Back to Journals » Journal of Pain Research » Volume 9
Efficacy, safety, and tolerability of fentanyl pectin nasal spray in patients with breakthrough cancer pain
Authors Ueberall M , Lorenzl S, Lux E, Voltz R, Perelman M
Received 10 February 2016
Accepted for publication 10 April 2016
Published 17 August 2016 Volume 2016:9 Pages 571—585
DOI https://doi.org/10.2147/JPR.S106177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Michael A Ueberall,1 Stefan Lorenzl,2 Eberhard A Lux,3,4 Raymond Voltz,5 Michael Perelman6
1Institute of Neurological Sciences, Nuremberg, Germany; 2Institute of Nursing Science and Practice, Paracelsus Private Medical University of Salzburg, Salzburg, Austria; 3Faculty of Medicine, Witten/Herdecke University, Witten, Germany; 4Clinic for Pain and Palliative Care Medicine, St.- Marien-Hospital, Luenen, Germany; 5Department of Palliative Medicine, University Hospital Cologne, Cologne, Germany; 6Archimedes Development Ltd., Nottingham, United Kingdom
Objective: Assessment of analgesic effectiveness, safety, and tolerability of fentanyl pectin nasal spray (FPNS) in the treatment of breakthrough cancer pain (BTcP) in routine clinical practice.
Methods: A prospective, open-label, noninterventional study (4-week observation period, 3 month follow-up) of opioid-tolerant adults with BTcP in 41 pain and palliative care centers in Germany. Standardized BTcP questionnaires and patient diaries were used. Evaluation was made of patient-reported outcomes with respect to “time to first effect”, “time to maximum effect”, BTcP relief, as well as changes in BTcP-related impairment of daily life activities, quality-of-life restrictions, and health care resource utilization.
Results: A total of 235 patients were recruited of whom 220 completed all questionnaires and reported on 1,569 BTcP episodes. Patients reported a significant reduction of maximum BTcP intensity (11-stage numerical rating scale [0= no pain, 10= worst pain conceivable]) with FPNS (mean ± standard deviation = 2.8±2.3) compared with either that reported at baseline (8.5±1.5), experienced immediately before FPNS application (7.4±1.7), or that achieved with previous BTcP medication (6.0±2.0; P<0.001 for each comparison). In 12.3% of BTcP episodes, onset of pain relief occurred ≤2 minutes and in 48.4% ≤5 minutes; maximum effects were reported within 10 minutes for 37.9% and within 15 minutes for 79.4%. By the end of the study, there had been significant improvements versus baseline in BTcP-related daily life activities (28.3±16.9 vs 53.1±11.9), physical (35.9±8.4 vs 26.8±6.5), and mental quality of life (38.7±8.5 vs 29.9±7.9) (P<0.001 for each comparison vs baseline); in addition, health care resource utilization requirements directly related to BTcP were reduced by 67.5%. FPNS was well tolerated; seven patients (3.2%) experienced eight treatment-emergent adverse events of which none was serious. There were no indicators of misuse or abuse.
Conclusion: FPNS provided rapid and highly effective BTcP relief in opioid-tolerant cancer patients with substantial improvements in daily functioning and quality of life. FPNS was well tolerated and associated with significant reductions in health care resource utilization and nursing assistance.
Keywords: breakthrough pain, cancer, fentanyl pectin nasal spray, intranasal administration, efficacy, safety, quality of life
Introduction
Moderate to severe pain is common among patients with cancer and remains a significant challenge to practitioners, despite advances in pain management and the widespread adoption of the World Health Organization (WHO) guidelines for cancer pain management.1,2 The WHO three-step “ladder” approach to cancer treatment includes the use of opioids with fixed, around-the-clock dosing in patients who do not respond to non-opioid analgesics.3,4 However, breakthrough cancer pain (BTcP), defined as a transient exacerbation of pain that occurs either spontaneously or in relation to specific predictable or unpredictable triggers despite relatively stable and adequately controlled background pain,5 is a common problem currently only minimally addressed by the WHO pain relief ladder.1,3 BTcP not only considerably impacts on daily activities, social relationships, quality of life (QoL), and well-being of afflicted patients6,7 but also places a substantial economic burden on society and the health care system.7–10
Fast-acting (rapid onset) fentanyl preparations administered on an “as needed” basis have become more common in BTcP control as they closely match the temporal dynamics of BTcP episodes.11,12 The high lipophilicity and low potential for local irritation of fentanyl13 permitted the development of new formulations with transmucosal administration routes, including the fentanyl pectin nasal spray (FPNS), PecFent® (Archimedes Pharma Ltd, Reading, UK). FPNS incorporates a proprietary pectin-based gelling agent that reduces drip and run-off.14 The nasal route avoids hepatic first-pass effects and thus offers better absorption; this formulation also benefits patients with oral mucositis and xerostomia and could be advantageous for patients experiencing nausea, vomiting, and impaired gastrointestinal function. Randomized controlled Phase II and Phase III studies in patients with BTcP have shown clinically important analgesic efficacy within 5–10 minutes following FPNS administration15–18 with safety/tolerability that is similar to placebo15,16 and faster meaningful pain relief than immediate-release morphine.17,18
Randomized clinical studies assess efficacy and safety of a particular medication within a restricted setting often with narrowly defined inclusion/exclusion criteria and treatment protocols to obtain a homogeneous study population. They should be complemented by noninterventional observational studies that are conducted in a “real world setting” and assess the routine administration of medications in the heterogeneous population of patients encountered in routine clinical practice. The current study investigated FPNS administration for BTcP episodes in opioid-tolerant patients under routine clinical practice conditions and was carried out in medical practices specializing in pain and palliative care.
