Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Efficacy, Safety, and Drug–Drug Interactions for Insomnia Therapy in COVID-19 Patients
Authors Saputra BD, Levita J , Mustarichie R
Received 29 September 2021
Accepted for publication 23 December 2021
Published 21 January 2022 Volume 2022:15 Pages 137—152
DOI https://doi.org/10.2147/JMDH.S337053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Billy Dwi Saputra,1 Jutti Levita,2 Resmi Mustarichie3
1Undergraduate Program of Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia; 2Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia; 3Department of Pharmaceutical Analysis and Medicinal Chemistry, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia
Correspondence: Resmi Mustarichie
Department of Pharmaceutical Analysis and Medicinal Chemistry, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia
Tel +6222-84288888 Ext 3510
Email [email protected]
Abstract: Coronavirus disease-19 (COVID-19) is a systemic viral infection. COVID-19 patients show diverse clinical presentations ranging from asymptomatic, mild symptoms to severe symptoms characterized by severe respiratory distress. Sleep disorders or insomnia is one of the psychiatric problems that arise during the COVID-19 pandemic. The term used to define this particular insomnia is coronasomnia or COVID-19 insomnia. Data show that the prevalence of this problem is increasing, especially in the confirmed COVID-19 patient group. Anti-insomnia drugs such as hypnotics, sedatives, and anxiolytics are the easiest option. As with drugs generally, anti-insomnia drugs are associated with various safety issues, especially in people with COVID-19. Therefore, their use may be hazardous. The literature review aims to make health practitioners aware of the anti-insomnia drugs that have the best efficacy and safety issues that are clinically relevant from the use of anti-insomnia drugs and the interactions of anti-insomnia drugs with various drugs used in the treatment of COVID-19. The articles were explored on PubMed and Cochrane Library, whereas the drug–drug interactions between the anti-insomnia and COVID-19 drugs were searched on Drugs.com Interaction Checker and Lexiomp-interact. Overall anti-insomnia drugs have efficacy in improving sleep parameters. Orexin receptor antagonist drugs have good efficacy in increasing WASO, LPS, and SE with an acceptable safety profile. Meanwhile, the combination of zolpidem, lorazepam, and diphenhydramine improved TST parameters better than other drugs. Side effects such as drowsiness and dizziness were among the most commonly reported effects. Therefore, attention and monitoring of the use of anti-insomnia drugs in COVID-19 patients need to be carried out by considering the side effects and interactions that are very risky.
Keywords: insomnia, COVID-19, polysomnography, efficacy, safety, interaction
Introduction
The Coronavirus Disease (COVID-19) pandemic is a condition that requires rapid handling and adjustment, especially in the health sector. Among various health fields, psychiatry is a medical field that is also affected by this pandemic. COVID-19 is a systemic viral infection that attacks many organs and work processes of the body. The respiratory tract is the main organ that is affected and disturbed. COVID-19 patients show diverse clinical presentations ranging from asymptomatic, mild symptoms to severe symptoms characterized by severe respiratory distress. COVID-19 is associated with hypoxic respiratory distress and can rapidly progress to acute respiratory distress syndrome (ARDS).1 The risk of severity and death from this disease increases in people with old age and comorbidities.2
COVID-19 patients have the possibility of experiencing psychiatric symptoms or problems due to the impact of diagnosis communication, forced isolation, the medical symptoms caused, and the risk of death. In addition, medical care and medication also trigger psychiatric problems.
Sleep disorders or insomnia is one of the psychiatric problems that arise during COVID-19 pandemic. The term used to define this particular insomnia is Coronasomnia or COVID-19 insomnia.3 COVID-19 insomnia is manifested by lack of sleep at night, sleepiness during the day, and an increased need for naps, which are associated with physical and psychological problems that occur during COVID-19 infection. This sleep disorder occurs due to disrupted circadian rhythm during isolation and increased cytokines due to infection that interferes with both non-REM and REM sleep sleep.4,5 Data shows that the prevalence of sleep problems during the COVID-19 pandemic is increasing and affecting society globally, especially in the confirmed COVID-19 patient group, which is 74.8%, followed by an increase in the use of sleeping pills.6,7
Anti-insomnia drugs such as hypnotics, sedatives, and anxiolytics are the easiest option to treat insomnia. However, as with drugs generally, anti-insomnia drugs are associated with various safety issues, especially in people with COVID-19. Therefore, their use may be hazardous. In addition, interactions with current medical treatment for COVID-19 and some of its side effects may worsen the course and outcome of the patient’s medical condition. Therefore, this literature review aims to alert the health practitioners (including doctors, psychiatrists, and pharmacists) of the best efficacy and safety anti-insomnia drugs that are clinically relevant from the use of anti-insomnia drugs, as well as the interactions of anti-insomnia drugs with various drugs used in the treatment of COVID-19.
