Back to Journals » International Journal of Nanomedicine » Volume 13
Efficient gene transfection to the brain with ultrasound irradiation in mice using stabilized bubble lipopolyplexes prepared by the surface charge regulation method
Authors Ogawa K, Fuchigami Y, Hagimori M, Fumoto S , Miura Y, Kawakami S
Received 17 November 2017
Accepted for publication 1 February 2018
Published 16 April 2018 Volume 2018:13 Pages 2309—2320
DOI https://doi.org/10.2147/IJN.S157375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Koki Ogawa,1,* Yuki Fuchigami,1,* Masayori Hagimori,1 Shintaro Fumoto,2 Yusuke Miura,1 Shigeru Kawakami1
1Department of Pharmaceutical Informatics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan; 2Department of Pharmaceutics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki, Japan
*These authors contributed equally to this work
Introduction: We previously developed anionic ternary bubble lipopolyplexes, an ultrasound-responsive carrier, expecting safe and efficient gene transfection. However, bubble lipopolyplexes have a low capacity for echo gas (C3F8) encapsulation (EGE) in nonionic solution such as 5% glucose. On the other hand, we were able to prepare bubble lipopolyplexes by inserting phosphate-buffered saline before C3F8 encapsulation. Surface charge regulation (SCR) by electrolytes stabilizes liposome/plasmid DNA (pDNA) complexes by accelerated membrane fusion. Considering these facts, we hypothesized that SCR by electrolytes such as NaCl would promote C3F8 encapsulation in bubble lipopolyplexes mediated by accelerated membrane fusion. We defined this hypothesis as SCR-based EGE (SCR-EGE). Bubble lipopolyplexes prepared by the SCR-EGE method (SCR-EGE bubble lipopolyplexes) are expected to facilitate the gene transfection because of the high amount of C3F8. Therefore, we applied these methods for gene delivery to the brain and evaluated the characteristics of transgene expression in the brain.
Methods: First, we measured the encapsulation efficiency of C3F8 in SCR-EGE bubble lipopolyplexes. Next, we applied these bubble lipopolyplexes to the mouse brain; then, we evaluated the transfection efficiency. Furthermore, three-dimensional transgene distribution was observed using multicolor deep imaging.
Results: SCR-EGE bubble lipopolyplexes had a higher C3F8 content than conventional bubble lipopolyplexes. In terms of safety, SCR-EGE bubble lipopolyplexes possessed an anionic potential and showed no aggregation with erythrocytes. After applying SCR-EGE bubble lipopolyplexes to the brain, high transgene expression was observed by combining with ultrasound irradiation. As a result, transgene expression mediated by SCR-EGE bubble lipopolyplexes was observed mainly on blood vessels and partially outside of blood vessels.
Conclusion: The SCR-EGE method may promote C3F8 encapsulation in bubble lipopolyplexes, and SCR-EGE bubble lipopolyplexes may be potent carriers for efficient and safe gene transfection in the brain, especially to the blood vessels.
Keywords: gene delivery, brain, bubble lipopolyplex, echo gas, spatial distribution
Introduction
Ultrasound-mediated gene transfection has been extensively studied for efficient gene therapy.1–3 In particular, other groups have reported that ultrasound-responsive bubble formulations and ultrasound irradiation cause blood–brain barrier (BBB) disruption by their cavitation energy and deliver plasmid DNA (pDNA) to the brain.4–6 Recently, Song et al7 have reported that the volume of echo gas in microbubbles may affect the degree of BBB opening. Therefore, the transfection efficiency is expected to be enhanced in the brain depending on the amount of echo gas in bubble formulations. In the field of in vivo gene transfection, cationic macromolecule/pDNA complexes (polyplexes) have been developed.8 However, these cationic polyplexes tend to have hematotoxicity and cytotoxicity owing to their strong interaction with blood components and cell membranes.9–11 Therefore, biocompatible complexes have been prepared by encapsulating cationic polyplexes with liposomes12,13 or coating cationic surfaces of polyplexes with anionic macromolecules via electrostatic interactions.14,15 However, these complexes themselves have no ability to target cells or tissues. To overcome this problem, we developed negatively charged ultrasound-responsive ternary bubble lipopolyplexes consisting pDNA, cationic polymers, and anionic liposomes (ALs).16
