Back to Journals » Drug Design, Development and Therapy » Volume 15
Efficacy, Pharmacokinetics, Biodistribution and Excretion of a Novel Acylated Long-Acting Insulin Analogue INS061 in Rats
Authors Pan K, Shi X, Liu K, Wang J, Chen Y
Received 6 May 2021
Accepted for publication 23 July 2021
Published 10 August 2021 Volume 2021:15 Pages 3487—3498
DOI https://doi.org/10.2147/DDDT.S317327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Kai Pan,1,2 Xiaolei Shi,2 Kai Liu,3 Ju Wang,2 Yijun Chen1
1State Key Laboratory of Natural Medicines and Laboratory of Chemical Biology, China Pharmaceutical University, Nanjing, Jiangsu Province, 211198, People’s Republic of China; 2Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, 222047, People’s Republic of China; 3Fujian Suncadia Medicine Co., Ltd, Xiamen, 361026, People’s Republic of China
Correspondence: Yijun Chen
State Key Laboratory of Natural Medicines and Laboratory of Chemical Biology, China Pharmaceutical University, 639 Longmian Avenue, Nanjing, Jiangsu Province, 210009, People’s Republic of China
Tel\Fax +86-25-86185919
Email [email protected]
Purpose: Long-acting insulin analogues are known to be a major player in the management of glucose levels in type I diabetic patients. However, highly frequent hypo- and hyperglycemic incidences of current long-acting insulins are the important factor to limit stable management of glucose level for clinical benefits. To further optimize the properties for steadily controlling glucose level, a novel long-acting insulin INS061 was designed and its efficacy, pharmacokinetics, biodistribution and excretion profiles were investigated in rats.
Methods: The glucose-lowering effects were evaluated in a streptozocin-induced diabetic rats compared to commercial insulins via subcutaneous administration. The pharmacokinetics, biodistribution, and excretion were examined by validated analytical methods including radioactivity assay and radioactivity assay after the precipitation with TCA and the separation by HPLC.
Results: INS061 exhibited favorable blood glucose lowering effects up to 24 h compared to Degludec. Pharmacokinetic study revealed that the concentration-time curves of INS061 between two administration routes were remarkably different. Following intravenous administration, INS061 was quickly distributed to various organs and tissues and slowly eliminated over time with urinary excretion being the major route for elimination, and the maximum plasma concentrations (Cmax) and systemic exposures (AUC) increased in a linear manner.
Conclusion: The present structural modifications of human insulin possessed a long-acting profile and glucose-lowering function along with favorable in vivo properties in rats, which establish a foundation for further preclinical and clinical evaluation.
Keywords: long-acting insulin, INS061, efficacy, pharmacokinetics, distribution, excretion
Introduction
As a metabolic disorder, Diabetes Mellitus (DM) is featured by unusually elevated level of blood glucose. According to the International Diabetes Federation’s statistics, the diabetes morbidity in people ranging from 20 to 79 years old has rocketed from 151 million in 2000 to 463 million in 2019; and the predicted number in 2030 will be 578 million, and in 2045, it will reach 700 million.1
In patients with DM, years of poorly controlled hyperglycemia can lead to several clinical complications, such as cardiovascular diseases,2 retinopathy,3 nephropathy,4 neuropathy,5 food disorders6 and dental diseases.7 These complications can cause the reduction of the quality of life and even disability. Furthermore, the patients with type 1 DM may shorten their life expectancy by 16 to 20 years, whereas those with type 2 DM suffer a loss of 4 to 6 years.8–10
To effectively manage diabetes, stable glycemic control is essential for avoiding or putting off the initiation and development of diabetes-related complications in patients. Currently, various drugs have been developed to treat type 2 DM, including PPAR agonists, aldose reductase inhibitors, PTP1B inhibitors, α-glucosidase inhibitors, GPCR agonists, DPP-4 inhibitors and SGLT inhibitors.11 However, the intravenous or subcutaneous administration of human insulin or its analogues is still the most effective and irreplaceable choice for the treatment of type 1 DM.
Due mainly to the stability issue, human insulin exhibits short half-life in patients, resulting in repeated dosing and treatment inconvenience. Thus, various approaches have taken to protect insulin from the degradation in the circulation. Among these efforts, the specific acylation of Lys residue at B29 with hexadecandioic acid has been demonstrated to effectively prolong the half-life of insulin for long-acting effects. Even though several long-acting insulin analogues with improved pharmacodynamic and pharmacokinetic profiles through subcutaneous administration have been approved by FDA for clinical uses in recent years, such as Glargine, Detemir and Degludec, these products still possess a high frequency of hypo- and hyperglycemic incidences and modest reduction in HbA1c in the clinics.12,13 Consequently, there remains an urgent demand for developing new human insulin analogue to better control the glucose level as well as to minimize the danger of hypoglycemia for type 1 diabetic therapy.14,15
Previous structure-function studies of human insulin have revealed that the sequence of B26-30 region in insulin is not crucial for its binding with insulin receptor for its glucose lowering activity. On the other hand, B26-30 region was found to be important to mediate the self-association to form hexamers for prolonging the time of duration.16,17 Therefore, we speculated that the change of amino acid sequence in this region in combination with the site-specific acylation may further improve the properties of insulin analogues for desired clinical benefits. Subsequently, we designed a series of new insulin analogues with various substitutions of B26-30 region.18 After the screening of receptor binding affinity compared to human insulin, a novel acylated insulin analogue (designated INS061) was identified, in which two acidic residues AspB28 and GluB30 were substituted to Pro and Thr respectively in addition to the acylation of LysB29 with a hexadecandioic acid (Figure 1).
