRI showed that the abnormal enhancement in the brain and leptomeningeal linear enhancement in the cerebellar hemisphere had disappeared (Figure 3D–F). As of November 2020, the patient had been treated with afatinib for 25 months without any progression of thoracic and leptomeningeal disease and without experiencing any severe adverse events.
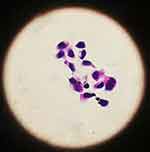 |
Figure 4 Lumbar puncture indicated positive cytology of cerebrospinal fluid (at high magnification 10 * 40). |
Discussion
LM is associated with a poor prognosis.12 A clinical study showed that afatinib was active in NSCLC that harbored certain types of uncommon EGFR mutations, especially G719Xaa; the objective response rate (ORR) was 77.8%, and the PFS was 10.7 months (95% CI 5.6–14.7).13 Preclinical data have shown that the concentrations of afatinib in the CSF and plasma are well correlated (r=0.844, p< 0.01),14 and the CSF penetration rate of afatinib (mean±SD) is 2.5±2.9%.10 A study showed that afatinib exhibits a beneficial effect in patients with LAC and LM harboring EGFR exon 18 p. G719A mutation.11 Brueckl et al15 reported a case of LAC with EGFR (p. G719A+L747V) mutation treated with afatinib, and PFS was 14.9 months. Therefore, in our case, as the patient had an uncommon EGFR mutation, we chose afatinib to treat leptomeningeal metastasis.
Previous studies reported that EGFR gene mutations and ALK rearrangements are mutually exclusive. Recently, co-alterations have been reported with increasing frequency as testing methods have become more sensitive. The prevalence of concomitant EGFR mutations and ALK alterations correlates with detection sensitivity. Along with the discovery of more sensitive gene detection technologies, such as NGS, the proportion of patients with EGFR/ALK co-alteration may be much higher. Currently, the reported incidence of concomitant EGFR mutation and ALK rearrangement ranges from 1.3% to 1.6% in patients with NSCLC.16
The precise mechanism underlying the coexistence of EGFR and ALK alterations remains to be clarified. Increasing evidence has shown that the genetic instabilities of cancer cells cause genetic and phenotypic heterogeneity in the tumor, suggesting that different genetic alterations might occur in different tumor cells rather than in a single clone. Moreover, in a study, the presence of different tumor cell clones with either ALK translocation or EGFR mutation was identified after ALK inhibitor treatment. In contrast, a cell line with homogeneous EML4-ALK rearrangement was found to have activation of EGFR, which suggests that multiple oncogenic pathways may be altered in a single clone of tumor cells.17 In our case, NGS revealed the EML4-ALK fusion gene, but IHC using the ALK (D5F3) CDx assay did not show ALK rearrangement, which might have been due to tumor heterogeneity.
How to treat EGFR/ALK co-alterations may critically depend on the biological roles of these oncodrivers. Based on previous research, EGFR mutations are considered one of the important resistance mechanisms of ALK-TKIs, and EGFR-TKIs combined with ALK-TKIs seem to exhibit more benefits. Koivunen et al18 reported that ALK rearrangement is a potential resistance mechanism to EGFR-TKIs. Yang et al16 assessed the response of patients with EGFR/ALK co-alterations to EGFR TKIs and the first-generation ALK-TKI crizotinib. For first-line EGFR-TKIs in patients with EGFR/ALK co-alterations, the ORR was 80%, and the median PFS was 11.2 months (95% CI 5.6–16.8); he concluded that the levels of phospho-EGFR and phospho-ALK could be prognostic indexes of EGFR-TKIs and crizotinib in NSCLC patients with both EGFR mutations and ALK rearrangements. Won et al17 suggested that ALK-TKIs can be selected first in patients with EGFR/ALK co-alterations. Schmid et al19 reported that EGFR-TKIs may yield better outcomes than ALK-TKIs in patients with EGFR/ALK co-alterations. In our case, the dizziness and neck pain symptoms, abnormal enhancement of the leptomeninges as evidenced by enhanced brain MRI, and identification of tumor cells by CSF cytology were indicative of LAC with LM. NGS of surgical specimens revealed EGFR exon 18 (p. G719A), EGFR exon 19 (p. L747V) and the EML4-ALK fusion gene, but IHC using the ALK (D5F3) CDx assay showed no ALK rearrangement, so we chose afatinib for EGFR/ALK co-alterations and observed a durable response for this patient with LAC and LM.
