Back to Journals » OncoTargets and Therapy » Volume 14
Efficacy with Trastuzumab Deruxtecan for Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutation in a Patient with a Poor Performance Status: A Case Report
Authors Kato Y, Kato Y, Minegishi Y, Suzuki T, Nakamichi S, Matsumoto M, Miyanaga A , Noro R, Kubota K, Terasaki Y , Seike M, Gemma A
Received 25 September 2021
Accepted for publication 15 November 2021
Published 23 November 2021 Volume 2021:14 Pages 5315—5319
DOI https://doi.org/10.2147/OTT.S341290
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Yuki Kato,1 Yasuhiro Kato,1 Yuji Minegishi,1,2 Takahiro Suzuki,1 Shinji Nakamichi,1 Masaru Matsumoto,1 Akihiko Miyanaga,1 Rintaro Noro,1 Kaoru Kubota,1 Yasuhiro Terasaki,3 Masahiro Seike,1 Akihiko Gemma1
1Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan; 2Department of Pulmonary Medicine, Mitsui Memorial Hospital, Tokyo, Japan; 3Department of Analytic Human Pathology, Nippon Medical School, Tokyo, Japan
Correspondence: Yasuhiro Kato
Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Sendagi 1-1-5 Bunkyo-ku, Tokyo, 113-8603, Japan
Email [email protected]
Abstract: Antibody–drug conjugate (ADC) was novel type of anticancer drugs. Trastuzumab deruxtecan (T-DXd), a human epidermal growth factor receptor 2 (HER2) targeting ADC, can be a novel treatment option for HER2 alternation (mutation, expression, amplification) advanced-stage non-small-cell lung cancer (NSCLC) from DESTINY-Lung01 result. Herein, we report a successful treatment with T-DXd for NSCLC harboring HER2 exon 20 insertion mutation in a patient with poor performance status (PS). We presented a case of a 52-year-old heavily pretreated female patient diagnosed with lung adenocarcinoma (cT1bN3M0, stage IIIB). After fifth-line pretreatment of systemic chemotherapy, primary tumor recurrence, pleural effusion, and miliary lung metastases were observed. The patient presented with hypoxia requiring oxygen therapy via nasal cannula at a flow rate of 4 L per minute, cancer pain, and cachexia requiring opioid treatment. Her Eastern Cooperative Oncology Group PS score was assessed 3. Comprehensive genomic profiling revealed HER2 exon 20 insertion mutation. After treatment with T-DXd was approved by the ethical review committee of Nippon Medical School Hospital, treatment was started. The tumor size decreased significantly, and her PS score decreased from 3 to 1, with improvement of hypoxia, cancer pain, and cachexia. The patient is still receiving treatment, without disease progression 6 months after starting treatment with T-DXd. Despite cases of poor PS, NGS should be performed and target therapy including ADCs should be considered.
Keywords: lung cancer, HER2 exon 20 insertion mutation, poor PS, trastuzumab deruxtecan
Introduction
Human epidermal growth factor receptor 2 (HER2) gene mutation is an oncogenic driver mutation that can be a treatment target.1 The recently developed anti-HER2 antibody–drug conjugate (ADC) was effective for the treatment of advanced-stage non-small-cell lung cancer (NSCLC) harboring HER2 mutation.1,2 However, to the best of our knowledge, no previous study has shown that ADCs including trastuzumab deruxtecan (T-DXd), which are used against advanced-stage NSCLC harboring HER2 mutation, are effective and safe in patients with a poor performance status (PS). Herein, we report a case of NSCLC harboring HER2 exon 20 insertion mutation in a patient who had significantly good tumor response and who experienced improvement in systemic conditions after treatment with T-DXd despite a poor PS. Treatment and this paper publication was approved by Ethics Committee Review Board of Nippon Medical School Hospital (approval no. B-2020-297).
