Back to Journals » OncoTargets and Therapy » Volume 9
Efficacy of three-dimensional conformal radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus
Authors Wu F, Lu H, Zhu S, Li Z, Zou L, Bai T, Chen J, Yang T, Liang S
Received 19 May 2016
Accepted for publication 30 July 2016
Published 1 December 2016 Volume 2016:9 Pages 7141—7147
DOI https://doi.org/10.2147/OTT.S113161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Fei-Xiang Wu,1–3,* Hui-Rong Lu,4,* Shao-Liang Zhu,1,* Zi-Hui Li,1 Ling Zou,1 Tao Bai,1 Jie Chen,1 Tian-Bo Yang,1 Shi-Xiong Liang4,5
1Department of Hepatobiliary Surgery, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, 2Guangxi Liver Cancer Diagnosis and Treatment Engineering and Technology Research Center, Nanning, 3Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, Shanghai, 4Department of Radiation Oncology, Affiliated Tumor Hospital of Guangxi Medical University, Nanning, 5Department of Radiation Oncology, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Objective: The current study aimed to evaluate the efficacy and outcomes of three-dimensional conformal radiotherapy (3DCRT) combined with transarterial chemoembolization (TACE) for treating patients with hepatocellular carcinoma involving portal vein tumor thrombus.
Methods: During 2000 and 2013, a total of 182 hepatocellular carcinoma patients with portal vein tumor thrombus were retrospectively analyzed: 68 patients were treated by 3DCRT alone (group A), 74 by TACE alone (group B), and 40 by a combination of 3DCRT + TACE (group C). The overall survival (OS) of the three groups was compared using the Kaplan–Meier method. The independent predictors of survival were identified using multivariate analysis.
Results: The total effective rate (complete response + partial response) among all patients was 44% (80/182). The objective response rate (complete response + partial response) was higher in group C than in group A or B, but the differences were not significant. OS rates at 1, 2, and 3 years were significantly higher in group C than in group A or B (P<0.05), while OS rates were similar between groups A and B. Multivariate analysis identified serum levels of alpha-fetoprotein <400 ng/mL and the use of 3DCRT + TACE as independent predictors of better OS.
Conclusion: These results suggest that combining 3DCRT with TACE may provide better OS than either technique alone in hepatocellular carcinoma patients with portal vein tumor thrombus.
Keywords: hepatocellular carcinoma, portal vein tumor thrombus, three-dimensional conformal radiotherapy, transarterial chemoembolization, overall survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related mortality worldwide.1 Portal vein tumor thrombus (PVTT) is found to have invaded the main trunk in 10%–15% of patients at the time of diagnosis with HCC.2–4 PVTT is a particularly frequent complication in patients with advanced HCC.5,6 It is often accompanied by portal vein hypertension, ascites, tumor dissemination, and deterioration of liver function, resulting in poor prognosis for HCC patients with PVTT, who survive a median of 2–3 months without treatment.3,7,8
Transarterial chemoembolization (TACE) is considered a standard treatment for patients with inoperable advanced HCC.9 Evidence suggests that TACE is relatively safe for HCC patients with PVTT who have good liver function and abundant collateral circulation,10 but the efficacy of TACE for such patients remains controversial.11–14
Radiotherapy although originally not used widely to treat HCC, because patients show low tolerance to whole-organ irradiation, has recently become attractive for comprehensive treatment of liver cancer due to the advent of precise, targeted radiotherapy techniques, including three-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiation therapy (IMRT), and stereotactic body radiotherapy (SBRT). These technologies can deliver maximal radiation doses to hepatic tumors without causing serious complications.15,16 Several studies have shown good local tumor control and long-term survival in HCC patients with PVTT treated using 3DCRT.17–21
In an effort to maximize therapeutic efficacy, investigators have combined 3DCRT with TACE for HCC patients with PVTT.22–24 While these studies suggest that the combined treatment modality can be safe and effective, whether it is superior to 3DCRT or TACE on their own is unclear because the studies lacked parallel control groups or were limited by short follow-up or small samples. To address this question more rigorously, we retrospectively analyzed the clinical data of 182 cases of HCC with PVTT to compare the efficacy and toxicity of the different treatments alone and in combination.
Patients and methods
Patients
This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was given by all participants for their clinical records to be used in this study and this was approved by the Institutional Review Board of Affiliated Tumor Hospital of Guangxi Medical University.