Materials and Methods
This review was based on the article published during 2011–2021, included publications from PubMed and Cochrane Library, and bibliography searches reviewed between March to April 2021 using the keywords: “insomnia”, “sleep”, “antipsychotic”, “hypnotic”, “sedative”, “psycholeptic”, “safety”, and “adverse.” The search was limited to publications in English and clinical trials of anti-insomnia drugs. Additional inclusion criteria included adult patients with insomnia or sleep disturbances and efficacy testing using the polysomnography method. The flowchart was used to identify and exclude manuscripts used in this review as depicted in Figure 1.
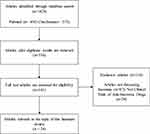 |
Figure 1 The Flowchart of Article Search. |
Information on drug–drug interactions between anti-insomnia and COVID-19 drugs search on the two databases Drugs.com Interaction Checker and Lexiomp-interact databases. In addition, drug–drug interactions were grouped into four groups based on the severity of high-risk (avoid), moderate, mild, and low.
Results
Of the 1029 articles identified, 24 studies (involving clinical studies with 5248 patients) were deemed to meet the eligibility criteria and included for analysis (Figure 1). There are 21 types of drugs involved, including benzodiazepines or BZDs (Lorazepam, Triazolam), “Z” drugs (Lorediplon, Eszopiclone, Zopiclone, Zolpidem, Sublingual Zolpidem), orexin receptor antagonists (ORA) (Almorexant, Seltorexant, Filorexant, Daridorexant, Lemborexant, Suvorexant), melatonin receptor agonists (Ramelteon and Melatonin), TCA antidepressants (Doxepin and Esmirtazapine) and anticonvulsants (Gabapentin and Pregabalin). The number of trials and sample sizes for each included drug detail are presented in Table 1.
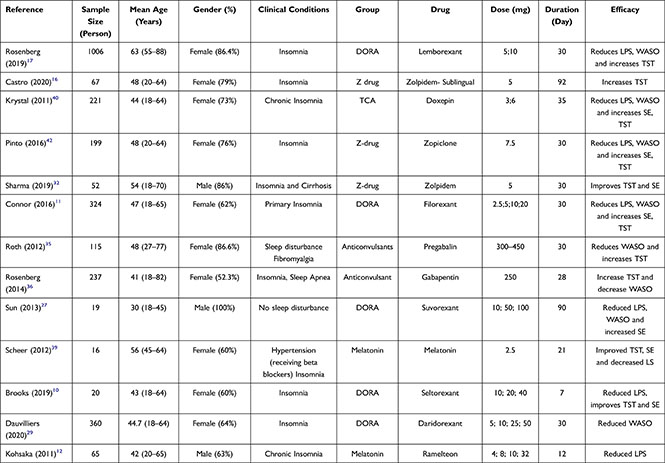 | 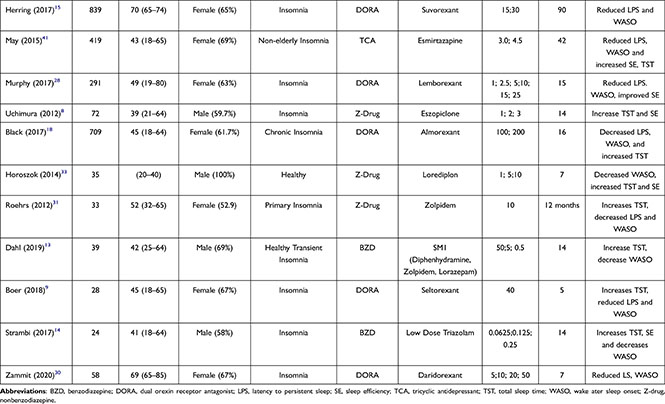 |
Table 1 Study Characteristics for Trials Comparing Anti-Insomnia Drug |
The articles used were published during 2011–2021. The mean age of the participants was 48 years, and most of the participants were females. A total of 5 studies included subjects with comorbidities (cirrhosis, fibromyalgia, sleep apnea, hypertension, depression), and 1 study examined elderly subjects. Most studies were conducted over 14 days.
