Back to Journals » Therapeutics and Clinical Risk Management » Volume 10
Efficacy of tramadol as a preincisional infiltration anesthetic in children undergoing inguinal hernia repair: a prospective randomized study
Authors Numanoğlu KV, Ayoğlu H, Tatli D, Er E
Received 6 February 2014
Accepted for publication 20 March 2014
Published 25 September 2014 Volume 2014:10 Pages 753—758
DOI https://doi.org/10.2147/TCRM.S62029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Kemal Varim Numanoğlu,1 Hilal Ayoğlu,2 Duygu Tatli,1 Ebubekir Er1
1Department of Pediatric Surgery, 2Department of Anesthesiology, Faculty of Medicine, Bülent Ecevit University, Kozlu, Zonguldak, Turkey
Background: Preincisional local anesthetic infiltration at the surgical site is a therapeutic option for postoperative pain relief for pediatric inguinal hernia. Additionally, tramadol has been used as an analgesic for postoperative pain in children. Recently, the local anesthetic effects of tramadol have been reported. The aim of this study was to determine both the systemic analgesic and the local anesthetic effects of tramadol and to determine how it differs from bupivacaine when administered preincisionally.
Methods: Fifty-two healthy children, aged 2–7 years, who were scheduled for elective herniorrhaphy were randomly allocated to receive either preincisional infiltration at the surgical site with 2 mg/kg tramadol (Group T, n=26) or 0.25 mL/kg 0.5% bupivacaine (Group B, n=26). At the time of anesthetic administration, perioperative hemodynamic parameters were recorded. The pain assessments were performed 10 minutes after the end of anesthesia and during the first 6-hour period, using pain scores. The time of first dose of analgesia and need for additional analgesia were recorded.
Results: Between T and B groups, the anesthesia time, perioperative hemodynamic changes, and pain scores were not statistically different. However, in group B, the postoperative analgesic requirement was higher than in group T.
Conclusion: Tramadol shows equal analgesic effect to bupivacaine and decreases additional analgesic requirement, when used for preincisional infiltration anesthesia in children undergoing inguinal herniorrhaphy.
Keywords: bupivacaine, postoperative analgesia, pain scores
Introduction
Inguinal hernia repair is the most frequent surgical procedure in early childhood. Postoperative pain is an important problem in children after inguinal hernia repair. Effective analgesia after herniorrhaphy in children is essential, and several techniques are used, such as administration of opioids, nonsteroidal anti-inflammatory drugs (NSAIDS), peripheral nerve block, caudal block, and wound infiltration with local anesthetics.
Analgesics or local anesthetics given before the surgical stimulus may prevent the increase in excitability of the central nervous system and prevent or attenuate postoperative pain.1,2 Preincisional wound infiltration with local anesthetics is an option for pain relief during the postoperative period. Preincisional wound infiltration of bupivacaine has been used to provide analgesia in patients undergoing inguinal herniorrhaphy.3 These infiltrations are fast and simple procedures, without serious side effects.
Intramuscular (IM) or intravenous (IV) tramadol (1–2 mg/kg) is effective in treating moderate to severe pain in children of 12 months or older who are undergoing pediatric surgical procedures.4 Tramadol is a racemic compound made up of two isomers that have opioid and nonopioid activities, and is used mainly for inhibition of pain. Additionally, the local anesthetic effect of tramadol has been demonstrated in both clinical and laboratory studies.4–9
Our study was based on the emerging theory that both the systemic analgesic and local anesthetic effects of tramadol could be exploited. The aim of this study was to compare the postoperative analgesic effects of tramadol versus bupivacaine, when used as a preincisional wound infiltration agent for postoperative pain relief in pediatric inguinal herniorrhaphy. Our hypothesis was based on the assumption that the efficacy of tramadol would be superior to bupivacaine, due to both its systemic and local anesthetic effects.