 |
Figure 1 Structure of INS061. |
In the present study, the blood glucose lowering effects of INS061 were evaluated compared to vehicle, recombinant human insulin and Degludec in STZ-induced diabetic rats.19 Moreover, various methods, radioactivity assay (RA), radioactivity assay after the precipitation by trichloroacetic acid (TCA-RA), and radioactivity assay after HPLC separation (HPLC-RA), were employed to study the pharmacokinetics, biodistribution, and excretion of INS061 following a single-dose subcutaneous or intravenous administration. Collectively, the present data provide a solid basis for further development of INS061 as a potential therapeutic agent for the treatment of type 1 DM.
Materials and Methods
Drugs and Reagents
INS061 (100IU/mL) was prepared by Jiangsu Hengrui medicine Co., Ltd. Na125I (99% radiochemical purity, specific activity 638 GBq/mg Iodide) was purchased from Amersham Pharmacia Biotech. The insulin receptor Ab70687 was obtained from Abcam Ltd. (Lot No. w3292-1229). Biacore T200 CM5 sensor chip (Serial No.10077015) was from GE Healthcare. Recombinant Human Insulin Injection (Humulin R, 3mL:300IU) was purchased from Lilly France. Degludec Injection (Tresiba, 3mL:300IU) was purchased from Novo Nordisk A/S Denmark. Other reagents were all analytical or chromatographic grade.
Animals
Since the animal facilities of both the university and the company were not available during the research period, we rented the animal facility of the Academy of Military Medical Sciences for the experiments with the institutional approval on animal ethics and welfares.
Wistar rats were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences, Beijing, China (license No.: SCXK-(Jun) 07–004). All animal experiments were carried out in strict accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Ethics Committee of the Academy of Military Medical Sciences, Beijing, China, according to the Laboratory animals-guidelines for ethical review of welfare (GB/T 35892–2018). All animals were housed in a controlled environment (temperature: 22 ± 3°C, relative humidity: 40–60%, light-dark cycle: 12 h) and raised with a normal diet with distilled water randomly throughout the experiments.
Effects on Blood Glucose-Lowering
The STZ-induced diabetic rat model was applied to evaluate the glucose lowering effects. STZ was prepared in 20 mM solution of sodium citrate buffer at pH 4.5 and injected to male rats at 100 mg/kg via tail vein. Blood glucose levels were determined after approximately 3 days. Rats with blood glucose levels in the range of 360−650 mg/dL were used for subsequent experiments. The blood glucose lowering effects of INS061 were evaluated in the animal model compared to vehicle (0.9% saline), recombinant human insulin (Humulin R) and Degludec (Tresiba) at an equal dose of 100 nmol/kg. Blood glucose levels of the rats were measured with a glucometer before the administration and up to 24 h after the administration. Total area under the curve (AUC) for glucose were calculated using trapezoidal rule to further calculate the efficacy.
Preparation and Characterization of 125I-INS061
INS061 was labeled with 125iodine by the Iodogen method.20 In the labelling experiment, 100 μL of INS061 (100IU/mL) and 10 μL of Na125I in phosphate-buffered saline (740 MBq/mL) were sequentially added to a reaction tube coated with Iodogen inside and gently mixed. Then, the tube was sealed, and the labeling reaction was allowed to proceed for 20 min at room temperature. After the reaction was completed, the labeled INS061 was purified by a SephadexTM G-50 gel filtration column. The eluted fractions were collected to determine γ-radioactivity (WALLAC 2470, PerkinElmer) and protein concentration with bicinchoninic acid method.21
The binding kinetics of INS061 to the receptor Ab70687 before and after labeling were analyzed by SPR method.22 Briefly, the experiments were performed using a Biacore™ T200 instrument and CM5 biosensor chips (GE Healthcare, Sweden), and the binding affinity constants were calculated accordingly.23
The specific activity of 125I-INS061 was 71.0 kBq/g with 125I-INS061 protein concentration of 787.6 μg/mL. The binding affinity constant (KD) values of INS061 to its receptor before and after 125I-labeling were 4.031×10−5 M and 4.495×10−5 M, respectively.
