Back to Journals » Drug Design, Development and Therapy » Volume 13
Efficacy, Safety And Feasibility Of Antiemetic Prophylaxis With Fosaprepitant, Granisetron And Dexamethasone In Pediatric Patients With Hemato-Oncological Malignancies
Authors Cabanillas Stanchi KM , Ebinger M , Hartmann U, Queudeville M , Feucht J, Ost M , Koch MS, Malaval C, Mezger M , Schober S, Weber S, Michaelis S, Lange V, Lang P, Handgretinger R, Döring M
Received 3 May 2019
Accepted for publication 29 August 2019
Published 30 September 2019 Volume 2019:13 Pages 3439—3451
DOI https://doi.org/10.2147/DDDT.S214264
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Cristiana Tanase
Karin Melanie Cabanillas Stanchi,1,* Martin Ebinger,1,* Ulrike Hartmann,2 Manon Queudeville,1 Judith Feucht,1 Michael Ost,1 Marie-Sarah Koch,1 Carmen Malaval,1 Markus Mezger,1 Sarah Schober,1 Simone Weber,1 Sebastian Michaelis,1 Veit Lange,1 Peter Lang,1 Rupert Handgretinger,1 Michaela Döring1
1Department of General Pediatrics, Hematology/Oncology, University Children‘s Hospital Tübingen, Tübingen 72076, Germany; 2University Pharmacy, Eberhard-Karls-University of Tübingen, Tübingen 72076, Germany
*These authors contributed equally to this work
Correspondence: Michaela Döring
University Hospital Tübingen - Children’s Hospital, Department I – General Pediatrics, Hematology/Oncology, Hoppe-Seyler-Str. 1, Tübingen 72076, Germany
Tel +49-(0)7071-2981355
Fax +49-(0)7071-295203
Email [email protected]
Background: Chemotherapy-induced nausea and vomiting (CINV) are a major burden for patients undergoing emetogenic chemotherapy. International guidelines recommend an antiemetic prophylaxis with corticosteroids, 5-HT3R-antagonists and NK1R-antagonists. The NK1R-antagonist fosaprepitant has shown favorable results in pediatric and adult patients. There is little pediatric experience with fosaprepitant.
Methods: This non-interventional observation study analyzed 303 chemotherapy courses administered to 83 pediatric patients with a median age of 9 years (2–17 years), who received antiemetic prophylaxis either with fosaprepitant and granisetron with or without dexamethasone (fosaprepitant group/FG; n=41), or granisetron with or without dexamethasone (control group/CG; n=42), during moderately (CINV risk 30–90%) or highly (CINV risk>90%) emetogenic chemotherapy. The two groups’ results were compared with respect to the safety and efficacy of the antiemetic prophylaxis during the acute (0-24hrs after chemotherapy), delayed (>24–120hrs after chemotherapy) and both CINV phases. Laboratory and clinical adverse events were compared between the two cohorts.
Results: Adverse events were not significantly different in the two groups (p>0.05). Significantly fewer vomiting events occurred during antiemetic prophylaxis with fosaprepitant in the acute (23 vs 142 events; p<0.0001) and the delayed (71 vs 255 events; p<0.0001) CINV phase. In the control group, the percentage of chemotherapy courses with vomiting was significantly higher during the acute (24%/FG vs 45%/CG; p<0.0001) and delayed CINV phase (28%/FG vs 47%/CG; p=0.0004). Dimenhydrinate (rescue medication) was administered significantly more often in the CG, compared to the FG (114/FG vs 320/CG doses; p<0.0001). Likewise, in the control group, dimenhydrinate was administered in significantly more (p<0.0001) chemotherapy courses during the acute and delayed CINV phases (79 of 150; 52.7%), compared to the fosaprepitant group (45 of 153; 29.4%).
Conclusion: Antiemetic prophylaxis with fosaprepitant and granisetron with or without dexamethasone was well tolerated, safe and effective in pediatric patients. However, larger prospective trials are needed to evaluate these findings.