However, no data regarding the efficacy of afatinib against LM in the treatment of patients with EGFR/ALK mutations are available. This case is the first report to describe the successful and durable response to afatinib in a patient harboring EGFR (p. G719A+L747V)/EML4-ALK co-alterations, suggesting that afatinib is a viable treatment option for this patient group.
Statement of Ethics
Written informed consent was obtained from the patient and his family regarding the publication of the case details and associated images. This is a retrospective case report, and institutional approval was not needed.
Acknowledgments
We thank the patient and his family for providing their information for this study.
Disclosure
The authors report no conflicts of interest.
References
1. Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19(1):e43–e55. doi:10.1016/S1470-2045(17)30689-7
2. Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11(11):1962–1969. doi:10.1016/j.jtho.2016.06.029
3. Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371–2376. doi:10.1093/annonc/mdt205
4. Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1683 patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(15):4273–4281. doi:10.1158/1078-0432.CCR-13-0318
5. Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology. 2007;242(3):882–888. doi:10.1148/radiol.2423051707
6. Shin HJ, Kho BG, Kim MS, et al. Co-alteration of EGFR mutation and ALK rearrangement in non-small cell lung cancer: case series. Medicine. 2019;98(9):e14699. doi:10.1097/MD.0000000000014699
7. Zhao Y, Wang S, Zhang B, et al. Clinical management of non-small cell lung cancer with concomitant EGFR mutations and ALK rearrangements: efficacy of EGFR tyrosine kinase inhibitors and crizotinib. Target Oncol. 2019;14(2):169–178. doi:10.1007/s11523-019-00628-6
8. Wang X, Zhong D. Advances in double mutations of EGFR and ALK gene in non-small cell lung cancer. Chin J Lung Cancer. 2018;21(9):686–691. doi:10.3779/j.issn.1009-3419.2018.09.07
9. Wecker H, Waller CF. Afatinib. Recent Results Cancer Res. 2018;211:199–215. doi:10.1007/978-3-319-91442-8_14
10. Tamiya A, Tamiya M, Nishihara T, et al. Cerebrospinal fluid penetration rate and efficacy of afatinib in patients with EGFR mutation-positive non-small cell lung cancer with leptomeningeal carcinomatosis: a Multicenter Prospective Study. Anticancer Res. 2017;37(8):4177–4182. doi:10.21873/anticanres.11806
11. Ma C, Wang S, Mu N, et al. Effective treatment with afatinib of lung adenocarcinoma with leptomeningeal metastasis harboring the Exon 18 p.G719A mutation in the EGFR gene was detected in cerebrospinal fluid: a case report. Front Oncol. 2020;10:1635. doi:10.3389/fonc.2020.01635
12. Lee J, La Choi Y, Han J, et al. Osimertinib improves overall survival in EGFR-mutated non-small cell lung cancer patients with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol. 2020;15(11):1758–1766. doi:10.1016/j.jtho.2020.06.018
13. Yang JCH, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015;16(7):830–838. doi:10.1016/S1470-2045(15)00026-1
14. Zhang SR, Zhu LC, Jiang YP, et al. Efficacy of afatinib, an irreversible ErbB family blocker, in the treatment of intracerebral metastases of non-small cell lung cancer in mice. Acta Pharmacol Sin. 2017;38(2):233–240. doi:10.1038/aps.2016.107
15. Brueckl WM, Laack E, Reck M. Efficacy of afatinib in the clinical practice: first results of the GIDEONtrial: a prospective non-interventional study (NIS) in EGFR mutated NSCLC in Germany. Ann Oncol. 2018;29:viii524. doi:10.1093/annonc/mdy292.071
16. Yang JJ, Zhang XC, Su J, et al. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20(5):1383–1392. doi:10.1158/1078-0432.CCR-13-0699
17. Won JK, Keam B, Koh J, et al. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2015;26(2):348–354. doi:10.1093/annonc/mdu530
18. Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–4283. doi:10.1158/1078-0432.CCR-08-0168
19. Schmid S, Gautschi O, Rothschild S, et al. Clinical outcome of ALK-positive non-small cell lung cancer (NSCLC) patients with de novo EGFR or KRAS co-mutations receiving Tyrosine Kinase Inhibitors (TKIs). J Thorac Oncol. 2017;12(4):681–688. doi:10.1016/j.jtho.2016.12.003
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.