Preparation of Standard Solutions and QC Samples in Rat Plasma, Tissues and Excretion
Working solution at a concentration of 100μg/mL (2 MBq/mL) was prepared by adding appropriate amount of 125I-labelled INS061 into INS061 injection for RA measurement. Standard samples were obtained by further dilution of the working solution with the homogenate of the blank rat biological matrix to yield concentrations of 0.5, 2.0, 5.0, 20, 50, 200 and 500 ng/mL for pharmacokinetic, tissue distribution and excretion study. Quality control (QC) samples were prepared following the same processing procedure listed above at the concentrations of 0.5, 1.5, 150 and 400 ng/mL.
The total radioactivities of standard samples or QC samples (100 μL) were measured by a γ-counter in duplicates. Similarly, for TCA-RA measurement, each standard sample or QC sample (100 μL) was vortex-mixed with 100 µL of TCA solution (20%, v/v), and then centrifuged at 3500 rpm for 20 min at 4°C. The precipitants were measured for the radioactivities after supernatants were removed.
Calibration standards for separation by HPLC were prepared by dilution of 100 µg/mL (16 MBq/mL) working solutions to concentrations of 0.5, 2.0, 5.0, 20, 50, 200 and 500 ng/mL. Twenty microliters of the sample was loaded onto a TSK Gel 2000 SWXL column using 0.02M PBS (pH 6.7) as a mobile phase at a flow of 1.0 mL/min. Eluents were collected at 30s intervals (approximately 0.5 mL/sample) and the associated radioactivity were determined by a γ-counter.
Validation of Bioanalytical Method
Linearity
The linearity was tested by analyzing the calibration curve in duplicate on three consecutive days. The calibration curves were constructed by plotting the net CPM versus the spiked concentrations, after which the regression equations were calculated by weighted (1/x2) least squares linear regression. All standard concentrations were within 15% deviation from nominal value (±20% for LLOQ).
Accuracy and Precision
Inter- and intra-assay precision and accuracy for both RA and TCA-RA assay was determined by analyzing 5 replicates at four different QC levels (0.5, 1.5, 150 and 400 ng/mL) on 3 consecutive days. The criteria for acceptability of the data included accuracy within ±15% deviation (SD) from nominal values and a precision of within ±15% relative standard deviation (RSD), except for LLOQ that should not exceed ±20% of standard deviation.
Recovery
The recovery of radioactivity for both RA and TCA-RA assay was determined by measuring 5 replicates of QC samples at 0.5, 1.5, 150 and 400 ng/mL, respectively.
Pharmacokinetic Study
The rats were randomly divided into four groups, and 6 rats (3 per gender) each for subcutaneous or intravenous administration to investigate the pharmacokinetics of INS061. Rats of the 3 groups individually received a single subcutaneous injection of INS061 at 0.75 IU/kg (33.3μg/kg), 1.5 IU/kg (66.7μg/kg) and 3 IU/kg (133.3μg/kg), respectively. Another group of rats received intravenous injection at a dose of 1.5 U/kg (66.7 μg/kg) via the tail vein. The dosing volume for all animals was 1.0 mL/kg and contained 125I-labelled INS061 (2MBq/mL). After administration, the vein was flushed with heparinized saline. Then, 200 μL blood sample was collected from the tail vein of the same animal at 0 (pre-dosing), 0.083, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, 16 and 24 h into heparinized plastic tubes. The time points for the groups of subcutaneous injection were 0 (pre-dosing), 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, 16 and 24 h. Blood collection tubes were immediately centrifuged at 4°C for 10 min at 3500 rpm. The plasma samples were stored at −20°C prior to measurement. The radioactivity of the plasma (100 μL) was measured for RA assay by a γ-counter. Subsequently, 100 μL of 20% TCA was added into the sample and mixed gently. The mixture was centrifuged at 3500 rpm for 20 min. The resulting precipitate was analyzed by TCA-RA method.
Similarly, for HPLC-RA method, 6 SD rats with equal number of gender received a single subcutaneous injection at 1.5 IU/kg (66.7 μg/kg). The dosing volume for all animals was 1.0 mL/kg and contained 125I-labelled INS061 (16 MBq/mL). Blood samples (200 μL) were harvested into heparinized tubes at 0 (pre-dosing), 0.083, 0.25, 0.5, 1, 2, 3, 4, 6, 8, 12, 16 and 24 h via tail vein of the rats. After centrifuged at 3500 rpm for 10 min, 20 μL of plasma samples were loaded and separated by TSK gel G2000SWXL column, the radioactivity content in eluent was measured by gamma counting.