Keywords: fosaprepitant, granisetron, pediatric, antiemetic prophylaxis, chemotherapy induced vomiting, children
Introduction
Chemotherapy-induced nausea and vomiting (CINV) belongs among the most burdensome and feared side effects of chemotherapy in hemato-oncological treatment and drastically reduces the patients’ quality of life.1,2
CINV is divided into two phases: the acute (0–24hrs after the administration of chemotherapy) and the delayed (>24–120hrs) CINV phase. The pathogenesis of CINV is mainly mediated by stimulation of different neurotransmitter receptors by chemotherapeutic agents: the 5-hydroxytryptamine-3 receptor (5HT3R), the neurokinin-1 receptor (NK1R) and the dopamine receptor D2. The activation of these receptors induces peripherally or centrally caused CINV.3
A chemotherapeutic agent’s emetogenic potential is classified according to the risk of emesis in the absence of antiemetic prophylaxis. The Pediatric Oncology Group of Ontario (POGO) has proposed a 4-stage risk classification system for pediatric patients: minimal, stage 1 (<10% emesis risk); low, stage 2 (10–<30%); moderate, stage 3 (30–90%) and high, stage 4 (>90%).4 International guidelines currently recommend a comprehensive antiemetic prophylaxis with corticosteroids (eg, dexamethasone), a 5-HT3R antagonist (eg, ondansetron or granisetron) and an NK1R antagonist (eg, aprepitant or fosaprepitant), for adult and pediatric patients undergoing moderately or highly emetogenic chemotherapy.2,5,6
Granisetron is a highly selective 5-HT3R antagonist which is both well tolerated by pediatric patients and effective as CINV prophylaxis.7,8 A study with healthy subjects did not identify any effects on granisetron’s pharmacokinetic properties from the concomitant administration of aprepitant.9
It was shown in positron emission tomography analyses of the NK1R occupancy, that over 90% of the NK1R in the brain are blocked for at least 48hrs after the administration of intravenous fosaprepitant; different comparative studies which included adult or pediatric patients, showed that single-dose fosaprepitant prior to chemotherapy, was just as effective as a three-day, oral antiemetic prophylaxis with aprepitant.10,11 CINV control has significantly improved following the approval of aprepitant/fosaprepitant.12–14
Randomized clinical trials have proven the safety and good efficacy of a CINV prophylaxis regimen with aprepitant or fosaprepitant for adult and pediatric patients after emetogenic chemotherapy, during both the acute and delayed CINV phases.13,15,16 However, there is few data on pediatric patients receiving an antiemetic prophylaxis with fosaprepitant during moderately or highly emetogenic chemotherapy.11,16,17
Based on fosaprepitant’s favorable properties, in terms of safety and efficacy, for adult patients and those of oral aprepitant in pediatric patients, the standard CINV prophylaxis regimen used for pediatric patients receiving moderately or highly emetogenic chemotherapy in the University Children’s Hospital Tübingen was gradually changed from a regimen of granisetron with or without dexamethasone, to a prophylaxis regimen with additional single-dose intravenous fosaprepitant directly before the first administration in the respective chemotherapy course of a moderately or highly emetogenic chemotherapeutic agent.
The primary objective of this work was to analyze these patients’ data in order to evaluate the efficacy, safety and feasibility of an antiemetic prophylaxis regimen with single-dose intravenous fosaprepitant and granisetron, with or without dexamethasone, in comparison to the standard prophylaxis regimen of granisetron with or without dexamethasone for pediatric patients receiving moderately and highly emetogenic chemotherapy.
Patients And Methods
Compliance With Ethical Standards
This analysis was performed in accordance with the Helsinki declaration and with the standards of the institutional ethics committee. This is a non-interventional observation study in accordance with the directive 2001/20/EC of the European Parliament and of the council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medical products for human use. Formal informed consent of the patients and an ethics vote of the institutional ethics committee are therefore not required for this study.18 Baseline demographics, clinical factors, and survival rates were abstracted from clinical and research records on all patients and maintained on a prospective basis.
Study Background And Design
The standard CINV prophylaxis regimen of granisetron, with or without dexamethasone, which had been used in the study center’s children’s hospital, was gradually changed during 2016 to a prophylaxis regimen with single-dose intravenous fosaprepitant for pediatric patients undergoing moderately or highly emetogenic chemotherapy. Initially, a weight-adapted fosaprepitant dosage (4.0 mg/kg bodyweight) was used for patients >12 years of age, in accordance with the MASCC/ESMO guidelines’ dosing recommendations for adult patients.2 After these patients demonstrated a good tolerability to fosaprepitant, younger patients (0.5–12 years) were also gradually moved to this prophylaxis regimen. In March 2018, the European Medicines Agency (EMA) reported a positive benefit-risk-ratio for fosaprepitant as CINV prophylaxis in pediatric patients (0.5–17 years of age).19 In April 2018, fosaprepitant was approved for pediatric patients (0.5–17 years of age) by the US Food and Drug Administration.14
The primary objective of this analysis was to evaluate the safety, efficacy and feasibility of an antiemetic prophylaxis regimen using fosaprepitant and granisetron, with or without dexamethasone, during moderately and highly emetogenic chemotherapy, compared to a standard regimen of only granisetron, with or without dexamethasone, for pediatric patients.
The data of all the pediatric patients who met the inclusion criteria and had received a CINV prophylaxis regimen with granisetron and fosaprepitant with or without dexamethasone, between August 2016 and September 2017 in the University Children’s Hospital Tübingen were analyzed (fosaprepitant group; FG) and compared with a pediatric cohort (control group; CG) who met the inclusion criteria and had received the standard CINV prophylaxis regimen of granisetron with or without dexamethasone, between August 2015 and July 2016.
The inclusion criteria were age between 0.5 and 17 years at the time of chemotherapy, administration of moderately to highly emetogenic chemotherapy during an in-patient stay, CINV prophylaxis with granisetron only, with or without dexamethasone only (CG), or granisetron, with or without dexamethasone, and single-dose fosaprepitant (FG).