Patients and methods
Study design and patients
This prospective, open-label, noninterventional observation was conducted in Germany in accordance with German Drug Law (AMG) §67 and in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and the requirements of National German Law. Study approval was granted by the independent ethics committee of the Medical Faculty Mannheim of Heidelberg University (file reference 2011-241N-MA). In accordance with legal requirements, the Bundesinstitut für Arzneimittel und Medizinprodukte (German Federal Institute of Drugs and Medical Devices), the National Association of Statutory Health Insurance Physicians, the National Association of Private Health Insurance Companies, and the Central Association of Health Insurances head organizations were notified. Patients provided written informed consent to the collection and release of anonymized data.
Patients were recruited at 41 treatment centers in Germany and included adult patients (age ≥18 years) with BTcP episodes despite a stable opioid regimen (≥60 mg/day of oral morphine or an equianalgesic dose of another opioid for ≥1 week) for whom a treatment decision to initiate FPNS had been made. Inclusion and exclusion criteria of the study were consistent with the European Summary of Product Characteristics (SmPC) for FPNS.19 Patient selection was at the discretion of the participating health care professionals who were experienced in the administration of strong opioids for cancer pain and who were directed to observe appropriate guidelines for the treatment of cancer pain and BTcP.20,21 To reduce selection bias, physicians were asked to enroll the first consecutive three to six eligible patients presenting with BTcP in their centers after study initiation.
Patients received FPNS in the course of routine clinical practice. Information pertaining to their health status and treatment was collected over a 4-week observation period using validated patient questionnaires. Additional information was gathered through standardized questionnaires, which were completed by the physicians during the study. A 3-month follow-up was scheduled to record possible unintended misuse/abuse indicators.
Study procedures
The 4-week core observation period comprised three study visits (baseline and weeks 1 and 4) and was complemented by a 3-month follow-up. At the baseline visit, participating patients provided data on their prestudy health, treatment, and pain; further questionnaires were completed at subsequent study visits. Patients also used a BTcP diary which contained detailed questions about their BTcP episodes. They completed the diary for up to eight BTcP episodes treated with FPNS during the 4-week observation. Patients were encouraged to independently complete the study questionnaires. However, proxy assessment/documentation by relatives was accepted (and recommended) in patients with advanced cancer stages or otherwise severe physical impairments. Physicians completed corresponding questionnaires at baseline, after 1 and 4 weeks of treatment, and after 3 months of follow-up. The study questionnaires were developed by the Institute of Neurological Sciences (IFNAP) on behalf of the German Pain Society and are based on standardized, validated questionnaires used for routine evaluation of (cancer) pain22 and palliative care patients.23,24
FPNS is available as a multidose nasal spray at 100 or 400 μg fentanyl/spray. It was prescribed after thorough instructions and advice (by the physician to the patient) according to the SmPC, patient information leaflet, and physician’s usual clinical procedures.19 Treatment was solely at the discretion of physician and patient. Initial doses were determined by the physician taking into account previous treatment, opioid dosage for background pain, previous experience with other rescue medications, and recommendations given in the SmPC. Titration was as deemed necessary for the individual patient to a dose providing adequate pain relief without any, or at least tolerable, side effects; dose adjustments were recorded throughout the study. Patients administered FPNS as needed. Adjunct therapies/rescue medications were used in accordance with the treatment center’s standard of care and clinical procedures; treatment changes were permitted and documented during the study as well as during a 3-month period afterwards to uncover potential abuse or misuse.
Assessments
Effectiveness of treatment was assessed with the measures “pain intensity” and “pain relief”.
- Pain intensity was rated by the patients on a validated 11-point numerical rating scale (NRS11; “0” = no pain, “10” = worst pain conceivable).24–27 Baseline documentation included current pain intensity status, background (chronic) pain intensity (“as it was during the last week”), and maximum pain intensity usually experienced during BTcP episodes. During the observation period, patients recorded their BTcP intensities experienced before FPNS administration and at maximum FPNS effect.
- The course of BTcP relief following FPNS administration was documented choosing from a list of time intervals for the two categories “time to first effect” and “time to maximum effect”. Furthermore, “speed of action”, “strength of action”, “tolerability”, and “ease of use” of FPNS treatment were rated on a 6-point Verbal Rating Scale from “very good” to “inefficient.”
- Patients who had previously used alternative rescue medications for BTcP episodes additionally compared FPNS effectiveness to their previous medication on a validated 7-point verbal Clinical Global Impression of Change scale from “very much better” to “very much worse.”
BTcP-related impairments of daily life activities and well-being were assessed at baseline and after 4 weeks with a modified version of the pain disability index (mPDI),28 and the German version of the 12-item short form QoL questionnaire (SF12).29 Patient-rated pain-related daily life activities impairments were assessed across the seven mPDI dimensions: “household and family”, “leisure and recreation”, “social activities”, “work”, “independence in personal hygiene and daily life”, “sleep”, and “quality of life”, each on an NRS11 ranging from 0= no disability to 10= complete disability. QoL was assessed using the SF12 physical component score (PCS) and mental component score (MCS) and compared to German reference data.30
Nursing assistance/help and individual health care resource utilization required by patients were recorded according to the checklist and proceedings of the German Hospice and Palliative Care Evaluation data collection, both at baseline and end of study.23
Safety was assessed throughout the observation period, including continuous treatment-emergent adverse event monitoring. Open-ended questions were part of the standard procedures performed at each individual study visit. In addition, patient diaries were routinely evaluated for any signs indicative of potential (hidden) adverse events. All adverse events were encoded with the Medical Dictionary for Regulatory Activities31 and evaluated for severity and relationship to FPNS.