Methods
This study was registered as a double-blinded clinical trial, with approval of the Zonguldak Karaelmas Research Hospital Ethics Committee, and written parental consent was taken before the operation. In this prospective study, 52 healthy children, American Society of Anesthesiologists (ASA) classification,10 class 1, aged 2–7 years, undergoing elective unilateral herniorrhaphy were enrolled. Children who had neurological, neuromuscular, psychiatric, convulsive or blood clotting disorders, or any drug allergy were excluded from the study. The patients were randomly assigned to either the tramadol group (T) or the bupivacaine group (B). After induction of general anesthesia, the groups received either locally administered 2 mg/kg tramadol in 0.2 mL/kg saline (group T, n=26) or 0.2 mL/kg of 0.25% bupivacaine (group B, n=6) 3 minutes before incision, by a surgeon.
All patients were premedicated 45 minutes before surgery with 0.5 mg/kg midazolam perorally. After cannulation of the dorsal hand vein with a 24-gauge cannula, standard patient monitoring included electrocardiogram, noninvasive blood pressure, pulse oximetry, and heart rate. General anesthesia was induced by 2.5–3.5 mg/kg propofol IV. Endotracheal intubation was performed with rocuronium bromide 0.3–0.5 mg/kg. Ventilation was controlled in all patients, with end-tidal carbon dioxide maintained between 30 and 40 mmHg after induction of anesthesia. Anesthesia was maintained with 2%–2.5% Sevoflurane (Sevorane, Abbott Laboratories, Abbott Park, IL, USA), 68% nitrous oxide, and 30% oxygen. The administration of IV fluids in the operating room followed standard guidelines.
Age, sex, body weight, duration of anesthesia, and perioperative hemodynamic changes of the patients were recorded. The Pain/discomfort Scale (PDS),11 which provides a score ranging from 0–10 points, evaluated the severity of pain. The evaluations were performed postoperatively, at 10, 20, and 30 minutes, in the postanesthesia care unit, by physicians who were unaware of group assignment (Table 1).11 After 30 minutes of observation, the patients were transferred to the pediatric surgical unit, where they were monitored for 4 hours before being discharged. In this postoperative period, an additional evaluation was performed using a Faces Pain Scale (FPS) (Figure 1). Pain was assessed by nurses who were unaware of the groups, from six face drawings (scoring from 0= no pain to 10= worst pain).12 The pain scores were noted at 0, 30, 60, and 120 minutes. Patients with FPS >4 were treated with a 30 mg/kg paracetamol suppository. Patients were discharged following the surgeon’s visit on the same day, when patients were calm and cooperative, pain scores were decreased, and there was no bleeding from the surgical site. A telephone interview with parents was performed 24 hours after the operation, in order to determine whether there were further complications, like vomiting, pruritus, local allergic reaction, problems with food or fluid intake, or additional analgesic requirements.
  | Table 1 Pain/discomfort Scale |
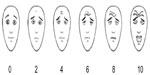  | Figure 1 Faces Pain Scale-Revised. |
Age, weight, height, sex, duration of surgery, duration of general anesthesia, perioperative hemodynamic parameters, time of first analgesic requirement, and average pain score were compared with the Mann–Whitney U test, using a statistical package program (SPSS for Windows, Version 10.0; SPSS, Inc., Chicago, IL, USA). Unless otherwise specified, data are given as arithmetic mean ± standard deviation (SD). The sample size was estimated using data from previous studies performed for pain scores in our institution. A difference of 1 point in the mean increase of pain scores between the groups and a SD of 0.8 were used for calculation. We considered a 30% increase in total postoperative analgesic efficacy to be clinically important. From preliminary data, we calculated, with significance set at 0.05, that 26 patients per group would give a statistical power of 98% between groups.
Results
All patients were eligible to complete the study and were included in the further analyses. Groups were similar with respect to age, weight, sex distribution, and ASA physical status. Fifty-two patients were studied, in two groups of 26 each. There were no significant differences between the groups according to demographic data and operation time (P>0.05) (Table 2). In addition, there were no hemodynamic alterations, including heart rate or mean arterial blood pressure, that required medication during the surgery or in the early postoperative period in either of the study groups.