Exclusion criteria were metastatic disease or chemotherapy with a palliative approach, vomiting or the administration of antiemetic medication in the 24hrs prior to the start of chemotherapy, medication with aprepitant, ondansetron or other NK1R or 5-HT3R antagonists, allergy to NK1 or 5-HT3-antagonists, abnormal liver (AST and ALT >2.5 times the upper normal limit) or kidney (serum creatinine >2.5 times the upper normal limit) function in the 24hrs prior to chemotherapy, contraindication for corticosteroids and conditioning chemotherapy before hematopoietic stem cell transplantation.
The chemotherapy courses’ emetogenic potential and their CINV risk were categorized using the scale proposed by Dupuis and colleagues.4 Each chemotherapy course’s overall emetogenic potential was defined as that of the administered agent with the highest emetogenic potential.
The acute CINV phase was defined as the first 24hrs after the first administration of the chemotherapeutic agent with the highest emetogenic potential in the respective course. The delayed CINV phase was defined as the subsequent 96hrs period (>24–120hrs after administration). The observation period included the acute and the delayed CINV phase.
Drug Administration
In accordance with international guidelines, pediatric patients in the CG received an antiemetic prophylaxis with granisetron with (in the case of highly emetogenic chemotherapy) or without (in the case of moderately emetogenic chemotherapy) dexamethasone.5,6,20 Pediatric patients in the FG additionally received fosaprepitant. Fosaprepitant, granisetron and dexamethasone were all administered through a central venous catheter.
Fosaprepitant (4.0 mg per kg bodyweight; max. 150 mg) was administered as a single intravenous (IV) infusion within 30 mins, at least 1hr before the first administration of a moderately or highly emetogenic agent in the respective chemotherapy course.
Granisetron was administered at 2 x 40 µg per kg BW and day, on all the days on which chemotherapy was administered. Granisetron was given during the administration of moderately emetogenic chemotherapy as a slow IV injection within 3 mins and was initially given at least 30 mins before the start of each chemotherapy course.
Dexamethasone was only administered during highly emetogenic chemotherapy courses at 2 x 0.1 mg per kg BW (max. 2 x 4 mg/d as a single dose; IV infusion within 20 mins in combination with granisetron). Dexamethasone was administered on each day of the respective chemotherapy course on which highly emetogenic chemotherapy was administered and was started at least 30 mins before beginning a chemotherapy course.
Rescue medication with dimenhydrinate was administered as a short infusion through a central venous catheter (1.0 mg per kg BW three times per day, max. 3 x 62 mg), on demand.
Dimenhydrinate (0.1 mg/kg BW/d; max. 4.0 mg, max. 4 times per day) and levomepromazine (0.1 mg/kg BW/d; max. 0.2 mg/kg BW/d as 24 hr infusion) were used to treat breakthrough CINV.
Safety And Tolerance
Adverse events during the observation period were registered and categorized as proposed by the United States National Cancer Institute’s Common Terminology Criteria for Adverse Events (US NCI CTCAE).21
Laboratory liver and kidney parameters and electrolytes were initially assessed on the first day of the in-patient stay prior to chemotherapy and antiemetic prophylaxis (Baseline); their maximums or minimums (Max/Min) and values at least 120hrs after the start of antiemetic prophylaxis (End) were assessed and analyzed. Normal serum concentrations of liver parameters were defined as: alanine aminotransferase (ALT) ≤39 U/L; aspartate aminotransferase (AST) ≤59 U/L; total bilirubin (normal range ≤1.1 mg/dL); direct bilirubin (normal range ≤0.3 mg/dL). Normal serum concentrations of kidney parameters were defined as creatinine ≤0.7 mg/dL and urea ≤46 mg/dL. Normal serum concentrations of electrolytes were defined as potassium 3.4–4.9 mmol/L; sodium 134 mmol/L - 145 mmol/L, and calcium 2.0–2.6 mmol/L.
An increase to >1.5 and >2.5-fold of the upper normal limits (ALT, AST, indirect bilirubin, direct bilirubin, creatinine and urea) was categorized as a clinically relevant increase beyond normal values. Likewise, clinically relevant changes in sodium concentrations were categorized by <134 mmol/L or <130 mmol/L, decreases in potassium were categorized by <3.4 mmol/L or <3.0 mmol/L, and decreases in calcium values were categorized by <2.0 mmol/L or <1.8 mmol/L. The results were used for comparisons with the baseline values.
Efficacy
For the efficacy analysis, the occurrence of vomiting and doses of dimenhydrinate administered (on-demand medication) were assessed and analyzed with respect to the CINV phases and the overall emetogenic potential of all moderately or highly emetogenic chemotherapy courses.
Primary efficacy endpoints were the total number of vomiting events, the number of chemotherapy courses in which vomiting occurred, the proportion of patients who experienced vomiting or received dimenhydrinate, and the number of dimenhydrinate doses administered. These endpoints were analyzed and compared with respect to the CINV phase, the relevant study cohort and the emetogenic potential of the administered chemotherapy course.
Statistical Analysis
Chi-square-tests (with Yates’ continuity correction) and Fisher’s exact test were used for 2-sample tests for equality of proportions and applied to the frequencies of clinical parameters in the two treatment groups (FG and CG). In addition, the package rateratio.test of R was used to compare the frequency of the vomiting events and the number of administered dimenhydrinate doses between the FG and the CG.