Binary pDNA/nanobubble complexes can be prepared in nonionic solutions such as 5% glucose to prevent aggregation.17 On the other hand, ternary bubble lipopolyplexes prepared in 5% glucose have a low capacity for echo gas (C3F8), suggesting that it may be difficult to prepare stable bubble formulations via electrostatic interactions because of their complicated structures. Although the detailed mechanism was unclear, we succeeded in preparing bubble lipopolyplexes with a sufficient capacity for C3F8 encapsulation using the post-inserted phosphate-buffered saline (PBS) method as follows. First, for the pre-C3F8-encapsulated form, ternary complexes were prepared in distilled water, and then a concentrated PBS solution was added before C3F8 encapsulation to be isotonic.16 The existence of electrolytes such as NaCl in nonionic solutions enabled preparation of stable cationic liposome/pDNA complexes (lipoplexes) with a small size via accelerated membrane fusion among liposomes by surface charge regulation (SCR) in lipoplexes.18,19 Therefore, we hypothesized that the existence of electrolytes such as NaCl would affect the preparation of bubble lipopolyplexes via facilitated fusion between liposomal membranes. Consequently, the encapsulation efficiency of C3F8 of bubble lipopolyplexes can be enhanced. We defined this method based on the hypothesis as SCR-based echo gas encapsulation (SCR-EGE) for C3F8 encapsulation. To test the hypothesis, we evaluated the encapsulation efficiency of C3F8 in bubble lipopolyplexes prepared by the SCR-EGE method (SCR-EGE bubble lipopolyplexes). Moreover, we expected that the SCR-EGE bubble lipopolyplexes would facilitate gene transfection in the brain by the promotion of C3F8 encapsulation, and we applied these bubble lipopolyplexes to the brain.
For efficient gene therapy of cerebral diseases, it is important to select an appropriate therapeutic gene depending on the distribution of transgene expression. Therefore, information about transgene distribution is necessary to develop a therapeutic strategy. However, such information in the brain using bubble formulations and ultrasound irradiation is lacking. We have developed an observation system for transgene expression in tissues using a tissue-clearing method and confocal microscopy.20 Tissue clearing enables deep imaging. Therefore, this method is suitable to evaluate the spatial distribution of transgene expression compared with conventional tissue sectioning. In this system, tissue-clearing reagents were selected for different purposes. For example, clear, unobstructed brain imaging cocktails (CUBIC)21 is suitable for deep observation, whereas ClearT2,22 or ScaleSQ23 is suitable for labeling biological structures such as blood vessels and the peritoneum using lipophilic dyes. Therefore, multicolor deep imaging of labeled structures and transgene expression was achievable in kidneys24 and peritoneal tissues.25 Considering these aspects, we applied this multicolor deep imaging system to clarify the three-dimensional distribution of transgene expression and blood vessels in the brain.
In this study, we first prepared SCR-EGE bubble lipopolyplexes and measured their physicochemical properties to evaluate whether the SCR-EGE method promotes C3F8 encapsulation. Then, the degree of membrane fusion was evaluated using fluorescent resonance energy transfer (FRET). Next, in vivo transgene expression was examined in mice administered SCR-EGE bubble lipopolyplexes followed by ultrasound irradiation of the brain. Moreover, the three-dimensional distribution of transgene expression in the brain was clarified by multicolor deep imaging using ScaleSQ. We also evaluated whether sustained transgene expression in the brain can be achieved by a CpG-depleted vector, because this is an approach to achieve sustained expression in tissues such as the lungs and liver.26,27
Materials and methods
Materials
1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-distearoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (DSPG) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). N-(carbonyl-methoxypolyethyleneglycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine (mPEG-DSPE) was purchased from NOF Co. (Tokyo, Japan). 4′,6-Diamidino-2-phenylindole (DAPI), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO), and protamine sulfate were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). N, N, N′, N′-tetrakis(2-hydroxypropyl)ethylenediamine was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Paraformaldehyde (PFA), formamide, urea, 2,2′,2′′-nitrilotriethanol, dimethyl sulfoxide, and polyoxyethylene (10) octylphenyl ether were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Animals
Five-week-old male ddY mice (25–30 g) were purchased from Kiwa Laboratory Animal Co., Ltd. (Wakayama, Japan), housed in cages in an air-conditioned room, and maintained on a standard laboratory diet (MF; Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. All animal experiments were performed in accordance with the guidelines for animal experimentation of Nagasaki University and approved by the Institutional Animal Care and Use Committee of Nagasaki University (approval number: 1308051086-6).
pDNA
The vector coding firefly luciferase under cytomegalovirus promoter (pCMV-Luc) was constructed as reported previously.28 pZsGreen1-N1 was purchased from Clontech-Takara Bio Inc. (Shiga, Japan). The CpG-depleted-Luc vector, which contains low level of unmethylated CpG dinucleotides, was constructed by subcloning the firefly luciferase cDNA fragment from pCMV-Luc into the pCpG free-MCS vector (Invivogen, Carlsbad, CA, USA). All pDNAs were amplified in Escherichia coli and purified using an EndoFree® Plasmid Giga kit (Qiagen NV, Venlo, the Netherlands).