Efficacy
All reviewed studies perform efficacy analyses. Efficacy parameters were obtained from the results of objective data using polysomnography. The data taken is data on changes in LPS (latency to persistent sleep), WASO (wakefulness after sleep onset), SE (sleep efficiency), and TST (total sleep time) values from the baseline Table 2. Overall efficacy of each drug decreased sleep-onset latency and the amount of time spent awake in bed after the first sleep attainment. In addition, the anti-insomnia drugs in the analyzed studies also improved sleep efficiency and total sleep time.
 |  |  |
Table 2 Efficacy of Changes in Sleep Parameters from Baseline |
The efficacy data used is the combined average of each drug based on the resulting parameters depicted in Figure 2A–D. It was found that eszopiclone decreased LPS the highest −54.9 minutes from baseline—followed by Seltorexant, which lowered LPS by −51.5 and Filorexant by −38.8 minutes. However, in lowering WASO, Seltorexant only decreased −11.3 minutes from baseline and became the second-lowest drug after Ramelteon which only decreased −3.8 minutes.8–12 The combination of Lorazepam, Diphenhydramine, and Zolpidem has the highest efficacy in reducing the largest WASO, which is −106.4 minutes from baseline. This drug also increased the TST time by 126.7 minutes from baseline and was the most significant change for TST of any other drug. For SE, the most significant increase was produced by the drug Filorexant, which increases sleep efficiency by 20.1%.11,13.
Triazolam showed lower efficacy in lowering LPS than Eszopiclone. The decrease in LPS with triazolam was only - 1.6 minutes from baseline. Suvorexant also showed low efficacy in increasing TST. TST results on Suvorexant only increased 15.5 minutes from baseline. Meanwhile, the lowest SE occurred in sublingual Zolpidem, which only increased sleep efficiency by 3% from baseline.14–16
Figure 2 Continue.
Safety
Safety studies were analyzed in the same study as efficacy. The most frequently reported side effects are non-serious, ranging from mild to moderate. However, several studies have reported severe but unrelated side effects.
Dizziness, excessive drowsiness, and somnolence were the most frequently reported side effects during the study. Almost all studies report this occurrence. Drugs from the Orexin Antagonist group reported the most events. Other side effects most commonly reported were falls, dizziness, diarrhea, and constipation.
Some studies report the occurrence of paralysis during sleep. For example, in therapy with Lemborexant 5 and 10 mg, mild sleep paralysis has been reported. However, there were no reports of deaths in this study,17 while another study of Lemborexant reported episodes of potential cataplexy lasting. 3 to 4 minutes after 14 hours of dosing and reported seizures after the second dose.28 Sleep paralysis also occurred in a study of Seltorexant receiving a dose of 40 mg at night, experiencing paralysis for 104 minutes.9
Studies on almorexant have reported brief episodes of hallucinations after the first 100 mg dose. Other side effects of almorexant have also been reported related to orthostatic hypotension with Seltorexant, but these events were more common in the comparison drug group.18 All studies indicated that the drug tested was well tolerated.
Drug–Drug Interaction
COVID-19 drugs included in the analysis are drugs commonly used in therapy. Among them, Remdesivir, Chloroquine, Hydroxychloroquine, Ivermectin, Lopinavir, Ritonavir, Azithromycin, Dexamethasone, Tocilizumab, Bamianvimab, Casirivimab, Colchicine, and Fluvoxamine.
The analysis shows that there are minor to high-risk interactions between anti-insomnia drugs and COVID-19 drugs. 37 interactions were obtained, with 14 being major, 21 moderate, and two minors. Fluvoxamine showed the most interactions with anti-insomnia drugs, 5 of which were high-risk.