Case Report
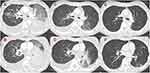
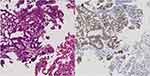
A 52-year-old female patient, a never smoker, was initially diagnosed with advanced-stage lung adenocarcinoma (cT1bN3M0, stage IIIB). Wild-type epidermal growth factor receptor (EGFR mutation), anaplastic lymphoma kinase (ALK) rearrangement, ROS 1 rearrangement, BRAF V600E mutation, and MET exon 14 skipping mutation were detected via accompanying diagnostic tests. Based on the assessment using transbronchial lung biopsy (TBB) samples, the programmed death-ligand 1 expression was <1%. She received concurrent chemoradiation (60 Gy/30 Fr with cisplatin plus S-1), and four systemic treatments including immune check point inhibitor (carboplatin plus paclitaxel plus bevacizumab plus atezolizumab, docetaxel plus ramucirumab, pemetrexed, and nanoparticle albumin-bound paclitaxel) for each relapse at recurrence. However, rapid disease recurrence was observed with the progression of left lower lobe primary tumor, miliary lung metastasis, bilateral malignant pleural effusion, and appearance of carcinomatous lymphangitis on computed tomography (CT) scan (Figure 1). The serum carcinoembryonic antigen (CEA) level increased to 14,333.9 ng/mL. The patient’s medical condition was affected as she presented with hypoxia, which required oxygen therapy via nasal cannula at a flow of 4 L per min, cancer pain, and cachexia, which needed opioid treatment. Her Eastern Cooperative Oncology Group PS score was assessed 3. TBB was again conducted for cancer genomic analysis using FoundationOne CDx® as next-generation sequencing and HER2 exon 20 insertion mutation (M774-775ins) and HER2 amplification was detected. Immunohistochemical staining of TBB specimens, which was performed by a pathologist, revealed HER2 positivity (Figure 2).
T-DXd was not approved for the treatment for NSCLC harboring HER2 mutation at that time in Japan. However, the patient’s condition was serious, and T-DXd had a promising therapeutic effect against NSCLC harboring HER2 insertion mutation. Therefore, treatment with T-DXd, which was covered using the patient’s own expense, was considered. After our cautious explanation about the risks and benefits of the drug was to the patient and her family, their informed consent was obtained. Treatment was started after the use of T-DXd was approved by Ethics Committee Review Board of Nippon Medical School Hospital (approval no. B-2020-297). Although participants received 6.4mg/kg in DISTNY Lung 01 trial, we conducted dose modification to 5.4 mg/kg of T-DXd for avoiding severe adverse event, considering her poor PS. CT scan performed 11 days after the administration of T-DXd presented that the size of the primary tumor decreased. Moreover, lung metastasis and pleural effusion improved (Figure 1). After 13 days the initiation of T-DXd, the need for oxygen administration was eliminated and the patient’s PS improved from 3 to 1. After 23 days, she was discharged without O2 administration, and T-DXd treatment was continued in the outpatient department. The serum CEA level significantly decreased to 873 ng/mL with the treatment. The patient experienced adverse events (AEs) such as grade 2 neutropenia and grade 2 nausea, which were correlated with T-DXd treatment. After adjusting for treatment schedule, the patient tolerated the AEs. The durable response of T-DXd was observed between 6 months follow-up terms from initiation of T-DXd and her response was evaluated partial response using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The treatment is still ongoing, and severe AEs including interstitial lung disease (ILD) have not been observed.
Discussion
ADCs are a novel type of anticancer agent. Their mechanism of action is considered to be as follows: The monoclonal antibodies recognize and bind to the extracellular domain of a cancer-specific transmembrane protein on the cell surface. Then, ADCs are internalized into the cell, thereby releasing the payload in an active state and exhibiting cytotoxicity. T-DXd is an ADC targeting somatic cancers harboring HER2 mutations that bind to trastuzumab (monoclonal antibody) and deruxtecan (cytotoxic agent).2
A previous study showed HER2 mutations in approximately 1–4% of the patients with NSCLC.3 Monoclonal antibodies including trastuzumab and pertuzumab as well as tyrosine kinase inhibitors such as afatinib, dacomitinib, and neratinib can be used as therapeutic agents targeting NSCLC harboring HER2 mutation. However, no agent has a sufficient treatment efficacy.4 The use of T-DXd in patients with NSCLC harboring HER2 mutation is currently investigated. DESTINY-Lung01, a Phase II study, showed a promising clinical activity, with a high objective response (55%: 95% CI, 44 to 65) and durable responses as the median duration of response was 9.3 months (95% CI, 5.7 to 14.7), median progression-free survival was 8.2 months (95% CI, 6.0 to 11.9) and median overall survival was 17.8 months (95% CI, 13.8 to 22.1).1 On the contrary, this clinical trial only included patients with PS score of 0 or 1. Our case suggested the efficacy and safety of T-DXd was maintained in patients with advanced-stage NSCLC who have PS score of 2 or higher. Some molecularly targeted agents, including osimertinib, which have a high response and disease control rates, can be effective against advanced-stage patients with NSCLC who have poor condition.5 Generally, ADCs including T-DXd, which use a cleavable linker payload system, are safety due to their tumor-specific effects.6,7 The Linker payload system of ADCs is generally cut specifically in tumors to release cytotoxic agents, which have antitumor effects. The number of molecules in cytotoxic agents delivered by the linker is regulated (8 DXd per antibody in T-DXd). In addition, “by-stander effect” occurs when the ADC drug is taken up by the target cell and released after degradation or when it is released into the extracellular space.8 The mechanism suggests that ADCs prevent the attenuation of therapeutic effects resulting from tumor heterogeneity. Hence, their antitumor effects are more tumor-specific than those of conventional cytotoxic drugs, and they are designed to address tumor heterogeneity without affecting normal cells. Therefore, based on our study, ADC treatment including T-DXd, when used as a target therapy of gene mutations, may be beneficial for patients with poor PS.