Biodistribution
SD rats were randomly divided into 4 groups with 6 animals per group (3 per gender). The rats were euthanized at 0.5, 2, 8, and 24 h after subcutaneous injection at dose of 1.5 U/kg (66.7 μg/kg). The dosing volume for all animals were 1.0 mL/kg and contained 125I-labelled INS061 (2 MBq/mL). Each tissue from lung, heart, liver, kidney, spleen, gonad, stomach, intestine, muscle, adipose, and brain was isolated and thoroughly rinsed with 0.9% saline, and then blotted to dry and subjected to dissection. The organ/tissue slices were scaled, recorded, and then subjected to homogenization with physiological saline at the rate of 1:4. The total radioactivity of each homogenate (400 μL) was directly determined by gamma counting (RA method). Each aliquot of tissue homogenate (400 μL) was then mixed with equal volume of 20% TCA and centrifuged at 3500 rpm at 4°C for 20 min. After centrifugation, the resulting precipitants were measured for its radioactivity (TCA-RA).
Excretion Study
Bile Excretion
The bile duct-cannulation method was used to treat six rats (3 per gender), which were anesthetized with 2% pentobarbital. The rats were administered with 125I-INS061 (1.5 IU/kg) by subcutaneous injection. The dosing volume for all animals were 1.0 mL/kg and contained 125I-labelled INS061 (2 MBq/mL). The collection time of bile samples were set at fixed time intervals from 0 to 1, 1 to 2, 2 to 4, 4 to 6, 6 to 8, 8 to 12, and 12 to 24 h. The volume of each collected sample was recorded and the total radioactivity was calculated.
Urine and Fecal Excretion
After subcutaneous injection of 125I-INS061 (1.5 IU/kg), 6 rats (3 per gender) were placed in a metabolic cage immediately. Then, the urine and fecal samples were gathered respectively and quantitated at time intervals of 0–4, 4–8, 8–12, 12–24, 24–48, 48–72, 72–96 and 96–120 h. The urine sample volumes were measured. The dried fecal samples were weighed, and then subjected to homogenization with 10-fold volumes of 0.9% saline for the detection of the radioactivity.
Statistical Analysis
In this study, DAS 2.1.1 was used for the analysis of pharmacokinetic parameters. All data are presented as mean ± standard deviation (SD). Concentration values below LLOQ from bioanalysis were not included. The absolute bioavailability was calculated by the ratio of AUC0–t of subcutaneous to intravenous administration. All data points are presented by mean ± SD values.
The differences in numeric variables between groups were examined with a two-side t test. P-value <0.05 indicated statistically significant difference.
Results
Blood Glucose-Lowering Efficacy in STZ-Induced Diabetic Rats
Blood glucose lowering profiles of insulin and the analogues in STZ-induced diabetic rats are shown in Figure 2. INS061 and the commercial products markedly exhibited glucose lowering effects compared to vehicle (p<0.001). As shown in Figure 2A, Recombinant Human Insulin (Humulin R) showed significant lowering of blood glucose at 2 h (p<0.001) after the administration, but its effects diminished at 6 h (p>0.05), indicating its nature of short-acting. Both INS061 and Degludec significantly decreased blood glucose levels for a longer period of time (from 2 to 16 h after the administration) with a peak effect at 4–8 h (P<0.05). Glucose levels in the Degludec group returned to baseline at 24 h, and a relatively lower average glucose level was found in IN061 group at the end of the experiment. As shown in Table 1, when AUC for glucose between Degludec and INS061 at same dose were compared in different time periods, overall exposures were similar. However, when AUC0-24 for glucose was compared, INS061 showed better exposures than Degludec with statistical significance (P<0.05).
 |
Table 1 AUC for Glucose at Different Time Periods After Subcutaneous Dosing of Insulins at a Dose of 100 Nmol/Kg in STZ-Induced Diabetic Rats (n=6) |
Validation of Bioanalytical Method
Linearity
The calibration curves showed a high level of linearity in the range of 0.5–500 ng/mL to analyte for rat plasma, organ/tissue (lung, heart, liver, kidney, spleen, gonad, stomach, intestine, muscle, adipose, and brain) and excretion (bile, urine and feces) with different pre-treatment methods, respectively. The typical plasma equation of standard curves was y = 79.06 x −337.6 (r=0.9996, RA). Deviations were all within ±15% for all regression equations.
Precision and Accuracy
The intra- and inter-day precision and accuracy of RA and TCA-RA were evaluated by analyzing QC samples at different concentrations in six replicates on the same day and on three different days, respectively. The intra- and inter-day accuracy was from 96.04% to 104.96% and coefficients of variation (CV) values for all the analytes were lower than 15%, indicating that all values were within the acceptable range and the method was confirmed to be accurate and precise.
Recovery
The recovery rates of the radioactivity at four concentration levels were calculated by analyzing five replicates of rat plasma with RA and TCA-RA, respectively. The mean recoveries of the radioactivity with different pretreatment methods from rat plasma were all above 88.2% and concentration independent. The data showed that the methods were suitable for the pharmacokinetic analysis.