Outcome measures
Primary outcome measures were patient-reported outcomes: change in BTcP intensity, time to onset (“time to first effect”), and time to maximum pain relief (“time to maximum effect”) following FPNS administration. Additionally, changes in BTcP-related impairments of daily functioning, QoL, and health care resource utilization requirements were evaluated.
Statistical analysis
Baseline, demographic, and efficacy analyses were performed for all enrolled patients who took at least one dose of FPNS and who had at least one postbaseline/postdose patient-reported outcome measure (efficacy analysis set [EAS]; modified intent-to-treat approach). Safety analyses were done for the complete study population (safety analysis set [SAS]).
Due to the explorative character of this analysis, no formal sample size estimation was performed. The last observation carried forward method was used to impute missing scores beyond baseline. For continuous variables, descriptive statistics were summarized by the number of patients, mean ± standard deviation (SD), and 95% confidence intervals of the mean, median, and range (minimum–maximum) values. For categorical and ordinal variables, data were summarized by frequency number and percentage of participants in each category; where appropriate, 95% confidence intervals were added.
For within group (eg, pre–post) comparisons of continuous/categorical variables paired samples, t-test/Fisher’s exact tests were performed. All statistical tests were carried out using a two-sided significance level of 0.05 and corrected for multiple significance comparisons with the Holm-Bonferroni method. All analyses were exploratory; no confirmatory analyses were performed nor statements derived. Test procedures were only used to evaluate the biometrical significance of differences found, not to confirm any a priori defined hypotheses.
Results
Study centers and patient disposition
Participating treatment centers were specialized in internal medicine (n=16), anesthesiology (n=13), general medicine (n=8), oncology/hematology (n=3), or surgery (n=1). They enrolled 235 patients (SAS), of whom 220 (93.6%) reported outcome measures for the efficacy analyses (EAS) creating a database of 1,569 BTcP episodes. Most patients were recruited by anesthesiologists (n=99, 42.1%) and internal specialists (n=89, 37.9%). Patient enrollment varied among centers engaged (on average, participating physicians recruited 5.6 [median 2] patients; 26 centers (63.4%) enrolled up to two patients, 15 centers [36.6%] enrolled three or more patients); however, statistical analysis revealed no “by center effect.”
The majority of patients (n=206, 87.7%) completed the 4-week observation period (see Figure 1). Reasons noted for premature discontinuation were “death due to progression of the underlying cancer” (n=6, 2.7%), “hospitalizations” (n=2, 0.9%), “cessation of BTcP” (n=3), “inadequate efficacy of the BTcP medication” (n=2, 0.9%), and “other reasons” (not further specified, n=1, 0.46%). No patient withdrew from the study because of an FPNS-related adverse effect.
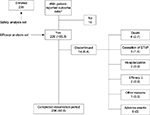  | Figure 1 Patient disposition. Notes: Data are presented as n or n (%). The downward arrow after efficacy indicates an insufficient efficacy. Abbreviation: BTcP, breakthrough cancer pain. |
Baseline situation
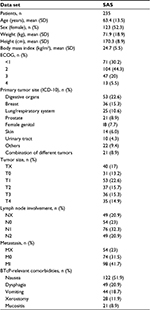
Demographic and clinical baseline characteristics of the SAS population are summarized in Table 1. Mean ± SD age was 63.4±13.5 years; 52.3% (n=123) were female. Eastern Cooperative Oncology Group (ECOG) performance status32 varied; 20.0% (n=47) of the patients had an ECOG score of 3 and 5.5% (n=13) of 4. The most common primary tumor sites were located in the gastrointestinal tract (n=53, 22.6%), breast (n=36, 15.3%), or lung/respiratory system (n=25, 10.6%); 21 patients (8.9%) suffered simultaneously from two different tumor types. In 108 patients (46.0%), the tumor spread into nearby structures (T2–4), in a fifth of patients (n=49, 20.9%) into distant lymph nodes (N2), and for 41.7% (n=98) distant metastasis were documented (M1).
More than half of the patients (n=134, 57.0%) reported at least one tumor or tumor treatment-related comorbidity with relevance for BTcP treatment, such as nausea (n=122, 51.9%), dysphagia (n=49, 20.9%), vomiting (n=44, 18.7%), xerostomy (n=28, 11.9%), and/or oral mucositis (n=21, 8.9%). Seven of ten patients (n=169, 71.9%) received outpatient care and a quarter (n=57, 24.3%) participated in a specialized ambulatory palliative care program.
Pain medication
All patients received background pain medication with strong opioids; fentanyl was the most commonly used, taken by 34.0% (n=80), followed by oxycodone (n=39, 16.6%), hydromorphone (n=36, 15.3%), and morphine (n=34, 14.5%). The average ± SD daily morphine equivalent dose was 162.2±107.9 mg (Table 2). More than half of patients (n=135, 57.4%) had not previously used BTcP medications; in those patients with previous experience (n=100), the most commonly prescribed BTcP medications were oral immediate-release opioids (n=48, 48.0%). Most patients were switched to FPNS owing to poor efficacy of previous BTcP treatments (n=52, 52.0%), inadequate speed of action (n=25, 25.0%), or because of intolerable side effects (n=11, 11.0%).