We retrospectively studied 182 patients with HCC involving PVTT treated between January 2000 and December 2013 in the Department of Radiation Oncology of the Affiliated Tumor Hospital of Guangxi Medical University (Nanning, People’s Republic of China). Patients were included in the study if they satisfied the following criteria: 1) age between 26 years and 75 years; 2) pathological or clinical diagnosis of HCC with PVTT; 3) Child-Pugh A or B liver function; 4) no history of liver radiotherapy; 5) Eastern Cooperative Oncology Group performance status of 0–2; 6) normal function of brain, kidneys, and other major organs; and 7) written informed consent before treatment.
PVTT was diagnosed on the basis of a filling defect in the portal vein or its branch on contrast-enhanced computed tomography (CT) or magnetic resonance imaging. The type of PVTT was classified according to ShuQun et al:25,26 type I, tumor thrombus involving the area proximal to the secondary branches of the portal vein; type II, tumor thrombus involving the primary branches; type III, tumor thrombus extending to the main portal vein but not to the superior mesenteric vein; and type IV, tumor thrombus extending to the superior mesenteric vein.
The clinicopathologic features of all patients are listed in Table 1. The median age across all patients was 47 years (range: 26–75 years). A total of 116 patients (63.7%) were diagnosed with type II PVTT and 66 (36.3%) with type III PVTT. Nearly equal numbers of patients were treated by 3DCRT alone (68, group A) or TACE alone (74, group B), and a smaller number was treated with the combination of 3DCRT + TACE (40, group C).
TACE procedure
Of all patients in the sample, 114 (62.6%) received a median of one course of TACE (range: 1–4) performed using the Seldinger technique. Guided by arterial angiography, the surgeon cannulated the proper hepatic artery through the femoral artery and then infused a mixture of chemotherapy drugs and iodine oil (5–20 mL) through the catheter. Finally, gelatin sponge pledgets were used to enhance the therapeutic effect.27 The following chemotherapy drugs and dosages were used: adriamycin, 50–60 mg/m2; cisplatin, 30–40 mg/m2; and mitomycin, 6–7 mg/m2 or HCPT, 10–15 mg/m2.
3DCRT procedure
3DCRT was performed using a Philips 8 MV X-ray linear accelerator and Shanghai Topslane three-dimensional treatment planning system or a Medical Precise 6 MV X-ray linear accelerator. Working together, a radiologist and a radiation oncologist outlined the visible gross tumor volume (GTV) consisting of PVTT and/or intrahepatic tumor. The planning target volume was defined as GTV + (0.5–2) cm. The organs at risk included normal liver tissue, duodenum, bilateral kidney, stomach, small intestine, pancreas, and spinal cord. The dose of each organ was not to exceed the corresponding tolerance dose. Cumulative dose–volume histograms were used to evaluate each treatment plan. All patients received 3DCRT 3–5 times per week at a median tumor dose of 50.6 Gy (range: 28–63 Gy) with a median fraction size of 4 Gy (range: 2–8 Gy). This translated to a biological effect dose of 38–91 Gy at an α/β ratio of 11.2 (Gy11.2).28
Clinical assessment and follow-up
The patients were evaluated on a weekly basis during treatment, once every 3 months during 1 year after treatment and every 6 months thereafter. The patients were evaluated by physical examination, routine blood analysis, analysis of liver and renal function, assay of carcinoembryonic antigen and alpha-fetoprotein (AFP), and imaging using plain chest film, abdominal CT, and abdominal B ultrasonography. Tumor and PVTT responses were determined from serial CT scans taken 1–2 months after the completion of treatment. Tumor response was assessed using the modified Response Evaluation Criteria in Solid Tumors.29 Complete disappearance of PVTT was defined as complete response (CR), >50% reduction of PVTT as partial response (PR), <50% reduction of PVTT as stable disease (SD), and >25% growth of PVTT as progressive disease (PD). All patients were followed up until death or December 31, 2013. The overall survival (OS) was calculated from the end of treatment until the date of the last follow-up.
Statistical analysis
Statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were compared using the chi-squared or Fisher’s exact tests as appropriate. Survival probability was calculated using the Kaplan–Meier method, and intergroup differences were assessed using the log-rank test. Multivariate analysis to identify predictors of OS was carried out using a Cox regression model. P<0.05 was considered statistically significant.