Pharmacokinetic Study in Rats
The mean plasma concentration-time profiles of INS061 in rats after a single subcutaneous (0.75, 1.5, 3.0 IU/kg) and intravenous (1.5 IU/kg) dose for RA and TCA-RA determinations were illustrated in Figure 3. The non-compartment model was used to analyze pharmacokinetic parameters and the main results are summarized in Tables 2 and 3.
 |
Table 2 The Mean Non-Compartmental Pharmacokinetic Parameters of 125I-INS061 After a Single Intravenous or Subcutaneous Injection in Rats by RA Method (n=6) |
 |
Table 3 The Mean Non-Compartmental Pharmacokinetic Parameters of 125I-INS061 After a Single Intravenous or Subcutaneous Injection in Rats by TCA-RA Method (n=6) |
Following three single subcutaneous dosing, plasma concentration profiles showed a quick absorption phase with mean tmax of 2.33–3.50 h (RA) and 2.17–2.50 h (TCA-RA). The calculated half-life (T1/2) was about 6.06–6.53 h (RA) and 4.81–5.61 h (TCA-RA), which represented a slow elimination of the drug. As the dose was increased from 0.75 to 3.0 IU/kg for subcutaneous administration, the values of Cmax and AUC increased in a dose-proportional manner with both RA method and TCA-RA method.
Following a single intravenous injection at 1.5 IU/kg dose, INS061 was rapidly distributed and then eliminated from rat plasma with t1/2 of 2.54 ± 0.422 h (RA) and 2.40 ± 0.613 h (TCA-RA). The absolute bioavailability for subcutaneous administration at 1.5 IU/kg dose was estimated to be 73.94% (RA) and 70.04% (TCA-RA).
The comparison of plasma concentration-time profiles and the derived pharmacokinetic parameters after subcutaneous administration in rats at 1.5 IU/kg dose by RA, TCA-RA and HPLC-RA methods are shown in Table 4. The values of Cmax and AUC generated by RA method were greater than that of TCA-RA method. However, the pharmacokinetic parameters determined by TCA-RA and HPLC-RA were comparable.
 |
Table 4 Comparison of Main Pharmacokinetic Parameters Determined by RA, TCA-RA and HPLC-RA Methods After a Single Subcutaneous Injection of 125I-INS061 at a Dose of 1.5 IU/Kg in Rats (n=6) |
Tissue Distribution After Subcutaneous Administration in Rats
As shown in Figure 4 and Table 5, the distributions of 125I-INS061 in different tissues at 0.5, 2, 8, and 24 h after subcutaneous injection at 1.5 IU/kg dose in rats were measured by RA and TCA-RA methods, and consequent accumulative amount [AUC (0–24 h)] of INS061 was calculated. The accumulative amount of INS061 [AUC (0–24h)] followed the order of kidney, serum, liver, lung, muscle, spleen, heart, intestine, stomach, adipose, and brain by RA method. Meanwhile, the descending order of AUC values by TCA-RA method was found to be kidney, serum, liver, lung, spleen, intestine, heart, muscle, stomach, adipose, and brain. INS061 was extensively exhibited in the urinary system, serum and fully perfused tissues, whereas the corresponding levels in adipose and brain were low.
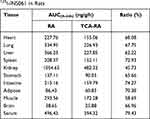 |
Table 5 Comparison of the Tissue Distribution Determined by RA and TCA-RA Methods After Subcutaneous Injection of 125I-INS061 in Rats |
The ratio of the AUC values of TCA precipitants to that of total tissues could reflect the amount of INS061 bound with various tissues. The ratio of AUC value in serum reached to 79.43%, indicating that 125I-INS061 is relatively stable in serum. However, the ratio in kidney was as low as 45.73%, suggesting that the majority of the radioactivity in kidney might be from the metabolic fragments.
Excretion Study in Rats
Figure 5 shows the excretion results after a single dose of 1.5 IU/kg subcutaneous injection from complete and bile duct cannulated rats. Urinary excretion was the major route for elimination after the administration, because 70.9±11.6% and 4.8±1.0% of the administered dose was excreted in urine and feces over 120 h.
 |
Figure 5 Cumulative excretion after subcutaneous administration of 125I-INS061 in rats. (A) urine and feces. (B) bile. |
When INS061 was injected in bile-duct cannulated rats, 1.40±0.37% of the injected radioactivity was egested to bile at 24 h post-dosing, which surpassed the corresponding amount in feces. Consequently, fecal excretion of the radioactivity was probably not owing to undigested drug but biliary excretion.