The most frequently recommended FPNS dose for initial use by SAS patients was 100 mg (n=170, 72.3%), followed by 200 mg (n=48, 20.4%) and 400 mg (n=16, 6.8%). However, initial dosages recorded “as used” for the first BTcP episode by EAS patients were 100 mg in 55.5% (n=122), 200 mg in 27.7% (n=61), and 400 mg in 16.8% (n=37) of patients. There was a moderate increase of the mean ± SD FPNS dose used between the first (178.5±108.7 mg) and eighth BTcP episode (222.2±116.9 mg). At study end, 45.5% of EAS patients (n=100) considered the 200 mg dose as “most effective” followed by 100 mg (n=66, 30.0%) and 400 mg (n=54, 24.5%).
BTcP characteristics and FPNS treatment effects
Most patients experienced more than one BTcP episode per day; the average ± SD daily frequency was 3.1±1.7 episodes (Table 2). About two-thirds of patients (n=151, 68.6%) reported a BTcP duration of 30–60 minutes; about a third of patients (n=76, 34.5%) experienced BTcP episodes of longer duration (>60 minutes). Mean ± SD NRS11 background pain intensity at baseline was 5.5±2.5 and therefore significantly higher than the patient-defined tailored treatment target of 3.5±1.6. Maximum BTcP intensity experienced without any rescue medication was 8.5±1.5 and 6.0±2.0 when rescue medication had been taken. Twenty-four patients (10.2%) reported predominantly “incidental”, 21.7% (n=51) mostly “spontaneous,” and 68.1% (n=160) both types of BTcP. In 77.4% (n=182), BTcP etiology was reported to be “cancer-related” and in 53.6% (n=126) of “mixed” pathophysiology (Table 2).
Average ± SD BTcP pain intensity improved significantly from 7.4±1.7 NRS11 before FPNS application to 2.9±2.3 NRS11 at the time of its maximum effect (see Figure 2; P<0.001) and differed significantly from that experienced without any BTcP treatments (8.5±1.5) as well as that achieved with prior BTcP medications (6.0±2.0; see Table 2; P<0.001 for each comparison). Mean ± SD absolute BTcP relief achieved with FPNS was 4.6±2.6 NRS11, and the corresponding relative improvement rate was 60.6%±35.2%.
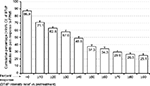
For the first eight BTcP episodes treated, the proportion of patients with intensity scores ≤3 NRS11 increased from 0.5%–2.2% at the time of FPNS use to 51.5%–64.7% at the time of its maximum effect (see Figure 3). BTcP relief associated with FPNS was rated as “complete” or “very strong” for 56.5% of episodes, “strong” for 25.4%, and “moderate” for 14.7%. Episodes in which the pain relief to FPNS was considered “mild” or “none” was only 3.0% or 0.4%. Percent relief reported at the maximum effect of FPNS versus the situation before administration was ≥30% in 57.6%, ≥50% in 37.3%, and ≥70% in 29.6% of BTcP episodes treated (see Figure 4).
Time to first and time to maximum effects
Time to first FPNS effect (ie, “onset of action”) was within 10 minutes for 78.9% of all BTcP episodes documented, within 5 minutes for 48.4%, and within 2 minutes for 12.3% (Figure 5). Time to “maximum effect” was ≤15 minutes for 79.4%, ≤10 minutes for 37.9%, ≤5 minutes for 19.3%, and ≤2 minutes for 6.0% of BTcP episodes. Titration had no overall impact on speed of onset and/or time to maximum effect.
Time intervals between BTcP onset and FPNS use shortened significantly over the first few treated BTcP episodes. Whereas only 21.8% of patients (n=48) initially used FPNS within 2 minutes after BTcP onset, this proportion increased considerably up to 65% (n=143, P<0.001) for the sixth episode treated/documented and remained there for the following BTcP episodes reported.
FPNS effects on daily life activities
BTcP treatment with FPNS was followed by a significant improvement in daily life activities (Table 3; Figure 6). Mean ± SD mPDI sum score improved from 53.1±11.9 at baseline to 28.3±16.9 at the end of the 4-week treatment period, corresponding to a relative improvement of BTcP-related restrictions in daily life of 46.7%±37.7% (P<0.001). In parallel, the proportion of patients reporting mPDI sum scores suggestive for severe BTcP-related disabilities in daily life (ie, those with sum score >43 points) decreased from 80.5% (n=177) at baseline to 22.7% (n=50) at study end (P<0.001), and the proportion of patients experiencing high/severe/extreme levels of BTcP-related disabilities (ie, those with >40/50/60 points) decreased significantly from 83.6%/65.9%/30.0% (n=184/145/66) to 22.7%/13.2%/2.7% (n=50/29/6; P<0.001 for each comparison).