Results
Tumor response
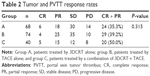
Tumor and PVTT responses were determined using serial CT scans taken 1–2 months after the completion of treatment. Based on modified Response Evaluation Criteria in Solid Tumors criteria, the 68 patients in group A showed the following distribution of clinical responses: CR, six (8.8%); PR, 18 (26.5%); SD, 30 (44.1%); and PD, 14 (20.6%). The objective response rate (CR + PR) in group A was 35.3%. The 74 patients in group B showed the following distribution of clinical responses: CR, four (5.4%); PR, 25 (33.8%); SD, 35 (47.3%); and PD, ten (13.5%). The objective response rate (CR + PR) in group B was 39.2%. The 40 patients in group C showed the following distribution of clinical responses: CR, five (12.5%); PR, 15 (37.5%); SD, 12 (30%); and PD, eight (20%). The objective response rate (CR + PR) in group C was 50%. The objective response rate in group C (50%, 20/40) was higher than the rates in group A (35.3%, 25/68) and group B (39.2%, 29/74), but the differences were not significant (P=0.315; Table 2).
Follow-up and survival
The median follow-up lasted 10 months (range: 1–102 months). During the follow-up, 33 of 182 patients (18.1%) experienced intrahepatic metastasis or lymph node metastasis in the abdominal aorta and 159 (87.4%) died. The median survival time was 7 months in group A, 6 months in group B, and 13 months in group C. The rates of OS at 1, 2, and 3 years were 28.8%, 12%, and 28.8% in group A; 28.7%, 10.5%, and 28.7% in group B; and 53.5%, 18.8%, and 9.4% in group C. The median survival time and OS rate were significantly higher in group C than in group A or B (P=0.017). In contrast, groups A and B were similar in terms of median survival time and OS rates. The survival curves are shown in Figure 1.
Univariate analysis
Univariate analysis identified serum AFP level <400 ng/mL and the use of 3DCRT + TACE as predictors of better OS (Table 3).
Multivariate analysis
Multivariate analysis using Cox regression identified serum AFP level <400 ng/mL (P=0.029) and the use of 3DCRT + TACE (P=0.015) as independent predictors of better OS (Table 4).
Adverse reactions
Treatment-related toxicities were assessed using the National Cancer Institute-Common Terminology Criteria for Adverse Events 3.0 classification scheme. Among all 182 patients, no treatment-related events of grade 4 or 5 acute toxicity were observed within 3 months after treatment. Among patients receiving 3DCRT, the most common adverse events were grades 1–2 nausea, vomiting, fatigue, and anorexia. Several patients treated with TACE experienced grades 1–2 acute bone marrow suppression.
Discussion
PVTT is a complication in many patients with advanced HCC, and it is associated with poor prognosis. The mean survival for HCC patients with PVTT is only 2.7–4 months, much shorter than the 24.4 months for HCC patients without PVTT.25,30 The patients with advanced HCC involving PVTT are ineligible for surgical resection or liver transplantation due to portal hypertension, deterioration of liver function, and distant metastasis. For such patients, only a few treatment options are recommended, including radiotherapy, TACE, and sorafenib. The Barcelona Clinic Liver Cancer group classifies HCC with PVTT as an advanced disease and recommends sorafenib as a standard systemic therapy.31 However, sorafenib is not widely used in the People’s Republic of China because of the low response rates,32,33 various adverse events,32,34 and high costs.
TACE has long been used as a palliative treatment for patients with technically unresectable or medically inoperable HCC. Even though some early clinical trials of TACE showed little survival benefit and serious side effects,35,36 recent reports have demonstrated safety and efficacy in HCC patients with PVTT.12,13 Tawada et al14 performed TACE on 49 HCC patients with PVTT and observed positive/negative rates of 20/13 for parenchymal response and 13/20 for PVTT response. The mean survival time was 11.1 months for patients positive for parenchymal response and 14 months for patients positive for PVTT response. The efficacy of TACE for HCC patients with PVTT may reflect its ability to alter the blood supply to tumors. Most (90%–95%) of the blood supply to intrahepatic tumors comes from the hepatic artery, while the blood supply in patients with PVTT comes from both the hepatic artery and portal vein. However, the establishment of collateral circulation after embolization and recanalization of the blood vessel37 can lead to tumor recurrence, making TACE less than satisfactory as a stand-alone treatment for HCC involving PVTT.