The high-risk interactions that occur are generally associated with increased levels of anti-insomnia drugs in the blood, thereby increasing the risk of drug side effects. The mechanism of action of the drug is equally potent inhibition of CYP450 3A4 so that when given concurrently, it will increase the half-life of the drug. One of these interacting drugs is eszopiclone and ritonavir, which will increase the half-life, peak plasma concentrations, and exposure to Eszopiclone. The increased levels will cause an increased risk of psychomotor disorders.19 This increase in plasma drug concentration also occurs in Ramelteon which, when used with fluvoxamine, will increase CYP450 1A2 levels.20 Lemborexant given with fluvoxamine or antivirals such as Ritonavir and Lopinavir will increase blood levels of Lemborexant. It also increases the risk of developing neurological disorders such as depression, sleep paralysis, hallucinations, complex sleep behaviors, and headaches. Therefore, elevated levels of these drugs should be of concern when prescribing them concurrently.21
P-glycoprotein inhibitors given concurrently with colchicine will increase the concentration of colchicine. Suvorexant will interact with colchicine which increases the colchicine level with a mechanism of reducing colchicine excretion due to the inhibition of the P-glycoprotein transporter in the gastrointestinal tract and excretory tract. Increased risk of neuromyopathy, rhabdomyolysis, hepatotoxicity. In COVID-19 patients, of course, this will be very dangerous and will further aggravate the symptoms.22
Doxepin is a QT-prolonging agent that also interacts with other drugs in the same group. The use of doxepin with other QT-prolonging drugs could initiate additive effects and the risk of ventricular arrhythmias such as torsade de pointes. This interaction is certainly a risk if used for COVID-19 patients treated with hydroxychloroquine/chloroquine showing a much higher rate of QT prolongation.23
The interaction between anticonvulsants and SSRIs such as fluvoxamine will increase the risk of hyponatremia in the body caused by inappropriate hormone secretion. So the use of these two drugs should be avoided if they are used together.24 Interactions between drugs have been included in Table 3.
 |
Table 3 Drug–Drug Interaction |
Discussion
Insomnia is a disorder characterized by difficulty initiating or maintaining sleep for three or more nights per week for three months.25 During this pandemic, there has been an increase in the number of insomnias occurring. Changes in sleep parameters during this pandemic were shown by the value of sleep onset latency which increased to 30.1 minutes, decreased sleep efficiency to 85.7%, and total sleep time to 7.2 hours.7
Cognitive Behavioral therapy is the first line that can be chosen for this insomnia. However, pharmacological treatment is still preferred and used because, in some cases, cognitive behavioral therapy is not very effective and difficult to access.26 Moreover, during the COVID-19 pandemic, the use of sleeping pills has also increased, both prescription and over-the-counter, compared to before the pandemic.7 BZDs are the most common class for treating insomnia. Other groups with different mechanisms are Z drugs or nonbenzodiazepines, ORAs, melatonin agonists, TCAs, and anticonvulsants.
The use of anti-insomnia drugs in COVID-19 patients must pay attention to the severity of the symptoms displayed. Covid 19 shows some mild to severe symptoms related to severe respiratory distress. Age and comorbid diseases such as heart disease, diabetes, respiratory disorders need to be considered in the selection of insomnia therapy.
Overall, the efficacy of anti-insomnia in each study was to increase the polysomnographic parameters of TST and SE and decrease WASO and LPS values. Dosage determines the efficacy of each drug. In most of the drugs analyzed, the higher the dose, the higher the drug efficacy or dose-dependent.