Construction of bubble lipopolyplexes
ALs were prepared as described previously16 and suspended in a 5% glucose solution. To construct bubble lipopolyplexes, pDNA, protamine, and ALs were mixed in a 5% glucose solution at a weight ratio of 1.0:1.5:7.0, respectively, and different volumes of saline (150 mM NaCl) were added to obtain the desired concentration. Then, C3F8 was encapsulated as described previously.16 The particle size (Z-average) and zeta potential were measured by a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). To evaluate the particle size and zeta potential of bubble lipopolyplexes, pCMV-Luc was used for preparing bubble lipopolyplexes as a model pDNA. Measurements of the particle size and zeta potential were performed in disposable folded capillary cells (DTS1070; Malvern Instruments) at 25°C. For size measurement, the number and duration of measurement were automatically adjusted by instruments. For the measurement of zeta potential, the voltage applied to cells was automatically set at 150 V and the instrument performed 10–100 runs per measurement. Each measurement was performed in triplicate.
FRET analysis
To evaluate the degree of membrane fusion among ALs, we performed FRET analysis.19,29 When DiO is excited at 484 nm, fluorescence from DiO can excite DiI. Therefore, we used DiO as a FRET donor, and DiI as a FRET acceptor. First, we prepared ALs labeled with DiO and DiI. Then, bubble lipopolyplexes were constructed with a mixture of labeled ALs and unlabeled ALs (1:3 [weight/weight]). To evaluate FRET from DiO to DiI, fluorescence intensity spectra were measured at an excitation wavelength of 484 nm using a spectrofluorophotometer (RF-5300PC; Shimadzu Co., Kyoto, Japan). A reduction in FRET between DiO to DiI was considered as an index of membrane fusion.
Quantification of C3F8 by gas chromatography–mass spectroscopy (GC–MS)
The amount of C3F8 in bubble lipopolyplexes was measured as described previously,30 with a slight modification. Briefly, 2 μL of bubble suspension was placed in a gas-tight vial. The sample was heated to disrupt the bubbles and then analyzed by a gas chromatographer (GC-2014; Shimadzu Co.) connected to a flame ionization detector.
Erythrocyte aggregation assay
Erythrocytes from mice were washed three times by suspending in PBS and centrifugation at 2,300 × g. Then, 2% (volume/volume) erythrocyte suspension was prepared. Erythrocyte suspensions were mixed with a 5% glucose solution as the negative control, protamine/pDNA complexes, or SCR-EGE bubble lipopolyplexes containing pCMV-Luc. Subsequently, the suspensions were incubated for 15 min and then observed using an AxioVert A1 microscope (Carl Zeiss Meditec AG, Jena, Germany) equipped with a ×20 objective lens.
In vivo gene transfection in the brain
Mice were anesthetized with three types of mixed anesthetic agents prepared as described previously.31 Scalp fur was removed carefully and 12.5, 25, 37.5, and 50 μg of bubble lipopolyplexes (in terms of pDNA) carrying pCMV-Luc or pCpG free-Luc were injected intravenously. Immediately after injection, ultrasound radiation was applied transdermally to the head using a Sonopore-4000 sonicator (Nepa Gene, Chiba, Japan) under the following conditions: frequency, 1.045 MHz; duty, 10%; intensity, 0.25, 0.5, 0.75, and 1 W/cm2; duration, 0, 5, 10, 20, and 120 s using a probe with a diameter of 20 mm.
Luciferase assay
At various time points after transfection, mice were sacrificed and their brain and other organs were harvested. A luciferase assay was performed as reported previously.20 Luciferase activity of 3 × 104 relative light units per gram tissues was regarded as the limit of quantitation.