Considering FPNS-related changes with respect to individual mPDI dimensions, the average ± SD mPDI scores improved from 7.0±2.5–8.1±2.0 at baseline to 3.4±2.6–4.4±2.6 NRS11 at the end of the 4-week observation period (see Table 3; P<0.001 for each comparison). In parallel, the proportion of patients reporting “none” or only “mild” BTcP-related mPDI restrictions (ie, scores ≤3) increased from 2.7%–10.9%
(n=6–24) to 39.1%–61.4% (n=86–135; P<0.001 for each mPDI dimension), whereas the proportion of patients experiencing “severe” or “extreme” restrictions (ie, scores ≥8) improved from 50.9%/65.9% (n=112/145) to 9.1%/14.1% (n=20/31; P<0.001 for each comparison). Greatest improvements were reported for BTcP-related restrictions in the mPDI dimensions “overall quality of life” (reduced by 4.5 NRS11) and “sleep” (reduced by 3.6 NRS11): the proportion of patients reporting “minor” or even “no” restrictions in these two mPDI dimensions increased from 2.7%/10.9% (n=6/24) at baseline to 51.4%/61.4% (n=113/135) after 4 weeks (P<0.001 for both).
FPNS effects on QoL
At baseline, study patients recorded severely impaired physical (PCS) as well as mental (MCS) SF12 component scores (see Figure 7). At the end of the 4-week observation period, average ± SD SF12 PCS and MCS improved significantly versus baseline – PCS: 35.9±8.4 versus 26.8±6.5 and MCS: 38.7±8.5 versus 29.9±7.9 (P<0.001 for each). In parallel, PCS and MCS distribution showed a considerable shift toward “normal” SF12 scores and the proportion of patients with PCS/MCS ≤2 SD below the German reference scores decreased significantly from 73.6%/75.9% (n=162/167) at baseline to 30.5%/29.6% (n=67/65) at study end (P<0.001 for both).
Average ± SD absolute/relative changes at study end versus baseline were comparable for both SF12 component scores: PCS: 9.1±8.8%/38.1±37.3% and MCS: 8.8±7.3%/33.1±28.5%; however, improvement varied with baseline scores and average ± SD FPNS-related percentage changes for both SF12 component scores were significantly higher in patients with “severe” (ie, scores ≤2 SD below reference) versus those with “moderate” QoL impairments (ie, scores ≤1 SD below reference) at baseline – PCS/MCS: 46.6±36.2%/38.0±27.5% versus 6.3±15.8%/8.5±19.3% (P<0.001 for each component).
FPNS effects on health care resource utilization
BTcP treatment with FPNS was associated with a substantial reduction of health care resource utilization. At the end of the 4-week period, physician-rated “overall nursing care requirements” were recorded as “very much less” due to FPNS for 31.8% of patients (n=70), and “much less” or “less” for a further 33.2% (n=73). Greatest changes versus baseline were reported for resource utilizations directly related to BTcP (67.5%; see Figure 8), followed by those related to stress/exertion (58.5%), anxiety (53.1%, P<0.001), and depression (46.0%; P<0.001 for each parameter).
Comparison with previous BTcP medications
Direct comparisons with prior BTcP rescue treatments were in favor of FPNS. Of those 88 patients of the EAS (40.0%) who had previously used other BTcP medications, 92.1% (n=81) rated FPNS as “better” than previous medications in terms of “speed of action”, 90.9% (n=80) with regard “strength of action”, 93.2% (n=82) with respect to its “tolerability,” and 56.8% (n=50) for its “ease of use”. Physicians also regarded FPNS as “superior” – that is, as “faster” in 96.6% (n=85) of those patients with prior experience, “stronger” in 88.6% (n=78), “better tolerated” in 58.0% (n=51), “safer” in 56.8% (n=50), and as “easier to use” in 50.0% (n=44).
Tolerability and safety
FPNS tolerability was rated positively by both patients (EAS) and physicians (SAS): a rating of “very good” was documented for EAS/SAS in 52.7%/43.8% (n=116 and 103), “good” in 34.6%/41.7% (n=76 and 98), and “satisfactory” in 10.9%/7.7% (n=24 and 18) of cases. Only in 1.8%/6.4% (n=4/15 patients) was FPNS tolerability rated as “poor”.
Seven patients (3.0%) reported eight treatment-emergent adverse events during the observation period – most common were “nausea” (n=2), “dizziness” (n=2), and “epistaxis” (n=2); “vomiting” and “dry mouth” were each reported once by two single patients. No event was considered serious or unlabeled; all were mild, transient, and resolved completely without any specific counter measures. None of these events led to a premature treatment discontinuation. Six patients died during the course of this study and two further patients were hospitalized. All of these eight events were attributed to the progression of the underlying cancer by the investigators and recorded as definitely “not FPNS-related.”
Neither during the 4-week observation period nor during the 3-month follow-up were any signs or indicators for an unintended misuse/abuse or an unapproved or accidental passing on of the study medication observed.
Discussion
Data from this study confirm that BTcP is associated with significant detrimental effects on daily functioning, physical, and mental QoL as well as a considerable demand for distinct health care resources in affected patients, despite an around the clock treatment with WHO step III opioids. Treatment with FPNS was highly efficacious, safe, well tolerated, and especially patient-friendly. FPNS-associated treatment effects were significant and not only translated directly into patient and physician perceived benefits, such as BTcP relief and related improvements in daily life activities as well as QoL, but also into medico-economic (indirect) factors, such as health care resource utilization.