The statistical comparison of the differences between the results and the normal range values for the liver and kidney parameters, as well as electrolytes, was performed by one-sample paired t-tests or one-sample Wilcoxon signed rank tests (depending on the results of the Shapiro–Wilk normality test), taking into account the 95% confidence intervals (CI).
The inferential statistical analysis between the baseline values, as well as the maximum and minimum values was performed with the Wilcoxon matched pairs signed rank test. Differences were only considered to be significant if they were clinically relevant, ie, significantly below (sodium, calcium and potassium) the reference values or above them (all other parameters).
Graphs and statistical tests were created with GraphPad Prism for Windows, version 7 (GraphPad Software Inc., La Jolla, CA, USA), or with R (The R Foundation for Statistical Computing, Institute for Statistics and Mathematics, Wirtschaftsuniversität Wien, Austria). P-values of p<0.05 (*), p<0.01 (**), p<0.001 (***), and p<0.0001 (****) were defined as statistically significant and are illustrated in the bar charts.
Results
Patient Characteristics
A total of 83 pediatric patients with a median age of 9 years (range 2–17) were enrolled in this analysis. Of these patients, 41 (49.4%) were enrolled in the fosaprepitant group (median age 9.5 years, range 2–17 years) and 42 (50.6%) were enrolled in the control group (median age 8 years, range 2–17 years).
The underlying diseases included acute lymphoblastic leukemia (ALL; n=15), acute myeloid leukemia (AML; n=3), astrocytoma (n=12), Burkitt lymphoma (n=5), choriocarcinoma (n=3), medulloblastoma (n=8), neuroblastoma (n=11), osteosarcoma (n=8), rhabdomyosarcoma (n=9) and other malignant neoplasms (n=9). The distribution of patient characteristics including age, sex and underlying disease was not significantly different in the two groups (p>0.05) (Table 1).
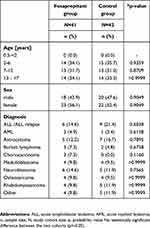 |
Table 1 Patient Characteristics |
Emetogenic Chemotherapy
All patients received chemotherapy courses with moderately or highly emetogenic chemotherapy. A total of 153 and 150 chemotherapy courses (not significantly different; p=0.908) were administered in the FG and the CG, respectively.
A median of 4 courses (range 1–4 courses) with a median duration of 4 days (range 1–9 days) were administered per patient in the CG and a median of 4 courses (range 1–4 courses) with a median duration of 4 days (range 1–8 days) in the FG. The chemotherapeutic agents used in these courses and the highest emetogenic potential of each course are summarized in Table 2. In the FG, 116 of 153 courses (75.8%) included highly emetogenic agents (risk of emesis>90%), and the other 37 courses (24.2%) contained moderately emetogenic agents (risk of emesis>30–90%). In the CG, 117 of 150 courses (78.0%) included highly emetogenic agents, and the other 33 courses (22.0%) contained moderately emetogenic agents (risk of emesis>30–90%). Significant differences could be detected in the administration of ifosfamide (p=0.0141) and irinotecan (0.0042) between the two cohorts. The significantly higher percentage of patients receiving irinotecan in the fosaprepitant group was caused by a significantly higher percentage of patients with relapsed neuroblastoma who received an irinotecan-based regimen (Table 2). However, the percentages of highly and moderately emetogenic chemotherapy courses administered were not significantly different between the two cohorts (p=0.7532) (Table 3).
 |
Table 2 Emetogenic Potential Of Chemotherapy |
 |
Table 3 Overall Emetogenic Potential |
Efficacy
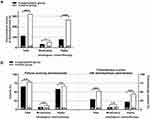
All the two groups’ 303 chemotherapy courses were included in the efficacy analysis. In the acute and the delayed CINV phase, 13 (31.7%) and 16 (39.0%) of the 41 patients in the fosaprepitant group, experienced vomiting during moderately and highly emetogenic chemotherapy, compared to 30 (71.4%) and 29 (69.0%) of the 42 patients in the control group. The differences were significant for both the acute (p=0.0004) and the delayed (p=0.0083) CINV phases. When only those patients undergoing moderately emetogenic chemotherapy were considered, significantly fewer patients in the fosaprepitant group experienced vomiting during the acute phases (CG: n=10 (23.8%) vs FG: n=0 (0.0%); p=0.0011) but not during the delayed CINV phase (CG: n=7 (16.7%) vs FG: n=3 (7.3%); p=0.3126). Of those patients undergoing highly emetogenic chemotherapy, significantly fewer patients in the fosaprepitant group experienced vomiting during the delayed phase (CG: n=22 (52.4%) vs FG: n=11 (26.8%); p=0.0248) but not during the acute CINV phase (CG: n=20 (47.6%) vs FG: n=13 (31.7%); p=0.1797) (Figure 1A).