Observation of transgene distribution in the brain
Twenty-four hours after transfection by bubble lipopolyplexes carrying pZsGreen1-N1, mice were anesthetized and fixed with 4% PFA via perfusion through the left ventricle. Vascular staining with DiI was performed according to a previously described procedure.32 Briefly, 10 mL of DiI solution (120 μM) was perfused through the left ventricle before the fixation. Brains, livers, and lungs were excised, and brains were coronally sectioned at approximately 2 mm thicknesses. Each organ was cleared with three types of tissue-clearing reagents, CUBIC,21 ScaleSQ,23 or ClearT2.22 The procedures for tissue clearing were performed according to the respective references. For nucleic acid staining by DAPI, tissues were immersed in CUBIC containing 5 μg/mL of DAPI for 24 h before the observation. Cleared samples were subsequently observed under a confocal laser scanning microscope (LSM 710; Carl Zeiss) equipped with ×10 or ×20 objective lenses. DAPI, DiI, and ZsGreen1 proteins were excited by lasers at 405, 549, and 493 nm, respectively. The acquisition software was ZEN2012.
Statistical analyses
Statistical comparisons were performed by Tukey’s test for multiple groups. P-values of <0.05 were considered as statistically significant.
Results
Physicochemical properties of bubble lipopolyplexes
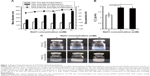
To elucidate whether the SCR-EGE method promotes C3F8 encapsulation in bubble lipopolyplexes, we evaluated the effect of the NaCl concentration on the size and zeta potential of bubble lipopolyplexes prepared in a 5% glucose solution. The size of pre-gas-encapsulated bubble lipopolyplexes was not affected by the addition of NaCl (Figure 1A). However, upon addition of more than 3 mM NaCl, the size of post-gas-encapsulated bubble lipopolyplexes was increased significantly; moreover, the zeta potential became less negative and close to neutral.
When we measured the amount of C3F8 in bubble lipopolyplexes by GC–MS, the amount of C3F8 was significantly higher in bubble lipopolyplexes with 6 or 30 mM NaCl than without NaCl (Figure 1B). Moreover, the appearance of bubble lipopolyplexes became cloudy by the addition of 6 or 30 mM NaCl (Figure 1C).
FRET analysis to evaluate membrane fusion
Bubble lipopolyplexes were prepared using ALs labeled with DiO and DiI, and then applied to FRET analysis to evaluate the degree of membrane fusion among ALs. We used the DiO/DiI fluorescent intensity ratio (F501/F565) to evaluate FRET between DiO and DiI. As shown in Figure 2A, no obvious change was observed by the addition of NaCl in the pre-C3F8-encapsulated state, and F501/F565 was 0.43 ± 0.04, 0.45 ± 0.04, and 0.43 ± 0.03 for lipopolyplexes with 0, 6, and 30 mM NaCl, respectively. In contrast, the reduction in FRET between DiO and DiI was observed in the post-C3F8-encapsulated state, and F501/F565 was 0.48 ± 0.06, 0.65 ± 0.12, and 0.83 ± 0.09 for bubble lipopolyplexes with 0, 6, and 30 mM NaCl, respectively (Figure 2B).
Erythrocyte aggregation assay
To evaluate hemagglutination, we added binary protamine/pDNA complexes or SCR-EGE bubble lipopolyplexes to erythrocyte suspensions. Aggregation was observed for binary protamine/pDNA complexes, whereas aggregations were not observed for SCR-EGE bubble lipopolyplexes (Figure S1A–C).
Transfection efficiency of bubble lipopolyplexes
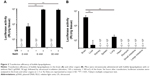
Next, we evaluated the transfection efficiency of SCR-EGE bubble lipopolyplexes. Bubble lipopolyplexes carrying pCMV-Luc were administered to mice, and then the mouse brain was irradiated by ultrasound. As shown in Figure 3A, mice transfected by bubble lipopolyplexes with 6 or 30 mM NaCl exhibited approximately 10-fold higher expression of luciferase compared with bubble lipopolyplexes without NaCl.
In addition, luciferase activity of the brain, liver, kidneys, spleen, lungs, heart, stomach, and small and large intestines was measured after transfection by bubble lipopolyplexes with 6 mM NaCl and ultrasound irradiation. As a result, luciferase activity in the brain was significantly higher than that in any other organs (Figure 3B).
Optimization of transfection conditions
To optimize transfection conditions, we evaluated the influence of the ultrasound duration, ultrasound intensity, and dose of SCR-EGE bubble lipopolyplexes containing 6 mM NaCl on transfection efficiency in the brain. Luciferase gene expression reached a plateau by ultrasound irradiation at 0.5 W/cm2 for 5 s (Figure 4A and B). In addition, luciferase gene expression tended to increase as the dose of pDNA was increased (Figure 4C). In subsequent experiments, mice were administered with 50 μg pDNA followed by ultrasound irradiation at 1 W/cm2 for 10 s.