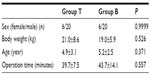  | Table 2 Patients characteristics and operation time |
The PDSs of the patients at 10, 20, and 30 minutes after surgery that were recorded in the postanesthesia care unit and the facial pain scores of the patients at 0, 30, 60, and 120 minutes that were assessed in the pediatric surgical unit were similar in both groups (Figures 2 and 3).
  | Figure 2 Pain/discomfort Scale11 of the two groups (bupivacaine versus tramadol) during the first 30 minutes after the operation. |
  | Figure 3 Faces Pain Scales (FPS)12 of the two groups (bupivacaine versus tramadol) during the first 120 minutes after the operation. |
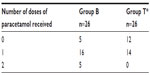
There was no difference between groups T and B for the average time of the first analgesics use. (P=0.059). The number of patients who required additional analgesic medication were as follows: 14 patients in group T required analgesic once, 16 patients in group B required analgesic once, and five patients in group B required additional postoperative analgesia twice (P=0.018) (Table 3). No further complications or side effects, like sleep disturbance or differences in food or fluid intake, were determined during the first postoperative day.
Discussion
In the last 2 decades, some progress has been made in alleviating pain in children. A growing body of knowledge about the nature of pain during intrauterine life and childhood has changed practices in pediatric pain treatment. However, the availability of potent analgesic medications labeled for use in children is limited. These medications include paracetamol, NSAIDs, and opioids. NSAIDs have restricted approval in children and may cause a bleeding tendency, renal impairment, and aggravate asthma. Opioids have the risk of respiratory depression. Systemic opioids are the cornerstone of postoperative pain management; however, the fear of serious adverse effects (in particular, respiratory depression) and other concerns have led to reluctance in administering parenteral opioids to children.13 Thus, pediatric surgeons and anesthesiologists mainly depend on paracetamol and regional techniques, including peripheral nerve block, caudal block, and wound infiltration with local anesthetics, especially in children having “day-case” surgery.14,15 A drug with different formulations, which could be effective in relieving moderate to severe pain in children, is still needed.
Tramadol is a synthetic 4-phenyl-piperidine analog of codeine that is marketed as a racemic mixture of (+) and (−) enantiomers. Tramadol exerts its action on central monoaminergic systems, and this mechanism may contribute to its analgesic effect. The opioid activity of tramadol results from low-affinity binding of the (+) enantiomer to μ-opioid receptors. The central analgesic effects of tramadol are partially reversed by naloxone.16 It was shown that after IM injection, tramadol was rapidly and almost completely absorbed. On average, peak serum concentrations were reached in 45 minutes. Serum concentrations adequate for the treatment of slight pain were already achieved, on average, after about 7 minutes.17 The recommended IM daily dose is between 50 and 100 mg every 4–6 hours. The elimination pharmacokinetics of tramadol are appropriately described by a two-compartment model, with a reported elimination half-life of 5.1 (±0.8) hours for tramadol and 9 hours for the metabolite 1 derivative after a single oral dose of 100 mg.7 The analgesic potency of tramadol is considered medium. It has one-tenth the potency of morphine, one-fifth the potency of nalbuphine, and has the equivalent potency of pethidine and oxycodone. Additionally, its potency is at least the equivalent of ketamine and NSAIDs.16,18
Preemptive analgesia is defined as an antinociceptive treatment that prevents the establishment of altered central processing of afferent input, which amplifies postoperative pain.2 By decreasing the capacity for altered central sensory processing, preemptive analgesia is thought to consequently decrease the incidence of hyperalgesia and allodynia after surgery.1 The clinical documentation of preemptive analgesia was reported after inguinal herniorrhaphy in children who received bupivacaine by wound infiltration.15 In another study, 14 children, aged 6–18 years, who were undergoing tonsillectomy and who received preincisional infiltration with 3 mL of 0.25% bupivacaine around the tonsils, demonstrated effective preemptive analgesia.19 A meta-analysis presented that preemptive analgesia showed an overall beneficial effect in selected analgesic regimens, which was most pronounced after epidural analgesia, local wound infiltrations, and systemic NSAID administration.20
Based on these observations, we believed that similar alterations may amplify and prolong postoperative pain in our patients and that the blockade of nociceptive stimuli with bupivacaine or tramadol before surgery may improve the duration and effectiveness of postoperative analgesia. For this reason, we planned the drug administration 3 minutes before incision, in the study groups.