Analyzing the data with respect to the chemotherapy courses, vomiting occurred in significantly more moderately and highly emetogenic chemotherapy courses during the acute phase (CG: 68 of 150 courses (45.3%) vs FG: 36 of 153 courses (23.5%); p<0.0001) and the delayed phase (CG: 71 of 150 courses (47.3%) vs FG: 42 of 153 courses (27.5%); p=0.0004). Vomiting occurred during significantly fewer moderately emetogenic chemotherapy courses in the fosaprepitant group during the acute phase (CG: n=16 (10.7%) vs FG: n=2 (1.3%); p=0.0005) but not in the delayed (CG: n=17 (11.3%) vs FG: n=13 (8.5%); p=0.4462) CINV phase. For highly emetogenic chemotherapy courses, vomiting occurred in significantly fewer chemotherapy courses in the fosaprepitant group during the acute phase (CG: n=52 (34.7%) vs FG: n=34 (22.2%); p=0.0215) and the delayed (CG: n=54 (36.0%) vs FG: n=29 (19.0%); p=0.0012) CINV phase (Figure 1B).
In the control group, the median number of vomiting events per course, during the courses in which vomiting occurred, was 2 (range 1–10) in the acute and 4 (range 1–21) in the delayed CINV phases. In the fosaprepitant group, the median number of vomiting events was 3 (range 1–3) during the acute and 3 (range 1–13) during the delayed CINV phases. The total number of vomiting events registered during moderately and highly emetogenic chemotherapy was significantly higher in the CG during the acute phase (142/CG vs 23/FG events; ratio=6.2; p<0.0001), the delayed phase (255/CG vs 71/FG events; ratio=3.6; p<0.0001), and both CINV phases (397/CG vs 94/FG events; ratio=4.2; p<0.0001), compared to the FG. In the control group, 31 and 76 vomiting events were recorded during moderately emetogenic chemotherapy, in the acute and the delayed phases, compared with 0 and 3 events (ratio=25.3), respectively, in the fosaprepitant group. The differences were significant (p<0.0001). During highly emetogenic chemotherapy, 111 and 179 vomiting events were registered during the acute and the delayed phases in the control group, compared with 23 (ratio=4.8) and 68 (ratio=2.6) events, respectively, in the fosaprepitant group. The differences were significant (p<0.0001). (Figure 1C).
A median of 4 dimenhydrinate doses (range 1–21 doses) were administered per chemotherapy course in the control group and a median of 1 dose (range 1–11 doses) in the fosaprepitant group, during all the moderately and highly emetogenic chemotherapy courses analyzed. Considered separately, a median of 4 doses (range 1–19) and 1 dose (range 1–11) were administered in the control group and the fosaprepitant group, respectively, during moderately emetogenic chemotherapy. In comparison, a median of 4 doses (range 1–21) and 1 dose (range 1–11) were administered in the control group and the fosaprepitant group, respectively, during highly emetogenic chemotherapy. During moderately and highly emetogenic chemotherapy, a total of 320 dimenhydrinate doses were administered in the control group, significantly more doses (p<0.0001) than the 114 doses in the fosaprepitant group. The results were similar for moderately emetogenic chemotherapy alone, with 53 (CG) versus 34 (FG) dimenhydrinate doses administered (p=0.0426), as well as during highly emetogenic chemotherapy alone, with 267 (CG) versus 80 (FG) administered doses (p<0.0001) (Figure 2A).
No significant difference could be detected when analyzing the data for the patients who received dimenhydrinate during one or more chemotherapy course of moderately and highly emetogenic chemotherapy (CG: 33 of 42 patients; 78.6% vs FG: 27 of 41 patients; 65.9%; p=0.2942), of moderately emetogenic chemotherapy alone (CG: 5 of 42 patients; 11.9% vs FG: 3 of 41 patients; 7.3%; p=0.7368), or of highly emetogenic chemotherapy alone (CG: 28 of 42 patients; 66.7% vs FG: 24 of 41 patients; 58.5%; p=0.5902) (Figure 2B). However, when analyzing the data with respect to the chemotherapy courses, dimenhydrinate was administered in significantly more (p<0.0001) chemotherapy courses in the control group (79 of 150; 52.7%), compared to the fosaprepitant group (45 of 153; 29.4%). The differences were not statistically significant (p=0.9606) during moderately emetogenic chemotherapy (emetogenic potential of 3), with 12 of 150 courses (8.0%) of the control group and 11 of 153 courses (7.2%) of the fosaprepitant group. However, for highly emetogenic chemotherapy (emetogenic potential of 4), dimenhydrinate was administered in significantly more (p<0.0001) chemotherapy courses in the control group (67 of 150; 44.7%), compared to the fosaprepitant group (34 of 153; 22.2%) (Figure 2B).