Distribution of transgene expression
The distribution of transgene expression in the brain was analyzed by confocal laser scanning microscopy after tissue clearing. To evaluate the dispersibility of transgene expression, the brain was cleared with CUBIC. Consequently, ZsGreen1 expression was detected in the entire observed area, and the degree of ZsGreen1 expression was apparently higher in mice transfected by bubble lipopolyplexes with 6 mM NaCl (SCR-EGE bubble lipopolyplexes) than those without NaCl (conventional bubble lipopolyplexes; Figure 5B and C). Moreover, the distribution of ZsGreen1 in the lungs and liver was evaluated when gene was transfected to the brain by SCR-EGE bubble lipopolyplexes with ultrasound. Consequently, few transgene expressions were detected in the lungs (Figure 5D and E) or liver (Figure 5F and G) when they were cleared with CUBIC (Figure 5D and F). Next, cerebrovascular staining was performed with DiI, followed by tissue clearing using ScaleSQ or ClearT2. When cleared with ScaleSQ (Figure 5H), ZsGreen1 transfected by both conventional and SCR-EGE bubble lipopolyplexes was observed mainly on blood vessels and partially outside of blood vessels (Figure 5I and J). Furthermore, the same trend of ZsGreen1 distribution was observed when the brain was cleared with ClearT2 (Figure 5K–M).
Duration of transgene expression
Bubble lipopolyplexes carrying pCMV-Luc or pCpG-depleted-Luc with or without NaCl were administered to mice, and then the brain was irradiated by ultrasound. Luciferase activity in the brain was determined at 1, 7, 14, and 28 days after transfection. Consequently, sustained expression was observed in brains transfected by bubble lipopolyplexes carrying CpG-depleted-Luc for at least 28 days after transfection (Figure 6). Conversely, for bubble lipopolyplexes carrying pCMV-Luc, the transgene expression was markedly decreased within 7 days. Moreover, at all time points, the expression levels of luciferase transfected by bubble lipopolyplexes with 6 mM NaCl was higher compared with lipopolyplexes without NaCl.
Discussion
The results in this study support the SCR-EGE hypothesis that SCR promotes membrane fusion of lipopolyplexes. Consequently, C3F8 encapsulation of bubble lipopolyplexes can be accelerated. The size and C3F8 content of bubble lipopolyplexes were increased, and the zeta potential became less negative and close to neutral (Figure 1A and B). Furthermore, their appearance became cloudy after the addition of NaCl (Figure 1C). Our results are supported by a study by Borden et al,33 in which an increase in the size of bubbles was observed after the addition of NaCl to a nonionic solution. Moreover, FRET between each fluorescent dye in liposomes was reduced by the addition of NaCl (Figure 2B). These results suggest that SCR promotes C3F8 encapsulation via accelerated membrane fusion of bubble lipopolyplexes. Recently, Song et al7 reported an increase in the extravasation of Evans blue from blood to brain tissue depending on the amount of injected gas. Therefore, promoting C3F8 encapsulation into bubble lipopolyplexes is also expected to enhance pDNA delivery to the brain, and the promotion of C3F8 encapsulation may result in a high transfection efficiency. The gene transfection efficiency of bubble lipopolyplexes with 6 or 30 mM NaCl was significantly higher than that of bubble lipopolyplexes without NaCl (Figure 3A). Although C3F8 capacity of SCR-EGE bubble lipopolyplexes was increased only 20% from the conventional bubble lipopolyplexes (Figure 1B), the transgene expression of SCR-EGE bubble lipopolyplexes was approximately 10 times higher than that of conventional bubble lipopolyplexes (Figure 3A). Therefore, the other factors may affect the transfection efficiency of bubble lipopolyplexes as well as the increase in C3F8 encapsulation. One possible factor is in vivo stability of the formulation. We previously reported that the SCR lipoplexes retained the primary structure longer than conventional bubble lipopolyplexes in the biological condition.18,19 Therefore, we speculated that SCR-EGE bubble lipopolyplexes could be more stable in the biological condition. When they are distributed to the brain, we think that SCR-EGE bubble lipopolyplexes deliver much higher C3F8 than conventional bubble lipopolyplexes; therefore, high cavitation energy can be generated in the brain in response to ultrasound irradiation. In general, a large formulation can greatly interact with cerebral capillaries. We consider that increasing the size of bubble lipopolyplexes also affects the transfection efficiency in the brain. In fact, the particle size of SCR-EGE bubble lipopolyplexes was increased about 70% from conventional bubble lipopolyplexes (Figure 1A), although the C3F8 capacity of SCR-EGE bubble lipopolyplexes was increased only 20% from conventional bubble lipopolyplexes. Taking these into consideration, we consider that, in addition to the C3F8 capacity, in vivo stability and size are important factors that affect the transfection efficiency to the brain. In addition, transgene expression was detected specifically in the brain (Figure 3B). Furthermore, bubble lipopolyplexes with 6 mM NaCl showed no aggregation with erythrocytes because of their negative surface charge (Figure S1A–C). This result is compatible with a previous study in which bubble lipopolyplexes were prepared in PBS.16 Our data suggest that the SCR-EGE method promotes C3F8 encapsulation in bubble lipopolyplexes, and SCR-EGE bubble lipopolyplexes may be a potent carrier for efficient and safe gene transfection to the brain.