Discussion
The aim of developing long-acting insulin analogues is to mimic the endogenous action of insulin. Currently, Degludec is considered as the best medication because of its ultra-longer duration of action than earlier insulin analogues, such as Glargine and Detemir. However, the frequent cause of nocturnal hypoglycemia has limited its clinical application.24,25 Although the newer long-acting insulin analogue Peglispro developed by Eli Lilly was terminated due to its liver toxicity in 2015,26 the discovery and development of better insulin analogues have still drawn a great attention and interest.
Structure-function studies on insulin analogues have revealed that amino acid residues in B26–30 region are not particularly crucial for the binding to insulin receptor. However, this region is extremely important to mediate the formation of insulin hexamer. Based on such information, structural modifications of insulin in this region would be expected to change in vivo duration with minimal alteration of receptor affinity.16,17,27,28 Therefore, we focused on B26-30 region of human insulin for rational design and substitutions of amino acid residues aiming for novel analogues with desired properties. After screening the affinity to insulin receptor, INS601, in which two acidic residues were substituted with neutral amino acids, was identified as the best candidate for further investigation.
To gain a better understanding of the therapeutic potential of INS061, we assessed its blood glucose-lowering efficacy, pharmacokinetics, tissue distribution and excretion in rats. As a result, INS601 indeed showed favorable effects and properties compared to Degludec in the present study.
In preclinical stage, favorable DMPK behaviors are the prerequisite for a drug candidate. Unlike small molecules, macromolecules are difficult to trace and quantitate with the methods of conventional mass spectrometry. In the case of insulin and its analogues, radioactive tracer techniques using 125Iodine isotope have been widely applied to the investigation and evaluation of pharmacokinetics, tissue distribution and excretion of macromolecules.29–32 Given the fact that free iodine or smaller protein fragments labeled with 125I could be released from the influence of metabolic system in RA method,33 TCA precipitation has been introduced to couple with radiolabeling (TCA-RA) for reliable quantification of protein drugs. Since peptide fragments are generally not precipitated by TCA, the measurement of the radioactivity of TCA-precipitated part could objectively reflect the content of prototype drug. Apart from the RA and TCA-RA methods, a set of HPLC-RA experiment was performed to further confirm the pharmacokinetic behaviors of the parent drug in this study. The mean plasma concentration-time profiles generated by HPLC-RA method were very close to the results from TCA-RA method. This indicated that INS061 was stable in plasma and the results generated by TCA-RA method were reliable.
Following subcutaneous administration at 3 different doses to rats, the pharmacokinetic behaviors of INS061 were exhibited in a dose-dependent manner. The t1/2 generated by TCA-RA method was within 5.40–6.43 h, which is longer than that of Degludec as reported to be 2.9 h and 2.3 h in rats.34,35 The results also indicated that INS061 should possess a longer in vivo duration compared to Degludec, which is a strong evidence for its long-acting effects. In addition, the long-acting efficacy of INS601 on glucose lowering in STZ-induced diabetic rats further supported its prolonged duration in vivo. Since Degludec has been used in diabetic patients and has shown good long-acting characteristics, we speculated that INS061 would have equivalent or better long-acting effects in humans.
The tissue distribution of INS601 was studied after a single subcutaneous administration in rats, which showed that INS061 distributed rapidly and widely in tissues within the experimental time course. The highest radioactivity level was detected around 2 h in most of the tissues after administration. Then, the amount of radioactivities in most tissues decreased gradually with the same elimination trend in plasma. The lowest radioactivity found in brain is a general phenomenon because insulin-like molecules are known to be difficult for the penetration of blood-brain-barrier.36,37
It is well known that two major clearance organs for insulin are liver and kidney. Endogenous insulin secreted by the pancreatic β-cells arrives in liver through portal vein circulation and is mainly metabolized by liver. However, when insulin or its analogue is exogenously administered, especially subcutaneous administration, the degradation profile is altered since insulin is no longer directly delivered to portal vein. Consequently, kidney becomes the major clearance organ.
While RA method is limited to determine total radioactivity, the most significant advantage for TCA-RA method is to detect the precipitated fractions for the elimination of the interference by free iodine and decomposed or metabolized fragments with small molecular weights. As depicted in Figure 4, the radioactivity found in kidney was much more than that in liver determined by both RA and TCA-RA methods at each time point, indicating that the amount of either parent drug or metabolites in kidney is greater than in liver. Moreover, based on the close correlation between TCA-RA and HPLC-RA, it is reasonable to conclude that kidney is the major clearance organ for INS061, which is similar to those reported in the literatures for other exogenous insulins.38–40
Given the species differences, we cannot simply infer the pharmacokinetic profiles in humans from that of rats at this point. Whether INS061 is superior to Degludec, in terms of efficacy and the duration of action in the human body, requires future clinical investigation. However, according to the comparison of PK behaviors of Degludec in rats and humans reported in the literatures,34,35,41 we are confident that INS061 is a potential effective and long-acting insulin analogue.