The ORAs are the most widely reviewed study in this article. Orexin is a receptor that regulates the sleep arousal cycle that increases awareness. Suvorexant is a drug that blocks orexin receptors. This drug shows efficacy at doses of 15 and 30 mg, which can be used in elderly patients. However, side effects such as drowsiness and headaches in the moderate category after administration of this drug need to be considered in its use.15,27
The Lemborexant study revealed an increase in PSG-on sleep onset and sleep maintenance. There was a decrease in sleep onset by 20 minutes and an increase in sleep time by 60 minutes, but it had no significant effect on sleep efficiency. The advantage of this drug is that long-term administration does not reduce the effects of the drug.17
Compared with its comparison drug, Lemborexant showed effective results in the first and last week of testing. Lemborexant also shows the minimal effect on the residual sleepiness felt in the morning. Despite cataplexy lasting 3 to 4 minutes, Lemborexant is well tolerated and indicates an acceptable safety profile.28
Treatment with Filorexant has conveyed an increase in sleep efficiency and WASO (Figure 2). The efficacy of this drug can be long-term. The effective doses for Filorexant were 10 and 20 mg in increasing sleep onset as measured by LPS. However, the use of high doses is associated with an increase in the side effects of this drug.11
Almorexant showed good efficacy, but it was lower than Filorexant and Seltorexant. The study of almorexant showed no disturbance in body performance the next day, rebound insomnia, or the effect of stopping the drug was also not seen, so the use of this drug is more acceptable, especially in adults and the elderly.18
The Seltorexant study showed no residual effect the following day, which may be related to the rapid clearance of Seltorexant. However, this rapid clearance resulted in a much shorter duration of action of the drug, and the efficacy of the drug in maintaining sleep was also limited. The incidence of side effects of Seltorexant was higher than that of placebo but overall was mild-moderate. Sleep paralysis that occurs can be controlled immediately.9 This drug can also reduce the incidence of hyperarousal, theoretically related to blocking Orexin receptors in the hypothalamus, thereby inhibiting hypothalamic processes.10
Daridorexant is a new ORA. Daridorexant has the efficacy to increase TST, reduce WASO and LPS. However, compared to other orexin antagonists such as Filorexant and Seltorexant, this drug manifests a lower efficacy. Improved sleep maintenance was demonstrated at doses of 10 and 50 mg of Daridorexant. The drug is well tolerated in the dose range studied. Incidences such as narcolepsy associated with orexin deficiency are relatively low.29,30
A typical drug commonly used to treat sleep disorders and used as a comparison in each study is Zolpidem, a Z-drug. Zolpidem in a 12-month study and discontinuation of the drug showed no rebound insomnia, and clinically significant symptoms occurred after discontinuation of the drug.31 The use of Zolpidem in cirrhotic patients is also likely to be safe, a study conducted on liver cirrhosis patients with insomnia for four weeks 5 mg zolpidem daily in CTP class A or B cirrhotic patients significant improvement in TST and sleep efficiency and improvement of polysomnographic parameters of sleep initiation and maintenance without significant changes in sleep architecture.32
Sublingual Zolpidem has overall efficacy comparable to that of oral Zolpidem. The efficacy in terms of decreasing sleep onset and improving the sleep of both oral and sublingual Zolpidem was not higher than that of the other drugs. However, the sublingually formulated Zolpidem has the ability for faster absorption and distribution, thus achieving higher concentrations and inducing sleep more quickly.16 Lorediplon 10 mg progressively reduces WASO during the first three-quarters of the night. Lorediplon showed a dose-dependent increase in sleep, whereas Zolpidem showed a more sustained WASO effect. No next-day hangover effects were observed. This sleep effect is also consistent with the pharmacokinetic profile of lorediplon.33
The addition of total sleep time for 2 hours was obtained in the combination of SM-1 drugs. This increased sleep duration is mediated by the strong effect on sleep maintenance of the combined use of Zolpidem and lorazepam, which acts on sleep receptors, and diphenhydramine, which acts on the wake side. The combination of drugs also has no effect the next day, and the side effects are still acceptable.13
The use of benzodiazepine drugs in patients with COVID-19 must be considered. In patients with severe conditions and comorbid or elderly patients should be avoided. This is associated with an increased risk of severe respiratory distress. The risk may be higher for highly sedating agents, especially at higher doses.34
Anticonvulsants that also can increase sleep onset and maintenance are gabapentin and pregabalin. Gabapentin resulted in a greater perceived PSG after 5 hours of use on Day 1 and Day 28 with no reduction the following day and greater sleep duration during home use. In addition, pregabalin tested in Fibromyalgia patients showed an increase in sleep duration. Fibromyalgia patients who were characterized by increased pain, sleep disturbances, and daytime sleepiness showed improvement in sleep also reported a decrease after pregabalin therapy.35,36 COVID-19 patients who also have fibromyalgia have an increased likelihood of pain severity. The use of antidepressants or anticonvulsants is preferable to using steroids associated with immunity.37
In hypertensive patients treated with beta-blockers, 3- week nightly melatonin supplementation significantly improved sleep quality, with no apparent tolerance and no return of sleep disturbances during discontinuation of melatonin supplementation (in fact, a positive carryover effect was demonstrated). Melatonin supplementation for three weeks significantly increased total sleep time, improved sleep efficiency, and decreased sleep onset latency as assessed by polysomnography.