The present study not only reinforces data observed in controlled clinical trials with FPNS but additionally provides “real world” experience on its efficiency in a study population typical of routine clinical practice: cancer patients managed in a pain/palliative care setting with ambulatory and outpatient scenarios. Under these conditions, patients reported a significant response to FPNS: 89.1% experienced a strong and 31.4% complete BTcP relief. Onset of pain relief was very fast: within 2 minutes after FPNS administration in 12.3% and within 5 minutes in 48.4% of BTcP episodes. Maximum pain relieving effects were achieved within 15 minutes after FPNS administration in 79.4% of BTcP episodes.
These FPNS effects were accompanied by significant improvements in BTcP-related restrictions with respect to daily life activities and QoL. Proportions of patients with severe BTcP-related disabilities in daily life (assessed with modified pain disability index) decreased from 80.5% to 22.7% and those with severe physical/mental QoL restrictions (measured with the SF12 PCS and MCS) improved from 73.6%/75.9% at baseline to 30.5%/29.6% at study end.
Improvement of daily functioning and QoL and the FPNS-related re-emergence of patient confidence in effectively encountering BTcP episodes without (external) help resulted in a significant reduction in health care resource utilization and nursing assistance. FPNS administration reduced health care resource requirements directly related to BTcP by 64.1% and those for stress/exertion by 58.5%. Average reduction of health care resource utilization needs was 46.0%±10.8% versus baseline. This is an important aspect of FPNS treatment, as BTcP complications usually not only add to patient morbidity33 but additionally increase the requirements for social and health care services, outpatient visits, inpatient admissions, and nursing assistance9 and thus direct and indirect treatment costs.
Almost half of our study patients reported previous experience with other BTcP medications, most commonly with oral immediate-release opioids. In general, FPNS was well accepted by these patients and worked significantly better than prior BTcP rescue medications: 92.1% of patients rated FPNS “faster” and 90.9% “stronger” than their prior treatments; in addition, 93.2% reported a better tolerability compared to previous rescue medications, and 56.8% documented improved ease of handling thus underlining the convenience of intranasal compared to oral/enteral administration routes.
FPNS showed an acceptable safety profile with an event pattern similar to that observed in controlled clinical trials; all reported adverse events were already known for the medication, were mild, transient, and resolved spontaneously without intervention. None were classified serious; no patient withdrew from the study because of an FPNS-associated safety concern. The frequency of events observed in our current study was lower than those reported in controlled and open-label extension studies,15–17,34,35 which probably reflects the shorter duration of this study, as well as differences in reporting procedures and study design.
In contrast to the controlled clinical trials with FPNS,15–17 participation in our study was not restricted to defined inclusion or exclusion criteria beyond those given in the SmPC. It is therefore interesting to note the demographic and baseline profile characteristics of the participants, as they directly reflect those of patients usually encountered in clinical practice and outpatient care. On average, patients in our study were considerably older than those in the controlled Phase II/III studies with FPNS reported by Portenoy et al,15 Taylor et al,16 and Davies et al.17 Patients in our study were also in a poorer state of health at the time of enrollment and reported a wider range of BTcP frequencies at enrollment (up to ten episodes per day) compared with those in the controlled studies, where a maximum of four BTcP episodes per day were allowed. All patients included in this study received background opioid dosages greater than the 60 mg morphine equivalent stated in the SmPC as a mandatory prerequisite for FPNS administration; in fact, average opioid dosages were 162.2 mg morphine equivalent per day. It is noteworthy that – despite these dosages – 61.8% (n=136) of our patients reported background pain intensities of ≥5 NRS11, which was significantly higher than their treatment target of 3.5 NRS11. These background pain intensity scores have to be taken into account when interpreting the data of this study – either as an indicator for inadequately controlled pain and thus a relative contraindication for any BTcP medication or a clinically driven indication for BTcP treatment because a significant number of our cancer pain patients obviously did not tolerate further opioid dose increases due to intolerable opioid-related side effects. Independent of this, FPNS provided significant BTcP relief with a good safety profile accompanied by improved daily functioning/QoL and with a reduced need for additional health care support.
To our surprise and despite repeated reference to the dosing guidance given in the SmPC and respective treatment guidelines, participating physicians frequently followed a dose-proportional, instead of the officially recommended step-wise titration, approach. However, in daily life and also in noninterventional studies, physicians are not mandated to follow such guidance and often try to speed up the titration process by tailoring the FPNS starting dose according to the daily dose of the underlying opioid background medication. In many ways, the fact that our study captures the real world management conditions of patients with BTcP is one of the advantages of an open-label noninterventional design. Similar discrepancies between official dosing recommendations given in the SmPC and practical use become increasingly known for all transmucosal fentanyl preparations for BTcP management.
Overall, the findings reported for our study add valuable information to our knowledge of FPNS and its effects given as a rescue medication to treat BTcP. However, there are several limitations to our study. Some are inherent to the open-label, single-arm design and include the lack of a placebo or active control group, and the relative lack of patient monitoring. The sample size was modest and study duration was just 4 weeks. Further evaluations with a larger patient population and a longer treatment period are warranted. However, our study population included the typical range of cancer patients encountered by pain and palliative care specialists in routine care, and treatment reflects the usual BTcP management in routine clinical practice. The study results should therefore be applicable under routine clinical practice conditions.