Based on the unmet medical need, a novel long-acting insulin analogue should exhibit improved and desired efficacy, pharmacokinetic behaviors and safety.25 Although the safety profiles of INS601 are absent in the present study, the safety evaluation of this novel long-acting insulin analogue is currently under investigation. Nevertheless, according to the efficacy, pharmacokinetics, tissue distribution and excretion profiles of INS601 in rats, this long-acting insulin analogue exhibits more favorable properties as newer generation of therapeutic agent for the treatment of type I diabetes.
Conclusion
We have designed a novel long-acting insulin analogue INS601 based on amino acid substitution and receptor binding affinity. The glucose-lowering effects of INS601 was confirmed to be better than Degludec in diabetic rats. By employing 125I-labeling coupled with TCA precipitation, the pharmacokinetic parameters, the data on tissue distribution and excretion of INS601 were obtained, which showed favorable preclinical characteristics as a novel long-acting insulin analogue. The present study provides a solid basis for the druggability of INS601, and also warrants further preclinical and clinical development of INS601 as a new therapeutic option for diabetic patients.
Acknowledgment
We would like to thank the Academy of Military Medical Sciences, Beijing, for the assistance on animal experiments.
Disclosure
Jiangsu Hengrui Medicine Co., Ltd., provided financial support for this study and owns the patent right for this work. Kai Pan, Xiaolei Shi, and Ju Wang are employees of Jiangsu Hengrui Medicine Co., Ltd.; Kai Liu is an employee of Fujian Suncadia Medicine Co., Ltd. The authors report no other conflicts of interest in this work.
References
1.. International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels,Belgium: 2019. Available from: https://www.diabetesatlas.org.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American diabetes association standards of medical care in diabetes. Ann Intern Med. 2018;168:640–651. doi:10.7326/M18-0222
3. Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27S1:S84–87. doi:10.2337/diacare.27.2007.S84
4. Wolf G, Ritz E. Diabetic nephropathy in type 2 diabetes prevention and patient management. J Am Soc Nephrol. 2003;14:1396–1405. doi:10.1097/01.ASN.0000065639.19190.CF
5. Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Inv. 2018;9:1239–1254. doi:10.1111/jdi.12833
6. American Diabetes Association. Microvascular complications and foot care: standards of medical care in diabetes-2018. Diabetes Care. 2018;41S1:S105–S118. doi:10.2337/dc18-S010
7. Wang Y, Xing L, Yu H, et al. Prevalence of dental caries in children and adolescents with type 1 diabetes: a systematic review and meta-analysis. BMC Oral Health. 2019;19:213. doi:10.1186/s12903-019-0903-5
8. Zorena K, Raczyńska D, Raczyńska K. Biomarkers in diabetic retinopathy and the therapeutic implications. Mediat Inflam. 2013;193604. doi:10.1155/2013/193604
9. Jacobson AM, Braffett BH, Cleary PA, et al. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life. Diabetes Care. 2013;36:3131–3138. doi:10.2337/dc12-2109
10. Trento M, Passera P, Trevisan M, et al. Quality of life, impaired vision and social role in people with diabetes: a multicenter observational study. Acta Diabetol. 2013;50:873–877. doi:10.1007/s00592-013-0470-1
11. Dowarah J, Singh VP. Anti-diabetic drugs recent approaches and advancements. Bioorg Med Chem. 2020;28:115263. doi:10.1016/j.bmc.2019.115263
12. Charbonnel B, Aroda VR, Westerbacka J, et al. 131-LB: differences in HbA1c reduction between insulin Glargine 300 U/mL (Gla-300) and insulin Degludec 100 U/mL (IDeg-100) in adults ≥70 years of age with T2DM in the BRIGHT trial. Diabetes. 2019;68:131–LB. doi:10.2337/db19-131-LB
13. Duttaroy A, Kanakaraj P, Osborn BL, et al. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54:251–258. doi:10.2337/diabetes.54.1.251
14. Bednarek E, Sitkowski J, Bocian W, et al. Structure and pharmaceutical formulation development of a new long-acting recombinant human insulin analog studied by NMR and MS. J Pharmaceut Biomed. 2016;135:126–132. doi:10.1016/j.jpba.2016.12.005
15. Dardano A, Bianchi C, Prato SD, et al. Insulin Degludec/insulin aspart combination for the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag. 2014;10:465–475.
16. Mayer JP, Zhang F, Dimarchi RD. Insulin structure and function. Biopolymers. 2007;88(5):687–713. doi:10.1002/bip.20734
17. Slieker LJ, Brooke GS, Dimarchi RD, et al. Modifications in the B10 and B26–30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997;40(2):S54–S61. doi:10.1007/s001250051402
18. Sun PY, Zhang LS, Liu JJ, et al. Human insulin analogue and acylated derivative thereof. European Patent EP2784085A1. 2014.
19. Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrol. 2007;12:261–266. doi:10.1111/j.1440-1797.2007.00796.x
20. Lv J, Pan Y, Li X, et al. The imaging of insulinomas using a radionuclide-labelled molecule of the GLP-1 analogue liraglutide: a new application of liraglutide. PLoS One. 2014;9:e96833. doi:10.1371/journal.pone.0096833
21. Kimuda SG, Biraro IA, Bagaya BS, et al. Characterising antibody avidity in individuals of varied Mycobacterium tuberculosis infection status using surface plasmon resonance. PLoS One. 2018;13:e0205102. doi:10.1371/journal.pone.0205102
22. Steinicke F, Oltmann-Norden I, Wätzig H. Long term kinetic measurements revealing precision and general performance of surface plasmon resonance biosensors. Anal Biochem. 2017;530:94–103. doi:10.1016/j.ab.2017.05.009
23. Korn M, Wohlfart P, Gossas T, et al. Comparison of metabolic and mitogenic response in vitro of the rapid-acting insulin lispro product SAR342434, and US- and EU-approved Humalog®. Regul Toxicol Pharm. 2019;109:104497. doi:10.1016/j.yrtph.2019.104497
24. Kerlan V, Gouet D, Marre M, et al. Use of insulin Degludec, a new basal insulin with an ultra-long duration of action, in basal–bolus therapy in type 1 and type 2 diabetes. Ann Dendocrinol. 2013;74:487–490. doi:10.1016/j.ando.2013.04.004
25. Standl E, Owen DR. New long-acting basal insulins: does benefit outweigh cost. Diabetes Care. 2016;39(S2):S172–S179. doi:10.2337/dcS15-3011
26. Riddle MC. Lessons from Peglispro: imagine how to improve drug development and affordability. Diabetes Care. 2016;39(4):499–501. doi:10.2337/dc15-2754
27. Meyts PD. Insulin and its receptor: structure, function and evolution. Bioessays. 2004;26:1351–1362. doi:10.1002/bies.20151
28. Gammeltoft S. Insulin receptors: binding kinetics and structure-function relationship of insulin. Physiol Rev. 1984;64(4):1321–1378. doi:10.1152/physrev.1984.64.4.1321
29. Sinclair AJ, Signore A, Bomanji J, et al. In vivo kinetics of 123I-labelled insulin: studies in normal subjects and patients with diabetes mellitus. Nucl Med Comm. 1987;8:779–786. doi:10.1097/00006231-198710000-00003
30. Iozzo P, Osman S, Glaser M, et al. In vivo imaging of insulin receptors by PET: preclinical evaluation of iodine-125 and iodine-124 labelled human insulin. Nucl Med Biol. 2002;29:73–82. doi:10.1016/S0969-8051(01)00286-4
31. Liu Y, Wang HY, Zhou L, et al. Biodistribution, activation, and retention of proinsulin-transferrin fusion protein in the liver: mechanism of liver-targeting as an insulin prodrug. J Control Release. 2018;275:189–191. doi:10.1016/j.jconrel.2018.02.030
32. Sommerman EF, Pritchard PH, Cullis PR. 125I labelled inulin: a convenient marker for deposition of liposomal contents. Biochem Biophys Res. 1984;122:319–324. doi:10.1016/0006-291X(84)90477-7
33. Hong G, Bazin-Redureau MI, Scherrmann JMG. Pharmacokinetics and organ distribution of cationized colchicine-specific IgG and Fab fragments in rat. J Pharm Sci. 1999;88:147–153. doi:10.1021/js970335n
34. Wronkowitz N, Hartmann T, Görgens SW, et al. LAPS Insulin115: a novel ultra-long-acting basal insulin with a unique action profile. Diabetes Obes Metab. 2017;19(12):1722–1731. doi:10.1111/dom.13006
35. Zhai J, Li L, Dong L, et al. Simultaneous quantitative determination of liraglutide and insulin degludec in rat plasma by liquid chromatography–tandem mass spectrometry method and its application. Biomed Chromatogr. 2020;34(10):e4921. doi:10.1002/bmc.4921
36. Li YH, Meng Q, Yang MB, et al. Current trends in drug metabolism and pharmacokinetics. Acta Pharm Sin B. 2019;9:1113–1144.
37. Banks WA, Morley JE, Lynch JL, et al. Insulin detemir is not transported across the blood-brain barrier. Peptides. 2010;31:2284–2288. doi:10.1016/j.peptides.2010.09.011
38. Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27(3):351–357. doi:10.1007/BF00304849
39. Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;5:608–624.
40. Piccinini F, Bergman RN. The Measurement of Insulin Clearance. Diabetes Care. 2020;43(9):2296–2302. doi:10.2337/dc20-0750
41. Tambascia MA, Eliaschewitz FG. Degludec: the new ultra-long insulin analogue. Diabetol Metab Syndr. 2015;7(1):57. doi:10.1186/s13098-015-0037-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