Repeated studies have examined postoperative analgesia following instillation of bupivacaine into the wound after herniorrhaphy and found a beneficial effect.21,22 This local anesthetic was chosen because of its high potency and long-lasting action. However, it should be kept in mind that serious systemic toxic reactions, like seizures and cardiovascular collapse, while unusual, may occur after inadvertent IV administration of large doses of bupivacaine.23
Clinical studies have also shown that tramadol has peripheral local anesthetic-type properties.15,24–26 By direct tramadol application to the sciatic nerve in rats, it was proven that tramadol exerts a local anesthetic effect.6 Altunkaya et al observed that tramadol had a local anesthetic action similar to that of lidocaine and because of its antinociceptive effect; it could be extended into the postoperative period.7 Demiraran et al presented an equal analgesic effect through subincisional injection of 2 mg/kg of tramadol and 0.25% bupivacaine, in children aged 1–6 undergoing herniorrhaphy.9 Gerçek et al showed that subcutaneous tramadol infiltration can provide effective analgesia and may have anti-inflammatory effects.27
In our study, we found no difference between groups T and B, for either pain scores or average time of the first analgesics use. However, supplemental postoperative analgesic requirements increased in group B (P=0.018). Major side effects of tramadol used for postoperative analgesia have been reported to be nausea and vomiting.28 None of these complications were observed in our study.
According to our data, the necessity for additional postoperative analgesia after wound infiltration with tramadol was decreased when compared with that following bupivacaine injection. The maintenance of analgesia during the early postoperative period was similar between tramadol and bupivacaine, but tramadol appeared to be slightly superior to bupivacaine in the late postoperative period. This might indicate that tramadol-related analgesia is achieved by both local effect and systemic absorption. The average time to first analgesic requirement and pain scores of the patients was similar in both groups. These results also suggest that preincisional bupivacaine infiltration did not provide any clinically perceptible benefits compared with preincisional infiltration of tramadol.
Conclusion
According to the results of our study, preincisional tramadol infiltration, an easy and reliable method that does not require additional experience, has the equivalent effects of a local anesthetic when compared with bupivacaine. Additionally, it decreases the postoperative analgesic requirements in children having inguinal hernia repair. We conclude that this technique may be a good alternative for postoperative analgesia in day-case operation, for children undergoing inguinal herniorrhaphy.
Disclosure
The authors report no conflicts of interest in this work.