Safety And Tolerance
None of the patients died during the observation period and the discontinuation of antiemetic prophylaxis was not indicated for any of the patients. Changes in the analyzed laboratory parameters were not significantly different between the two cohorts (Table 4, Figure 3). Increases in ALT ≥1.5-fold of the upper normal value (39 U/L) were seen in 12.4% (n=19) of the FG patients’ chemotherapy courses and in 19.3% (n=29) of the CG patients’ courses. ALT increases ≥2.5-fold of the upper normal value were observed in 2.0% (n=3) of the FG’s courses and in 3.3% (n=5) of the CG’s courses. Increases in AST ≥1.5-fold of the upper normal limit (59 U/L) were seen in 3.3% (n=5) and 9.3% (n=14) of the FG and the CG courses, respectively. However, the mean and median AST and ALT levels did not increase significantly (p>0.05) beyond the upper normal limits.
 |
Table 4 Adverse Events |
It was possible to detect increases ≥1.5-fold of the upper normal limit of indirect bilirubin during 2.0% (n=3) of the CG’s chemotherapy courses. There was no significant difference between the two groups’ results (p=0.1201). Increases in direct bilirubin were significantly different (p=0.0106) between the two study groups, occurring in 1.3% (n=2) and 7.3% (n=11) of the FG and the CG courses, respectively. However, neither the CG nor the FG’s median and mean, direct and indirect, bilirubin values (baseline, maximum and end point values), increased beyond the normal values. Renal parameters did not relevantly change and only affected 4 of 153 courses (2.6%) in the FG, with increased creatinine ≥1.5-fold of the upper normal limit. No further increases in creatinine or urea, ≥1.5-fold or 2.5-fold of the upper normal value, could be detected in either study group. The differences were not statistically significant (p>0.5). The median and mean creatinine and urea values (baseline, maximum and end point values) of both the CG and the FG did not increase beyond the normal values.
No clinically relevant decreases in single values of potassium below 3.0 mmol/L, sodium below 130 mmol/L or calcium below 2.0 mmol/L, could be detected in either study group.
Similarly, clinical adverse events were low in both study groups and included headache (FG: n=2 vs CG: n=0), sweating (FG: n=3 vs CG: n=1), hiccups (FG: n=2 vs CG: n=0) and constipation (FG: n=4 vs CG: n=3). No neuropathy symptoms were observed in either group. The occurrence of adverse clinical events was not significantly different in the two study groups (p>0.05; Table 3).
Discussion
To the best of our knowledge, this is the first report on the use of an antiemetic prophylaxis regimen with intravenous fosaprepitant and granisetron, with or without dexamethasone, in pediatric patients receiving moderately or highly emetogenic chemotherapy.
Oral administration of drugs to pediatric patients is often difficult, eg, in younger children and infants or due to chemotherapy-related adverse effects, such as oral complications (eg, mucositis) or CINV.22–24 The availability of the intravenous form of aprepitant is therefore a suitable alternative for children who cannot or do not want to take oral aprepitant. Several controlled trials with adult patients have proven the superior efficacy and a favorable side effects profile of an antiemetic prophylaxis regimen with additional intravenous fosaprepitant, in comparison to a prophylaxis regimen with 5-HT3R antagonists and corticosteroids alone.12,13,25–29
The use of fosaprepitant in pediatric patients was initially reported in 2014 in a retrospective chart analysis: seven patients with a median age of 14 years (13–17 years) and emetogenic chemotherapy tolerated and responded well to intravenous fosaprepitant. No drug-related side effects were observed.30 A chart analysis of 35 pediatric patients (0.8–18 years of age) showed that an antiemetic CINV prophylaxis with the 5-HT3R antagonist ondansetron and fosaprepitant (4 mg/kg BW), led to a complete emesis control during the acute phase (89% of the patients), the delayed phase (63% of the patients) and both CINV phases (60% of the patients).17 A recently published randomized, double-blind placebo-controlled trial substantiated these findings: 163 pediatric patients between 1 and 12 years of age who were receiving moderately or highly emetogenic chemotherapy received a CINV prophylaxis regimen with ondansetron (before start of chemotherapy: intravenously, 1x0.15–0.3 mg/kg; max. 16 mg | after chemotherapy: orally, administered hourly; total dose 0.3 mg/kg per 8hrs), dexamethasone (before start of chemotherapy: intravenously, 1x0.075 mg/kg | after chemotherapy: orally, administered hourly; total dose 0.15 mg/kg per 8hrs) and a placebo (control group) or with ondansetron, dexamethasone and fosaprepitant (as a single dose prior to chemotherapy; 3 mg/kg bodyweight; therapy group). Complete control (absence of vomiting) was significantly higher (p<0.001) in the therapy group than the control group during the acute phase (86% vs 60%), the delayed phase (79% vs 51%) and both CINV phases (70% vs 41%).16
Likewise, the fosaprepitant-based regimen was seen to be similarly effective in this analysis, with 68% and 61% of the patients not experiencing vomiting in the acute and the delayed CINV phases, respectively, as opposed to 29% and 31% in the control group. Analyzing the chemotherapy courses in which no vomiting occurred, the fosaprepitant-based regimen was also superior, compared with a prophylaxis regimen with granisetron (and dexamethasone) alone, with 76% and 72% in the two CINV phases in the fosaprepitant group, compared with 55% and 53% in the control group, respectively. Additional fosaprepitant could achieve a complete absence of vomiting during moderately emetogenic chemotherapy, for almost all patients or chemotherapy courses during the acute CINV phase. The most striking result was the significant reduction of vomiting events during the antiemetic prophylaxis with fosaprepitant in the acute (6.2-fold reduction), and the delayed (3.6-fold reduction) CINV phases, compared to the control group. The ratios in the acute (0 vs 31 events), and the delayed (25.3-fold reduction), CINV phases were most pronounced during moderately emetogenic chemotherapy courses. Analyzing all moderately and highly emetogenic chemotherapy courses, the number of dimenhydrinate doses administered were 2.8-fold lower under fosaprepitant; during moderately emetogenic chemotherapy by 1.6-fold, and during highly emetogenic chemotherapy by 3.3-fold.