The use of melatonin in COVID-19 patients is recommended to be used as adjuvant therapy. In addition to having the effect of increasing sleep parameters. Melatonin has anti-inflammatory and immunomodulatory effects.38
There is no effect on the long-term use of melatonin; the side effects increase with increasing the dose.39 Another drug in the melatonin group is Ramelteon. The effect of Ramelteon in reducing sleep latency lasted up to 6 months, but the effect was less potent in total sleep time. These drugs promote sleep initiation without affecting other sleep parameters, possibly due to their circadian shift effects. As for the side effects of Ramelteon as a whole, there is nothing serious and related to therapy.12
Doxepin and Esmirtazapine are TCA drugs that are used to treat insomnia. Studies show that DXP 3 mg and 6 mg improve sleep maintenance, including in the last hours of the night, in adults with no residual effects the following day. Meanwhile, Esmirtazapine at doses of 3.0 and 4.5 mg was associated with consistent and sustained improvements in sleep in adults with primary insomnia. The effect of Esmirtazapine on sleep maintenance was also demonstrated by a significant reduction compared to placebo in WASO as measured by PSG. Esmirtazapine is associated with minimal residual daytime effects. However, there are reports of weight gain in this Esmirtazapine study.40,41
Zopiclone and Eszopiclone are cyclopyrrolone drugs whose mechanism of action differs from Zolpidem by acting on the a1 and a2 subunits of the GABA-A receptor. This drug has shown efficacy in treating early chronic insomnia or sleep maintenance and is well tolerated by the elderly. Eszopiclone is well tolerated at 1–3 mg doses, with the most commonly observed side effect being mild dysgeusia. The effects of eszopiclone 2 mg and 3 mg are comparable to the effects of Zolpidem 10 mg. Eszopiclone is an efficacious and generally well-tolerated treatment for sleep onset and sleep maintenance in the non-elderly patient population.8,42 The side effects of each drug are generally mild. Dizziness and excessive drowsiness become effects that often appear, and some are moderate. Drug interactions which are generally in the form of increasing drug levels in the body, must be a concern when prescribing or giving this drug to COVID-19 patients.
The use of anti-insomnia drugs in COVID-19 patients should be monitored thoroughly. The absence of research on the efficacy and safety of anti-insomnia drugs directly in COVID-19 patients is the limitation of this article. However, from the various clinical studies reviewed in this article, it can be considered the choice of therapy based on the results of existing studies regarding the efficacy and safety of each anti-insomnia drug.
Conclusion
The efficacy obtained from the use of anti-insomnia drugs, in general, is an increase in TST, SE, and a decrease in WASO and LPS based on testing using polysomnography. Orexin receptor antagonist drugs have good efficacy in increasing WASO, LPS, and SE with an acceptable safety profile. Meanwhile, the combination of Zolpidem, Lorazepam, and Diphenhydramine improved TST parameters better than other drugs. Melatonin can be chosen as an adjunct therapy in COVID-19 patients besides improving sleep parameters, it can be used for anti-inflammatory and immunomodulatory properties. Side effects such as drowsiness and dizziness were among the most commonly reported effects. Caution and monitoring are needed if anti- insomnia drugs are used together with COVID-19 drugs because there are several high-risk and dangerous interactions.
Acknowledgments
The authors thank the Rector of Universitas Padjadjaran for funding the publication fee via the Unpad Internal Academic-Leadership Grant of Prof. Resmi Mustarichie batch 2021 managed by the Directorate of Research and Community Engagement.
Disclosure
The authors declared no potential conflicts of interest to the research, authorship, or publication of this article.
References
1. Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Coronavirus disease (COVID-19): comprehensive review of clinical presentation. Front Public Heal. 2021;8. doi:10.3389/fpubh.2020.582932
2. Alonso-Lana S, Marquié M, Ruiz A, Boada M, Martínez-Pinilla E. Cognitive and Neuropsychiatric Manifestations of COVID-19 and Effects on Elderly Individuals With Dementia. Front Aging Neurosci. 2020;12:12. doi:10.3389/fnagi.2020.588872
3. Osterweil N. Coronasomnia: pervasive sleeplessness, self-medicating raise concerns of sleep experts. CHEST Physician; 2021.
4. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463(1):121–137. doi:10.1007/s00424-011-1044-0
5. Tahara Y, Shibata S. Entrainment of the mouse circadian clock: effects of stress, exercise, and nutrition. Free Radic Biol Med. 2018;119:129–138. doi:10.1016/j.freeradbiomed.2017.12.026
6. Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(2):299–313. doi:10.5664/jcsm.8930
7. Robillard R, Dion K, Pennestri M, et al. Profiles of sleep changes during the COVID‐19 pandemic: demographic, behavioural and psychological factors. J Sleep Res. 2021;30(1). doi:10.1111/jsr.13231
8. Uchimura N, Kamijo A, Kuwahara H, et al. A randomized placebo-controlled polysomnographic study of eszopiclone in Japanese patients with primary insomnia. Sleep Med. 2012;13(10):1247–1253. doi:10.1016/j.sleep.2012.08.015
9. De Boer P, Drevets WC, Rofael H, et al. A randomized Phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J Psychopharmacol. 2018;32(6):668–677. doi:10.1177/0269881118773745
10. Brooks S, Jacobs GE, de Boer P, et al. The selective orexin-2 receptor antagonist seltorexant improves sleep: an exploratory double-blind, placebo controlled, crossover study in antidepressant-treated major depressive disorder patients with persistent insomnia. J Psychopharmacol. 2019;33(2):202–209. doi:10.1177/0269881118822258
11. Connor KM, Mahoney E, Jackson S, et al. A Phase II Dose-Ranging Study Evaluating the Efficacy and Safety of the Orexin Receptor Antagonist Filorexant (MK-6096) in Patients with Primary Insomnia. Int J Neuropsychopharmacol. 2016;19(8):pyw022. doi:10.1093/ijnp/pyw022
12. Kohsaka M, Kanemura T, Taniguchi M, et al. Efficacy and tolerability of ramelteon in a double-blind, placebo-controlled, crossover study in Japanese patients with chronic primary insomnia. Expert Rev Neurother. 2011;11(10):1389–1397. doi:10.1586/ern.11.128
13. Dahl T, Chen LB, Zammit G, Ahmad M, Roth T. Efficacy of SM‐1 in a transient insomnia model. Hum Psychopharmacol Clin Exp. 2019;34(6). doi:10.1002/hup.2713
14. Ferini Strambi L, Marelli S, Zucconi M, Galbiati A, Biggio G. Effects of different doses of triazolam in the middle-of-the-night insomnia: a double-blind, randomized, parallel group study. J Neurol. 2017;264(7):1362–1369. doi:10.1007/s00415-017-8530-z
15. Herring WJ, Connor KM, Snyder E, et al. Suvorexant in Elderly Patients with Insomnia: pooled Analyses of Data from Phase III Randomized Controlled Clinical Trials. Am J Geriatr Psychiatry. 2017;25(7):791–802. doi:10.1016/j.jagp.2017.03.004
16. Castro LS, Otuyama LJ, Fumo-dos-Santos C, Tufik S, Poyares D. Sublingual and oral zolpidem for insomnia disorder: a 3-month randomized trial. Brazilian J Psychiatry. 2020;42(2):175–184. doi:10.1590/1516-4446-2019-0389
17. Rosenberg R, Murphy P, Zammit G, et al. Comparison of Lemborexant With Placebo and Zolpidem Tartrate Extended Release for the Treatment of Older Adults With Insomnia Disorder: a Phase 3 Randomized Clinical Trial. JAMA Netw open. 2019;2(12):e1918254. doi:10.1001/jamanetworkopen.2019.18254
18. Black J, Pillar G, Hedner J, et al. Efficacy and safety of almorexant in adult chronic insomnia: a randomized placebo-controlled trial with an active reference. Sleep Med. 2017;36:86–94. doi:10.1016/j.sleep.2017.05.009
19. Giri P, Naidu S, Patel N, Patel H, Srinivas NR. Evaluation of In Vitro Cytochrome P450 Inhibition and In Vitro Fate of Structurally Diverse N-Oxide Metabolites: case Studies with Clozapine, Levofloxacin, Roflumilast, Voriconazole and Zopiclone. Eur J Drug Metab Pharmacokinet. 2017;42(4):677–688. doi:10.1007/s13318-016-0385-7
20. Obach RS, Ryder TF. Metabolism of ramelteon in human liver microsomes and correlation with the effect of fluvoxamine on ramelteon pharmacokinetics. Drug Metab Dispos. 2010;38(8):1381–1391. doi:10.1124/dmd.110.034009
21. Landry I, Aluri J, Nakai K, et al. Evaluation of the CYP3A and CYP2B6 drug‐drug interaction potential of lemborexant. Clin Pharmacol Drug Dev. 2021;10(6):681–690. doi:10.1002/cpdd.915
22. Garrouste C, Philipponnet C, Kaysi S, Enache I, Tiple A, Heng AE. Severe colchicine intoxication in a renal transplant recipient on cyclosporine. Transplant Proc. 2012;44(9):2851–2852. doi:10.1016/j.transproceed.2012.09.028
23. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1036. doi:10.1001/jamacardio.2020.1834
24. Gandhi S, McArthur E, Mamdani MM, et al. Antiepileptic drugs and hyponatremia in older adults: two population-based cohort studies. Epilepsia. 2016;57(12):2067–2079. doi:10.1111/epi.13593
25. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Vol. 53.