Distinct measures were undertaken to ensure that participating patients and physicians as well as therapeutic processes result in a representative picture of the routine clinical practice for the treatment of BTcP. Study centers were regionally distributed in Western Germany and the allocation of physicians/sites based on professional qualifications and center settings with a special focus on office-based physicians. The number of enrolled patients per physician was limited to avoid compromises of the representativeness and centers were told to include the first three to six consecutive patients eligible for the study to prevent significant selection effects. Center effect analyses revealed only minor and statistically insignificant differences with respect to demographic characteristics, FPNS use, and/or treatment effects.
Due to a lack of information on the number and characteristics of eligible, but for some reason, not enrolled patients, some selection bias cannot be formally excluded. However, the comparison of our patient population with those evaluated in other studies did not reveal any issues (eg, less advanced disease stages, minor pain problems, special care settings). In fact, the patients in our study were considerably older, in a poorer state of health, and presented with a broader range of BTcP episodes than those reported in the controlled trials.15–17 This renders a positive selection bias (eg, of patients with “easier to treat” BTcP) fairly unlikely.
Finally, the evaluations conducted in this study may also be limited by the inherently subjective nature of the patient-reported outcomes used. However, the NRS11 is a widely employed, validated, and highly reliable instrument of pain treatment outcomes.27 Regarding the temporal aspects of pain relief assessed in the present study, it should be noted that the parameters “time to first effect” and “time to maximum effect” have not been formally validated and warrant thorough reliability assessments. A comparison of these results with those of a comparably designed study using the same instruments,36 however, showed evident benefits of BTcP management with FPNS despite these methodological shortcomings.
Conclusion
The fast-acting intranasal fentanyl pectin formulation FPNS closely matches the temporal dynamics of BTcP episodes and thus offers several advantages over alternative treatment options. The real world data in the present study complement previously reported clinical data. Treatment with FPNS provided rapid pain relief accompanied by improved daily functioning and QoL and reduced requirements for additional health care support. FPNS was well tolerated. These results suggest that FPNS should be considered a treatment option in routine clinical practice for opioid-tolerant cancer patients with BTcP episodes.
Acknowledgments
This study was sponsored by Archimedes Pharma GmbH. Archimedes Pharma GmbH was responsible for both the design and the conduct of the study. Archimedes Pharma GmbH funded the statistical analysis and medical writing/editing assistance for this manuscript. Statistical analysis was carried out by O.Meany-MDPM GmbH, an independent Contract Research Organization, (sponsored by Archimedes Pharma GmbH).
We thank all investigators from the 41 centers involved and all patients who took part in this trial: Akrivakis K Hamburg; Arnold R Beucha; Bauermeister H Erfurt; Behrendt J Brandenburg; Benrath J Mannheim; Bluhm M Wedel; Böttcher B Potsdam; Felber J Berlin; Freidt A Bautzen; Gastmeier K Potsdam-Babelsberg; Golla I Quedlinburg; Hait B Unna; Ha-Phuoc H Olpe; Hauch U Erfurt; Heits F Rotenburg; Helmbrecht J Bochum; Heβling J Berlin; Hildebrandt S Ratzeburg; Hladik R-J Ludwigshafen; Kagalovska T Castrop-Rauxel; Kindler M Berlin; Kindler B Berlin; Kolitsch K Katzhütte; Mansfeld-Nies R Siegen; Meixner M Witzenhausen; Mühlmann U Leipzig; Münker A Herne; Niknafs K Hilden; Oestereicher M Frankfurt; Otremba B Delmenhorst; Nüvit Özmen M Berlin; Papke J Neustadt i. Sa.; Richter T Neustrelitz; Römmele U Nürtingen; Ruffert K Jena; Schütze B Cottbus; Schwittay A Böhlen; Sittig H-B Geesthacht; Stern H Neuss; Zarth R Albstadt; and Zimmermann M Frankfurt.
Author contributions
MAU and MP were responsible for the study design and execution. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Within the last 12 months: MAU has received speaker’s honoraria and or consultancy fees from Archimedes, Almirall, Bene-Arzneimittel, Grünenthal, Janssen-Cilag, Menarini, Mundipharma, Pfizer, Pharm Allergan, Philips, ProStrakan, and TEVA. SL has received speaker’s honoraria from Boehringer, TEVA and UCB, and scientific grants from TEVA. EAL has received speaker’s honoraria and advisory fees from Archimedes, Beta-Pharma, Grünenthal, Mundipharma, Pfizer, and TEVA. RV has received speaker’s honoraria and advisory fees from Archimedes, Munidpharma, Pfizer and TEVA, and fees for a clinical trial from TEVA. MP was an employee of Archimedes Development Ltd. and acts now as a consultant to ProStrakan which acquired Archimedes Development Ltd in 2015. The authors report no further conflicts of interest in this work.