Radiotherapy originally found limited application in treating HCC because of low liver tolerance to radiation, but the advent of 3DCRT, IMRT, and SBRT has made radiotherapy a widely used treatment for patients with advanced HCC involving PVTT. Various studies have examined 3DCRT in such patients, but the effects have been quite variable. Huang et al21 reported median survival time of only 3.8 months, a response rate of 25.2%, and 1-year OS rate of 16.7% in HCC patients with PVTT after 3DCRT or IMRT. In contrast, Rim et al19 reported median survival time of 16.7 months, a response rate of 62.3%, and 1-year OS rate of 63.7% in HCC patients with PVTT after 3DCRT.
Several considerations have led clinicians to investigate the benefit of combining 3DCRT with TACE for HCC patients with PVTT. First, deposition of iodized oil after TACE may help reveal minute lesions and determine the GTV boundary. Second, the decreased tumor volume after TACE may reduce radiation exposure of normal liver tissue during 3DCRT. Third, chemotherapy drugs may increase radiation sensitivity. Yoon et al38 reported median survival time of 10.6 months and OS rates of 42.5% at 1 year and 22.8% at 2 years for 412 HCC patients with PVTT who underwent 3DCRT + TACE. Park et al24 reported median survival time of 13 months and an objective response rate of 44.4% in 18 patients with unresectable HCC and PVTT who underwent 3DCRT + TACE. The OS rates in that study were significantly higher among responders than among nonresponders. These studies suggest that combining 3DCRT and TACE may lead to greater efficacy than either therapy on its own for HCC patients with PVTT, but the lack of parallel comparison arms weakens this conclusion.
Therefore, we conducted a retrospective clinical study in which we compared safety and efficacy of the two techniques on their own and together in parallel groups of patients recruited from the same medical center over the same period. The median survival time and OS rates were significantly higher for the group that received 3DCRT + TACE (group C) than for the groups receiving either treatment on its own. The objective response rate tended to be higher in the combined treatment group (50%) than in the other two groups (35.3% for group A and 39.2% for group B), although the difference was not significant. Multivariate analysis also identified the use of 3DCRT + TACE as an independent predictor of better OS. Our findings from this parallel-comparison study, conducted in a cohort of Chinese patients from a region of the world with one of the highest incidences of HCC, provide some of the strongest evidence to date that combining 3DCRT with TACE can be more effective than either treatment alone for HCC patients with PVTT.
The other predictor of better OS identified in our multivariate analysis was serum AFP <400 ng/mL. This is consistent with the results of Huang et al21 indicating that higher AFP is associated with poorer survival in HCC patients. Several European and Japanese reports have stressed the importance of preoperative AFP levels, leading to their incorporation into clinical prognostic scores.39,40
The current study has some limitations. First, the small size of the combined-treatment arm and differences in follow-up duration among the three parallel arms raise the risk of bias in our efficacy analysis. Second, this was a retrospective study in which patients chose their treatment rather than being randomly assigned, increasing the risk of selection bias. Our results should be verified and extended in larger, prospective, randomized trials.
Conclusion
Our results indicate that the combination of 3DCRT and TACE is a feasible and safe treatment modality for HCC patients with PVTT. This combination therapy may be associated with higher objective response rate and OS than either treatment on its own.