Thus far, there have been no reports about transfection to the brain using SCR-EGE bubble lipopolyplexes and ultrasound irradiation. Therefore, we optimized the transfection conditions, focusing on the ultrasound duration, ultrasound intensity, and dose of SCR-EGE bubble lipopolyplexes. We found sufficiently high transgene expression under the following conditions: ultrasound duration, 10 s; intensity, 1 W/cm2; pDNA dose; 50 μg (Figure 4A–C). Therefore, we used these conditions in subsequent experiments to evaluate the transgene distribution and sustained expression.
To develop a therapeutic strategy with bubble lipopolyplexes, we evaluated the distribution of transgene expression achieved by the combination of bubble lipopolyplexes and ultrasound irradiation using tissue clearing. First, we evaluated the spatial dispersibility of transgene expression in the brain using tissue-clearing reagent CUBIC, because the reagent provides high transparency (Figure 5A). Consequently, transgene expression was observed at a depth of 700 μm from the surface and in the entire observed area (Figure 5B and C). The degree of transgene expression was apparently higher in mice transfected with SCR-EGE bubble lipopolyplexes compared with conventional bubble lipopolyplexes. These data support the quantitative result of luciferase expression in Figure 3A. On the other hand, the number of transgene positive cells appeared to be small (Figure 5C), as compared to the result of luciferase expression (Figure 3A). In the current study, ultrasound was irradiated to whole brain using planner ultrasound; therefore, the density of transgene expression may be low by dispersing the energy of ultrasound. In Figure 5E and G, in the control organs such as the lungs and liver, few transgene expressions were detected. The results are corresponding to Figure 3B. Taking these into considerations, SCR-EGE bubble lipopolyplexes with ultrasound irradiation to brain may transfect gene efficiently and selectively to brain. Next, to evaluate the spatial positional relationship between cerebral blood vessels and transgene expression, we cleared the brain using ClearT2 (Figure 5K) and ScaleSQ (Figure 5H), followed by vascular staining with DiI, because these reagents do not contain a detergent and retain DiI. So far, for gene transfection to the brain using microbubbles and ultrasound irradiation, it has been reported that transgene expression was observed in extravascular cells such as astrocytes and neurons by evaluation through tissue sectioning and immunohistochemical analysis.5,6 Therefore, we expected that bubble lipopolyplexes mediated the extravascular transgene expression. In fact, transgene expression was observed mainly on DiI-labeled blood vessels and partially outside of blood vessels in mice transfected with both conventional and SCR-EGE bubble lipopolyplexes when the brain was cleared with ScaleSQ (Figure 5I and J). Furthermore, the same trend of transgene expression was observed when the brain was cleared with ClearT2 (Figure 5L and M). These results are incompatible with other reports using microbubbles. We consider that this difference may be derived from the bubble size. Tung et al34 reported that larger microbubbles have a higher ability for BBB opening, because they tend to contact vascular walls. The size of bubble lipopolyplexes was 400 nm even when C3F8 encapsulation was improved. Therefore, in bubble lipopolyplexes, sonoporation of endothelial cells might mainly occur. Overall, our spatial evaluation using tissue clearing succeeded in clarifying that the SCR-EGE method enhances the transfection efficiency of bubble lipopolyplexes with a tendency to transfect blood vessels.