References
Kumar N. WHO Normative Guidelines on Pain Management. Report of a Delphi Study to determine the need for guidelines and to identify the number and topics of guidelines that should be developed by WHO. Geneva: World Health Organization; 2007. Available from: http://www.who.int/medicines/areas/quality_safety/delphi_study_pain_ guidelines.pdf. Accessed January 16, 2016. | ||
Margarit C, Juliá J, López R, et al. Breakthrough cancer pain – still a challenge. J Pain Res. 2012;5:559–566. | ||
World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed. Geneva: World Health Organization; 1996. Available from: http://whqlibdoc.who.int/publications/9241544821.pdf. Accessed January 16, 2016. | ||
World Health Organization. WHO’s Pain Ladder for Adults. Available from: http://www.who.int/cancer/palliative/painladder/en/. Accessed January 16, 2016. | ||
Davies AN, Dickman A, Reid C, et al. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13(4):331–338. | ||
Caraceni A, Martini C, Zecca E, et al; Working Group of an IASP Task Force on Cancer Pain. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat Med. 2004;18(3):177–183. | ||
Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81:129–134. | ||
Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J Pain Symptom Manage. 2000;20:87–92. | ||
Fortner BV, Okon TA, Portenoy RK. A survey of pain-related hospitalizations, emergency department visits, and physician office visits reported by cancer patients with and without history of breakthrough pain. J Pain. 2002;3:38–44. | ||
Abernethy AP, Wheeler JL, Fortner BV. A health economic model of breakthrough pain. Am J Manag Care. 2008;14:S129–S140. | ||
Hanks GW, Conno F, Cherny N, et al; Expert Working Group of the Research Network of the European Association for Palliative Care. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84:587–593. | ||
Prommer E. The role of fentanyl in cancer-related pain. J Palliat Med. 2009;12:947–954. | ||
Dale O, Hjortkjær R, Kharasch ED. Nasal administration of opioids for pain management in adults. Acta anesthesiol Scand. 2002;46:759–770. | ||
Castille J, Cheng YH, Simmons B, Perelman M, Smith A, Watts P. Development of in vitro models to demonstrate the ability of PecSys®, an in situ nasal gelling technology, to reduce nasal run-off and drip. Drug Dev Ind Pharm. 2013;39(5):816–824. | ||
Portenoy RK, Burton AW, Gabrail N, Taylor D; Fentanyl Pectin Nasal Spray 043 Study Group. A multicenter, placebo controlled, double-blind, multiple-crossover study of Fentanyl Pectin Nasal Spray (FPNS) in the treatment of breakthrough cancer pain. Pain. 2010;151(3):617–624. | ||
Taylor D, Galan V, Weinstein SM, Reyes E, Pupo-Araya AR, Rauck R; Fentanyl Pectin Nasal Spray 043 Study Group. Fentanyl pectin nasal spray in breakthrough cancer pain. J Support Oncol. 2010;8(4):184–190. | ||
Davies A, Sitte T, Elsner F, et al. Consistency of efficacy, patient acceptability, and nasal tolerability of fentanyl pectin nasal spray compared with immediate-release morphine sulfate in breakthrough cancer pain. J Pain Symptom Manage. 2011;41(2):358–366. | ||
Fallon M, Reale C, Davies A, et al; Fentanyl Nasal Spray Study 044 Investigators Group. Efficacy and safety of fentanyl pectin nasal spray compared with immediate-release morphine sulphate tablets in the treatment of breakthrough cancer pain: a multicentre, randomised, controlled, double-blind, double-dummy multiple-crossover study. J Support Oncol. 2011;9(6):224–231. | ||
PecFent® (pectin fentanyl nasal spray) Summary of product characteristics. European Medicines Agency; October 25, 2012. | ||
Caraceni A, Hanks G, Kaasa S, et al; for the European Palliative Care Research Collaborative (EPCRC); on behalf of the European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–e68. | ||
Mercadante S, Radbruch L, Davies A, et al. Comparison of intranasal fentanyl spray with oral transmucosal fentanyl citrate for the treatment of breakthrough cancer pain: an open-label, randomised, crossover trial. Curr Med Res Opin. 2009;25:2805–2815. | ||
German Pain Questionnaire and German Pain Diary; Nagel B, Gerbershagen HU, Lindena G, Pfingsten M. Development and evaluation of the multidimensional German pain questionnaire. Schmerz. 2002;16:263–270. | ||
Stiel S, Pollok A, Elsner F, et al. Validation of the Symptom and Problem Checklist of the German Hospice and Palliative Care Evaluation (HOPE). J Pain Symptom Manage. 2012;43(3):593–605. | ||
Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126. | ||
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. | ||
Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage. 2003;25:406–411. | ||
Dworkin RH, Turk DC, Farrar JT, et al; IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. | ||
Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil. 1987;68:438–441. | ||
Bullinger M, Kirchberger I. SF-36 Fragebogen zum Gesundheitszustand – Manual. Göttingen: Hogrefe; 1998. | ||
Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. J Clin Epidemiol. 1998;51(11):1171–1178. | ||
US Food and Drug Administration. Medical Dictionary for Regulatory Activities, Version 14.1. Available from: http://www.meddra.org/. Accessed April 29, 2016. | ||
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. | ||
Skinner C, Thompson E, Davies A. Clinical features. In: Davies A, editor. Cancer-Related Breakthrough Pain. Oxford: Oxford University Press; 2006:13–22. | ||
Portenoy RK, Raffaeli W, Torres LM, et al; Fentanyl Nasal Spray Study 045 Investigators Group. Long-term safety, tolerability, and consistency of effect of fentanyl pectin nasal spray for breakthrough cancer pain in opioid-tolerant patients. J Opioid Manage. 2010;6(5):319–328. | ||
Radbruch L, Torres LM, Ellershaw JE, et al. Long-term tolerability, efficacy and acceptability of fentanyl pectin nasal spray for breakthrough cancer pain. Support Care Cancer. 2012;20(3):565–573. | ||
Überall MA, Müller-Schwefe GH. Sublingual fentanyl orally disintegrating tablet in daily practice: efficacy, safety and tolerability in patients with breakthrough cancer pain. Curr Med Res Opin. 2011;27(7):1385–1394. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.