Acknowledgment
This study was supported by the Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education, People’s Republic of China (GKE2015–ZZ06) and the Scientific Research Fund of the Ministry of Health of Guangxi Province (2016–5).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Miller K, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. | ||
Cheung TK, Lai CL, Wong BC, Fung J, Yuen MF. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther. 2006;24(4):573–583. | ||
Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12(47):7561–7567. | ||
Park KW, Park JW, Choi JI, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008;23(3):467–473. | ||
Kuo YH, Lu SN, Chen CL, et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer. 2010;46(4):744–751. | ||
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. | ||
Nakazawa T, Adachi S, Kitano M, et al. Potential prognostic benefits of radiotherapy as an initial treatment for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels. Oncology. 2007;73(1–2):90–97. | ||
Schoniger-Hekele M, Muller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48(1):103–109. | ||
Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. | ||
Ban D, Shimada K, Yamamoto Y, et al. Efficacy of a hepatectomy and a tumor thrombectomy for hepatocellular carcinoma with tumor thrombus extending to the main portal vein. J Gastrointest Surg. 2009;13(11):1921–1928. | ||
Kim JH, Yoon HK, Kim SY, et al. Transcatheter arterial chemoembolization vs. chemoinfusion for unresectable hepatocellular carcinoma in patients with major portal vein thrombosis. Aliment Pharmacol Ther. 2009;29(12):1291–1298. | ||
Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18(12):1517–1526; quiz 1527. | ||
Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. | ||
Tawada A, Chiba T, Ooka Y, et al. Efficacy of transarterial chemoembolization targeting portal vein tumor thrombus in patients with hepatocellular carcinoma. Anticancer Res. 2014;34(8):4231–4237. | ||
Feng M, Ben-Josef E. Radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21(4):271–277. | ||
Tanaka Y, Nakazawa T, Komori S, et al. Radiotherapy for patients with unresectable advanced hepatocellular carcinoma with invasion to intrahepatic large vessels: efficacy and outcomes. J Gastroenterol Hepatol. 2014;29(2):352–357. | ||
Mornex F, Girard N, Beziat C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies – mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. 2006;66(4):1152–1158. | ||
Tse RV, Guha C, Dawson LA. Conformal radiotherapy for hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008;67(2):113–123. | ||
Rim CH, Yang DS, Park YJ, Yoon WS, Lee JA, Kim CY. Effectiveness of high-dose three-dimensional conformal radiotherapy in hepatocellular carcinoma with portal vein thrombosis. Jpn J Clin Oncol. 2012;42(8):721–729. | ||
Toya R, Murakami R, Baba Y, et al. Conformal radiation therapy for portal vein tumor thrombosis of hepatocellular carcinoma. Radiother Oncol. 2007;84(3):266–271. | ||
Huang YJ, Hsu HC, Wang CY, et al. The treatment responses in cases of radiation therapy to portal vein thrombosis in advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;73(4):1155–1163. | ||
Xu LT, Zhou ZH, Lin JH, et al. Clinical study of transarterial chemoembolization combined with 3-dimensional conformal radiotherapy for hepatocellular carcinoma. Eur J Surg Oncol. 2011;37(3):245–251. | ||
Merle P, Mornex F. [Transarterial chemoembolization and conformal radiotherapy for hepatocellular carcinoma]. Cancer Radiother. 2011;15(1):69–71. French. | ||
Park MK, Gwak GY, Lim DH, et al. The efficacy of combined transarterial chemoembolization and 3-dimensional conformal radiotherapy for hepatocellular carcinoma with main portal vein thrombosis. Hepatogastroenterology. 2010;57(101):801–806. | ||
ShuQun C, Mengchao W, Han C, et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54(74):499–502. | ||
Shi J, Lai EC, Li N, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18(1):74–80. | ||
Niu ZJ, Ma YL, Kang P, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29(4):2992–2997. | ||
Zeng ZC, Jiang GL, Wang GM, Tang ZY, Curran WJ, Iliakis G. DNA-PKcs subunits in radiosensitization by hyperthermia on hepatocellular carcinoma hepG2 cell line. World J Gastroenterol. 2002;8(5):797–803. | ||
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. | ||
Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62–67. | ||
Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. | ||
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. | ||
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. | ||
Nakazawa T, Hidaka H, Takada J, et al. Early increase in alpha-fetoprotein for predicting unfavorable clinical outcomes in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol. 2013;25(6):683–689. | ||
Trevisani F, De Notariis S, Rossi C, Bernardi M. Randomized control trials on chemoembolization for hepatocellular carcinoma: is there room for new studies. J Clin Gastroenterol. 2001;32(5):383–389. | ||
Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS. Chemoembolization of hepatocellular carcinoma: results of a meta-analysis. Am J Clin Oncol. 2003;26(4):344–349. | ||
Seong J, Park HC, Han KH, et al. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47(5):1331–1335. | ||
Yoon SM, Lim YS, Won HJ, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82(5):2004–2011. | ||
Liver Cancer Study Group of Japan. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer. 1994;74(10):2772–2780. | ||
Manghisi G, Elba S, Mossa A, et al. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology. 1998;28(3):751–755. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.