26. Buenaver LF, Townsend D, Ong JC. Delivering Cognitive Behavioral Therapy for Insomnia in the Real World. Sleep Med Clin. 2019;14(2):275–281. doi:10.1016/j.jsmc.2019.01.008
27. Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36(2):259–267. doi:10.5665/sleep.2386
28. Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. doi:10.5664/jcsm.6800
29. Dauvilliers Y, Zammit G, Fietze I, et al. Daridorexant, a new dual orexin receptor antagonist to treat insomnia disorder. Ann Neurol. 2020;87(3):347–356. doi:10.1002/ana.25680
30. Zammit G, Dauvilliers Y, Pain S, Sebök Kinter D, Mansour Y, Kunz D. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94(21):e2222–e2232. doi:10.1212/WNL.0000000000009475
31. Roehrs TA, Randall S, Harris E, Maan R, Roth T. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;26(8):1088–1095. doi:10.1177/0269881111424455
32. Sharma MK, Kainth S, Kumar S, et al. Effects of zolpidem on sleep parameters in patients with cirrhosis and sleep disturbances: a randomized, placebo-controlled trial. Clin Mol Hepatol. 2019;25(2):199–209. doi:10.3350/cmh.2018.0084
33. Horoszok L, Baleeiro T, D’Aniello F, et al. A single-dose, randomized, double-blind, double dummy, placebo and positive-controlled, five-way cross-over study to assess the pharmacodynamic effects of lorediplon in a phase advance model of insomnia in healthy Caucasian adult male subjects. Hum Psychopharmacol Clin Exp. 2014;29(3):266–273. doi:10.1002/hup.2395
34. Kirmeier E, Eriksson LI, Lewald H, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7(2):129–140. doi:10.1016/S2213-2600(18)30294-7
35. Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res. 2012;64(4):597–606. doi:10.1002/acr.21595
36. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093–1100. doi:10.5664/jcsm.4108
37. Mohabbat AB, Mohabbat NML, Wight EC. Fibromyalgia and chronic fatigue syndrome in the age of COVID-19. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):764–766. doi:10.1016/j.mayocpiqo.2020.08.002
38. Ramos E, López-Muñoz F, Gil-Martín E, et al. The Coronavirus Disease 2019 (COVID-19): key Emphasis on Melatonin Safety and Therapeutic Efficacy. Antioxidants. 2021;10(7):1152. doi:10.3390/antiox10071152
39. Scheer FAJL, Morris CJ, Garcia JI, et al. Repeated melatonin supplementation improves sleep in hypertensive patients treated with beta-blockers: a randomized controlled trial. Sleep. 2012;35(10):1395–1402. doi:10.5665/sleep.2122
40. Krystal AD, Lankford A, Durrence HH, et al. Efficacy and safety of doxepin 3 and 6 mg in a 35-day sleep laboratory trial in adults with chronic primary insomnia. Sleep. 2011;34(10):1433–1442. doi:10.5665/SLEEP.1294
41. Ivgy-May N, Ruwe F, Krystal A, Roth T. Esmirtazapine in non-elderly adult patients with primary insomnia: efficacy and safety from a randomized, 6-week sleep laboratory trial. Sleep Med. 2015;16(7):838–844. doi:10.1016/j.sleep.2015.04.001
42. Pinto L, Bittencourt L, Treptow E, Braga L, Tufik S. Eszopiclone versus zopiclone in the treatment of insomnia. Clinics. 2016;71(1):5–9. doi:10.6061/clinics/2016(01)02
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