Cerebral blood vessels are expected to be a potent target of therapeutic gene transfection for the treatment of some cerebral diseases. Transfection of glial cell-derived neurotrophic factor (GDNF) into brain capillary endothelial cells and secretion of GDNF into brain parenchyma are reported to exert protective effects against Parkinson’s disease.35,36 For efficient gene therapy of cerebral diseases, sustained therapeutic transgene expression is desired because these diseases tend to be chronic. A CpG-depleted plasmid vector is known to extend the duration of transgene expression by minimizing the inflammatory response mediated by Toll-like receptor 9.26,27 Therefore, we evaluated the duration of transgene expression in the brain transfected with bubble lipopolyplexes containing a CpG-depleted vector. As shown in Figure 6, sustained transgene expression mediated by pCpG-depleted-Luc was observed for at least 28 days. In contrast, transgene expression mediated by pCMV-Luc was drastically decreased within 7 days. However, the transgene expression level in mice transfected with SCR-EGE bubble lipopolyplexes was higher than that in mice transfected with conventional bubble lipopolyplexes at all time points. These results suggest that sustained high transgene expression is achieved by SCR-EGE bubble lipopolyplexes carrying a CpG-depleted vector. Based on the results of the transgene distribution and sustained expression, we believe that a potent therapeutic strategy can be expected by vascular transfection using SCR-EGE bubble lipopolyplexes carrying CpG-depleted pDNA and subsequent sustained secretion of therapeutic proteins from blood vessels.
Conclusion
In this study, we clarified that the SCR-EGE method can promote C3F8 encapsulated in bubble lipopolyplexes, and that SCR-EGE bubble lipopolyplexes may be potent carriers for efficient and safe transfection to the brain in combination with ultrasound irradiation of the brain. Moreover, spatial evaluation using tissue clearing succeeded in clarifying the three-dimensional distribution of transgene expression in the brain with high transgene expression found on cerebral blood vessels. These findings are valuable to develop therapeutic strategies using bubble lipopolyplexes with ultrasound irradiation.
Acknowledgments
We thank Professor Kazuo Maruyama (Teikyo University) for measuring C3F8 by GC–MS. We also thank Mitchell Arico from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript. This work was supported by JSPS KAKENHI (grant number 16K18862), the Shin-Nihon Foundation of Advanced Medical Research, and Takeda Science Foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
Kawakami S, Hashida M. Glycosylation-mediated targeting of carriers. J Control Release. 2014;190:542–555. | ||
Fumoto S, Kawakami S. Combination of nanoparticles with physical stimuli toward cancer therapy. Biol Pharm Bull. 2014;37(2):212–216. | ||
Newman CM, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14(6):465–475. | ||
Negishi Y, Yamane M, Kurihara N, et al. Enhancement of blood-brain barrier permeability and delivery of antisense oligonucleotides or plasmid DNA to the brain by the combination of bubble liposomes and high-intensity focused ultrasound. Pharmaceutics. 2015;7(3):344–362. | ||
Mead BP, Mastorakos P, Suk JS, Klibanov AL, Hanes J, Price RJ. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J Control Release. 2016;223(10):109–117. | ||
Huang Q, Deng J, Xie Z, et al. Effective gene transfer into central nervous system following ultrasound-microbubbles-induced opening of the blood-brain barrier. Ultrasound Med Biol. 2012;38(7):1234–1243. | ||
Song K-H, Fan AC, Hinkle JJ, Newman J, Borden MA, Harvey BK. Microbubble gas volume: a unifying dose parameter in blood-brain barrier opening by focused ultrasound. Theranostics. 2017;7(1):144–152. | ||
Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Deliv Rev. 2001;53(3):341–358. | ||
Dekie L, Toncheva V, Dubruel P, Schacht EH, Barrett L, Seymour LW. Poly-L-glutamic acid derivatives as vectors for gene therapy. J Control Release. 2000;65(1):187–202. | ||
Breunig M, Lungwitz U, Liebl R, Goepferich A. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc Natl Acad Sci U S A. 2007;104(36):14454–14459. | ||
Gebhart CL, Kabanov AV. Evaluation of polyplexes as gene transfer agents. J Control Release. 2001;73(2–3):401–416. | ||