The limitation of the manuscript was a case report of only one patient. In particular, some reports have shown that T-DXd is associated with a high risk for ILD based on clinical trials about breast, gastric, and lung cancer. Moreover, and there may be concerns about its safety in patients with poor condition.2,3,9 The mechanism is considered that HER2 expression in human normal lung cells caused T-DXd toxicity in normal lung cell specifically.6 In the DISTNY-lung 01 trial, 26% of the participants experienced ILD.1 On the contrary, the DESTINY-Breast01 trial showed that 13.6% of the participants.3 These discrepancies between the two trials are associated with dose of T-DXd. Therefore, our dose modification had a positive impact on the results of this case. In addition, 80% of the patients experienced ILD presented with less than grade 2 ILD.1,3 In our case, it was possible to get a durable response without the onset of ILD despite poor PS. With consideration of the high efficacy in the current case, T-DXd could be beneficial in patients with NSCLC harboring HER2 mutation with a poor PS. Further case accumulation and analysis must be performed to assess the efficacy and safety of T-DXd in patients with advanced-staged somatic cancer including NSCLC harboring HER2 mutation who have a poor PS.
Conclusion
Our case suggested T-DXd may be beneficial and safe for patients with NSCLC harboring HER2 exon20 insertion mutation who have a poor PS. Despite cases of poor PS, NGS should be performed and target therapy including ADCs should be considered. Currently, several clinical trials (NCT: 04644237, NCT: 04447118) are ongoing for lung cancer with HER2 alternation. Patients with NSCLC harboring HER2 mutation must be included more frequently as possible in clinical trials.
Abbreviations
HER2, human epidermal growth factor receptor 2; ADC, antibody–drug conjugate; NSCLC, non-small-cell lung cancers; T-DXd, trastuzumab deruxtecan; PS, performance status; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; TBB, transbronchial lung biopsy; CT, computed tomography; CEA, carcinoembryonic antigen; AE, adverse event; RECIST, Response Evaluation Criteria in Solid Tumors; ILD, interstitial lung disease.
Highlights
*ADCs are novel type of anticancer drugs for molecular targeting treatment.
*T-DXd developed as ADCs can be a novel treatment option for HER2-positive advanced-stage non-small-cell lung cancer.
*There are few reports on the administration of targeting ADCs including T-DXd to patients with poor PS.
*We report a case of NSCLC harboring HER2 exon 20 insertion mutation received durable response with T-DXd despite a poor PS.
Consent for Publication
We obtain informed consent from the patient for the publication of this case report and associated images.
Funding
No funding to declare.
Disclosure
AG and KK received lecture fee from Daiichi-Sankyo. MS reports grants and/or personal fees from AstraZeneca, MSD K.K, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Novartis, Kyowa Hakko Kirin, Nippon Kayaku, and Daiichi-Sankyo Company, during the conduct of the study. All other authors have no conflicts of interest in this work.
References
1. Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2021. doi:10.1056/NEJMoa2112431
2. Smit EF, Nakagawa K, Nagasaka M, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): interim results of DESTINY-Lung01. J Clin Oncol. 2020;38(15):9504. doi:10.1200/JCO.2020.38.15_suppl.9504
3. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi:10.1056/NEJMoa1914510
4. Mazieres J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003. doi:10.1200/JCO.2012.45.6095
5. Nakashima K, Ozawa Y, Daga H, et al. Osimertinib for patients with poor performance status and EGFR T790M mutation-positive advanced non-small cell lung cancer: a phase II clinical trial. Invest New Drugs. 2020;38(6):1854–1861. doi:10.1007/s10637-020-00943-0
6. Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–5108. doi:10.1158/1078-0432.CCR-15-2822
7. Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18(1):3–19. doi:10.1158/1541-7786.MCR-19-0582
8. Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–1742. doi:10.1038/bjc.2017.367
9. Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–2430. doi:10.1056/NEJMoa2004413
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.