The fosaprepitant dose reported in this observational study (4 mg/kg BW) is higher than the current FDA-approved pediatric dose (3 mg/kg BW).14 However, there was no significant difference in potential regimen-related adverse events between the two study cohorts, which is in accordance with previously reported data.16,17 A simultaneous administration of ifosfamide and aprepitant or fosaprepitant, can potentially cause neurological side effects, such as seizures or neuropathy, as has been shown in several studies.31–34 In this study, fosaprepitant was administered during 25 of the 153 (22.3%) chemotherapy courses containing ifosfamide. Neurological adverse events were not observed during these courses. However, fosaprepitant was no longer administered during ifosfamide chemotherapy because of the experiences of, and recommendations from, other study sites and the later published data.32 New or different, potential, fosaprepitant-related adverse events, as described in previous studies, were not observed.15,16,28,35
Since this study was conducted in a non-randomized, observational setup, the conclusions that can be drawn are limited. Due to inconsistencies in the chart documentation and the difficulties with younger pediatric patients (under 4 years of age) reporting nausea, a valid assessment of nausea was not possible. Therefore, the efficacy analysis was based on the registered vomiting events only. Prospective randomized controlled trials are needed to evaluate these findings and to increase the evidence of the results.
Conclusion
Antiemetic prophylaxis with single-dose fosaprepitant (4.0 mg/kg) in addition to granisetron with or without dexamethasone was safe, effective and favorable tolerated in pediatric patients receiving moderately or highly emetogenic chemotherapy, compared to the standard regimen of granisetron (and dexamethasone) alone. In particular, the total number of vomiting events recorded and the doses of rescue medication (dimenhydrinate) administered during moderately and highly emetogenic chemotherapy were significantly reduced by additional, single-dose fosaprepitant, underlining the clinical benefit of this regimen for the patients and the medical staff treating them.
Abbreviations
5-HT3R, 5-hydroxytryptamine-3 receptor; ALL, acute lymphoblastic leukemia; ALT, alanine aminotransferase; AML, acute myeloid leukemia; ASCO, American Society for Clinical Oncology; AST, aspartate aminotransferase; BW, bodyweight; CG, control group; CINV, chemotherapy-induced nausea and vomiting; EP, emetogenic potential; FG, fosaprepitant group; IV, intravenous; kg, kilogram; L, liter; MASCC/ESMO, Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology; mg, milligram; µg, microgram; n, sample size; NK1, neurokinin-1; NK1R, neurokinin-1 receptor; p, p-value, probability value; U/L, units per liter; vs, versus.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Patel P, Robinson PD, Thackray J, et al. Guideline for the prevention of acute chemotherapy-induced nausea and vomiting in pediatric cancer patients: A focused update. Pediatr Blood Cancer. 2017;64:10. doi:10.1002/pbc.26542
2. Einhorn LH, Rapoport B, Navari RM, Herrstedt J, Brames MJ. 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following multiple-day chemotherapy, high-dose chemotherapy, and breakthrough nausea and vomiting. Support Care Cancer. 2017;25(1):303–308. doi:10.1007/s00520-016-3449-y
3. Frame DG. Best practice management of CINV in oncology patients: I. Physiology and treatment of CINV. Multiple neurotransmitters and receptors and the need for combination therapeutic approaches. J Support Oncol. 2010;8(2 Suppl 1):5–9.
4. Dupuis LL, Boodhan S, Sung L, et al. Guideline for the classification of the acute emetogenic potential of antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2011;57(2):191–198. doi:10.1002/pbc.23114
5. Dupuis LL, Boodhan S, Holdsworth M, et al. Guideline for the prevention of acute nausea and vomiting due to antineoplastic medication in pediatric cancer patients. Pediatr Blood Cancer. 2013;60(7):1073–1082. doi:10.1002/pbc.24508
6. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl_5):v119–v133. doi:10.1093/annonc/mdw270
7. Hsu ES. A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics. Am J Ther. 2010;17(5):476–486. doi:10.1097/MJT.0b013e3181ea7821
8. Phillips RS, Friend AJ, Gibson F, et al. Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood. Cochrane Database Sys Rev. 2016;2:Cd007786. doi:10.1002/14651858.CD004158.pub3
9. Blum RA, Majumdar A, McCrea J, et al. Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjects. Clin Ther. 2003;25(5):1407–1419.
10. Celio L, Ricchini F, De Braud F. Safety, efficacy, and patient acceptability of single-dose fosaprepitant regimen for the prevention of chemotherapy-induced nausea and vomiting. Patient Prefer Adherence. 2013;7:391–400. doi:10.2147/PPA.S31288
11. Saito Y, Kumamoto T, Arima T, et al. Evaluation of aprepitant and fosaprepitant in pediatric patients. Pediatr Int. 2019. doi:10.1111/ped.13780.
12. Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97(12):3090–3098. doi:10.1002/cncr.11433
13. Aapro M, Carides A, Rapoport BL, Schmoll HJ, Zhang L, Warr D. Aprepitant and fosaprepitant: a 10-year review of efficacy and safety. Oncologist. 2015;20(4):450–458. doi:10.1634/theoncologist.2014-0229
14. US Food and Drug Administration. EMEND - Fosaprepitant Dimeglumine. NDA 022023 - SUPPL-17. SUPPL-17 2018; FDA Approval. Available from: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM605696.pdf.
15. Okumura LM, D’Athayde Rodrigues F, Ferreira MAP, Moreira LB. Aprepitant in pediatric patients using moderate and highly emetogenic protocols: a systematic review and meta-analyses of randomized controlled trials. Br J Clin Pharmacol. 2017;83(5):1108–1117. doi:10.1111/bcp.13193
16. Radhakrishnan V, Joshi A, Ramamoorthy J, et al. Intravenous fosaprepitant for the prevention of chemotherapy-induced vomiting in children: A double-blind, placebo-controlled, phase III randomized trial. Pediatr Blood Cancer. 2018;e27551.
17. Timaeus S, Elder J, Franco K. Evaluation of the use of fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in pediatric patients. J Pediatr Hematol Oncol. 2018;40(7):527–531. doi:10.1097/MPH.0000000000001213
18. European Parliament and Council. Directive 2001/20/EC of the European Parliament and of the Councol of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medical Products for Human Use. In. Vol 2001/20/EC2001. European Parliament; 2001:34–44.
19. European Medicines Agency (EMA) - Committee for Medicinal Products for Human Use (CHMP). Assessment report Ivemend - Procedure No. EMEA/H/C/000743/II/0037. 2018; https://www.ema.europa.eu/documents/variation-report/ivemend-h-c-743-ii-0037-epar-assessment-report-variation_en.pdf.
20. NCCN Clinical Practice Guidelines in Oncology. NCCN Guidelines - Antiemesis. Version 2.2017. National Comprehensive Cancer Network; 2017.
21. U.S. NIH -NCI. Common Terminology Criteria for Adverse Events v4.03 (CTCAE) 2010. May 28, 2009, 2010.
22. Ethier MC, Regier DA, Tomlinson D, et al. Perspectives toward oral mucositis prevention from parents and health care professionals in pediatric cancer. Support Care Cancer. 2012;20(8):1771–1777. doi:10.1007/s00520-011-1274-x
23. Wong HM. Oral complications and management strategies for patients undergoing cancer therapy. TheScientificWorldJournal. 2014;2014:581795. doi:10.1155/2014/581795
24. Cheng KK, Goggins WB, Lee VW, Thompson DR. Risk factors for oral mucositis in children undergoing chemotherapy: a matched case-control study. Oral Oncol. 2008;44(11):1019–1025. doi:10.1016/j.oraloncology.2008.01.003
25. Gralla RJ, de Wit R, Herrstedt J, et al. Antiemetic efficacy of the neurokinin-1 antagonist, aprepitant, plus a 5HT3 antagonist and a corticosteroid in patients receiving anthracyclines or cyclophosphamide in addition to high-dose cisplatin: analysis of combined data from two Phase III randomized clinical trials. Cancer. 2005;104(4):864–868. doi:10.1002/cncr.21222
26. Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin–the aprepitant protocol 052 study group. J Clin Oncol. 2003;21(22):4112–4119. doi:10.1200/JCO.2003.01.095
27. Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer. 2010;18(4):423–431. doi:10.1007/s00520-009-0680-9
28. Saito H, Yoshizawa H, Yoshimori K, et al. Efficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Ann Oncol. 2013;24(4):1067–1073. doi:10.1093/annonc/mds541
29. Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23(12):2822–2830. doi:10.1200/JCO.2005.09.050
30. Shillingburg A, Biondo L. Aprepitant and fosaprepitant use in children and adolescents at an academic medical center. J Pediatr Pharmacol Ther. 2014;19(2):127–131. doi:10.5863/1551-6776-19.2.127
31. Aapro MS, Walko CM. Aprepitant: drug-drug interactions in perspective. Ann Oncol. 2010;21(12):2316–2323. doi:10.1093/annonc/mdq149
32. Patel P, Leeder JS, Piquette-Miller M, Dupuis LL. Aprepitant and fosaprepitant drug interactions: a systematic review. Br J Clin Pharmacol. 2017. doi:10.1111/bcp.13322
33. Kataria PS, Kendre PP, Patel AA. Ifosfamide-induced encephalopathy precipitated by aprepitant: a rarely manifested side effect of drug interaction. J Pharmacol Pharmacother. 2017;8(1):38–40. doi:10.4103/jpp.JPP_182_16
34. Dushenkov A, Kalabalik J, Carbone A, Jungsuwadee P. Drug interactions with aprepitant or fosaprepitant: review of literature and implications for clinical practice. J Oncol Pharm Pract. 2017;23(4):296–308. doi:10.1177/1078155216631408
35. Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol–EASE. J Clin Oncol. 2011;29(11):1495–1501. doi:10.1200/JCO.2010.31.7859
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.