References
Dahl JB, Møiniche S. Pre-emptive analgesia. Br Med Bull. 2004;71:13–27. | |
Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology. 2002;96(3):725–741. | |
Bay-Nielsen M, Klarskov B, Bech K, Andersen J, Kehlet H. Levobupivacaine vs bupivacaine as infiltration anaesthesia in inguinal herniorrhaphy. Br J Anaesth. 1999;82(2):280–282. | |
Bozkurt P. Use of tramadol in children. Paediatr Anaesth. 2005;15(12):1041–1047. | |
Pang WW, Mok MS, Chang DP, Yang TF, Lin CH, Huang MH. Intradermal injection of tramadol has local anesthetic effect: a comparison with lidocaine. Acta Anaesthesiol Sin. 1998;36(3):133–136. | |
Tsai YC, Chang PJ, Jou IM. Direct tramadol application on sciatic nerve inhibits spinal somatosensory evoked potentials in rats. Anesth Analg. 2001;92(6):1547–1551. | |
Altunkaya H, Ozer Y, Kargi E, et al. The postoperative analgesic effect of tramadol when used as subcutaneous local anesthetic. Anesth Analg. 2004;99(5):1461–1464. | |
Mert T, Gunes Y, Guven M, Gunay I, Ozcengiz D. Comparison of nerve conduction blocks by an opioid and a local anesthetic. Eur J Pharmacol. 2002;439(1–3):77–81. | |
Demiraran Y, Ilce Z, Kocaman B, Bozkurt P. Does tramadol wound infiltration offer an advantage over bupivacaine for postoperative analgesia in children following herniotomy? Paediatr Anaesth. 2006;16(10):1047–1050. | |
Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2(3):281–284. | |
Hannallah RS, Broadman LM, Belman AB, Abramowitz MD, Epstein BS. Comparison of caudal and ilioinguinal/iliohypogastric nerve blocks for control of post-orchiopexy pain in pediatric ambulatory surgery. Anesthesiology. 1987;66(6):832–834. | |
Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–183. | |
Ekemen S, Yelken B, Ilhan H, Tokar B. A comparison of analgesic efficacy of tramadol and pethidine for management of postoperative pain in children: a randomized, controlled study. Pediatr Surg Int. 2008;24(6):695–698. | |
Splinter WM, Bass J, Komocar L. Regional anaesthesia for hernia repair in children: local vs caudal anaesthesia. Can J Anaesth. 1995;42(3):197–200. | |
Schindler M, Swann M, Crawford M. A comparison of postoperative analgesia provided by wound infiltration or caudal analgesia. Anaesth Intensive Care. 1991;19(1):46–49. | |
Halfpenny DM, Callado LF, Hopwood SE, Bamigbade TA, Langford RM, Stamford JA. Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured by real time voltammetry. Br J Anaesth. 1999;83(6):909–915. | |
Lintz W, Beier H, Gerloff J. Bioavailability of tramadol after i.m. injection in comparison to i.v. infusion. Int J Clin Pharmacol Ther. 1999;37(4):175–183. | |
Duthie DJ. Remifentanil and tramadol. Br J Anaesth. 1998;81(1):51–57. | |
Umuroglu T, Eti Z, Ciftçi H, Yilmaz Gögüs F. Analgesia for adenotonsillectomy in children: a comparison of morphine, ketamine and tramadol. Paediatr Anaesth. 2004;14(7):568–573. | |
Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100(3):757–773. | |
Reid MF, Harris R, Phillips PD, Barker I, Pereira NH, Bennett NR. Day-case herniotomy in children. A comparison of ilio-inguinal nerve block and wound infiltration for postoperative analgesia. Anaesthesia. 1987;42(6):658–661. | |
Hashemi K, Middleton MD. Subcutaneous bupivacaine for postoperative analgesia after herniorrhaphy. Ann R Coll Surg Engl. 1983;65(1):38–39. | |
Albright GA. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51(4):285–287. | |
Jaffe RA, Rowe MA. A comparison of the local anesthetic effects of meperidine, fentanyl, and sufentanil on dorsal root axons. Anesth Analg. 1996;83(4):776–781. | |
Acalovschi I, Cristea T, Margarit S, Gavrus R. Tramadol added to lidocaine for intravenous regional anesthesia. Anesth Analg. 2001;92(1):209–214. | |
Kapral S, Gollmann G, Waltl B, et al. Tramadol added to mepivacaine prolongs the duration of an axillary brachial plexus blockade. Anesth Analg. 1999;88(4):853–856. | |
Gerçek A, Eti Z, Gögüs FY, Sav A. The analgesic and anti-inflammatory effects of subcutaneous bupivacaine, morphine and tramadol in rats. Agri. 2004;16(3):53–58. | |
Shipton EA. Tramadol – present and future. Anaesth Intensive Care. 2000;28(4):363–374. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.