Schäfer J, Hobel S, Bakowsky U, Aigner A. Liposome-polyethylenimine complexes for enhanced DNA and siRNA delivery. Biomaterials. 2010;31(26):6892–6900. | ||
Pinnapireddy SR, Duse L, Strehlow B, Schäfer J, Bakowsky U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf B Biointerfaces. 2017;158:93–101. | ||
Kurosaki T, Kitahara T, Fumoto S, et al. Ternary complexes of pDNA, polyethylenimine, and γ-polyglutamic acid for gene delivery systems. Biomaterials. 2009;30(14):2846–2853. | ||
Koyama Y, Yamada E, Ito T, Mizutani Y, Yamaoka T. Sugar-containing polyanions as a self-assembled coating of plasmid/polycation complexes for receptor-mediated gene delivery. Macromol Biosci. 2002;2(6):251–256. | ||
Kurosaki T, Kawakami S, Higuchi Y, et al. Development of anionic bubble lipopolyplexes for efficient and safe gene transfection with ultrasound exposure in mice. J Control Release. 2014;176(28):24–34. | ||
Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials. 2010;31(30):7813–7826. | ||
Kawakami S, Ito Y, Fumoto S, Yamashita F, Hashida M. Enhanced gene expression in lung by a stabilized lipoplex using sodium chloride for complex formation. J Gene Med. 2005;7(12):1526–1533. | ||
Fumoto S, Kawakami S, Ito Y, Shigeta K, Yamashita F, Hashida M. Enhanced hepatocyte-selective in vivo gene expression by stabilized galactosylated liposome/plasmid DNA complex using sodium chloride for complex formation. Mol Ther. 2004;10(4):719–729. | ||
Fumoto S, Nishimura K, Nishida K, Kawakami S. Three-dimensional imaging of the intracellular fate of plasmid DNA and transgene expression: ZsGreen1 and tissue clearing method CUBIC are an optimal combination for multicolor deep imaging in murine tissues. PLoS One. 2016;11(1):e0148233. | ||
Susaki EA, Tainaka K, Perrin D, et al. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell. 2014;157(3):726–739. | ||
Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development. 2013;140(6):1364–1368. | ||
Hama H, Hioki H, Namiki K, et al. ScaleS: an optical clearing palette for biological imaging. Nat Neurosci. 2015;18(10):1518–1529. | ||
Oyama N, Fuchigami Y, Fumoto S, et al. Characterization of transgene expression and pDNA distribution of the suctioned kidney in mice. Drug Deliv. 2017;24(1):906–917. | ||
Nishimura K, Fumoto S, Fuchigami Y, Hagimori M, Maruyama K, Kawakami S. Effective intraperitoneal gene delivery system using nanobubbles and ultrasound irradiation. Drug Deliv. 2017;24(1):737–744. | ||
Hodges BL, Taylor KM, Joseph MF, Bourgeois SA, Scheule RK. Long-term transgene expression from plasmid DNA gene therapy vectors is negatively affected by CpG dinucleotides. Mol Ther. 2004;10(2):269–278. | ||
Yew NS, Zhao H, Przybylska M, et al. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol Ther. 2002;5(6):731–738. | ||
Nomura T, Yasuda K, Yamada T, et al. Gene expression and antitumor effects following direct interferon (IFN)-γ gene transfer with naked plasmid DNA and DC-chol liposome complexes in mice. Gene Ther. 1999;6(1):121–129. | ||
Yefimova SL, Kurilchenko IY, Tkacheva TN, et al. Microspectroscopic study of liposome-to-cell interaction revealed by förster resonance energy transfer. J Fluoresc. 2014;24(2):403–409. | ||
Oda Y, Suzuki R, Mori T, et al. Development of fluorous lipid-based nanobubbles for efficiently containing perfluoropropane. Int J Pharm. 2015;487(1–2):64–71. | ||
Kawai S, Takagi Y, Kaneko S, Kurosawa T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim. 2011;60(5):481–487. | ||
Li Y, Song Y, Zhao L, Gaidosh G, Laties AM, Wen R. Direct labeling and visualization of blood vessels with lipophilic carbocyanine dye DiI. Nat Protoc. 2008;3(11):1703–1708. | ||
Borden MA, Caskey CF, Little E, Gillies RJ, Ferrara KW. DNA and polylysine adsorption and multilayer construction onto cationic lipid-coated microbubbles. Langmuir. 2007;23(18):9401–9408. | ||
Tung YS, Vlachos F, Feshitan JA, Borden MA, Konofagou EE. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J Acoust Soc Am. 2011;130(5):3059–3067. | ||
Huang R, Ma H, Guo Y, et al. Angiopep-conjugated nanoparticles for targeted long-term gene therapy of Parkinson’s disease. Pharm Res. 2013;30(10):2549–2559. | ||
Jiang C, Koyabu N, Yonemitsu Y, et al. In vivo delivery of glial cell-derived neurotrophic factor across the blood-brain barrier by gene transfer into brain capillary endothelial cells. Hum Gene Ther. 2004;14(12):1181–1191. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.