Back to Journals » Cancer Management and Research » Volume 12
Efficacy of the Preoperative Albumin–Bilirubin Grade for Predicting Survival and Outcomes of Postoperative Chemotherapy for Advanced Gastric Cancer
Authors Zhu C, Wang X, Chen S, Yang X, Sun J, Pan B, Zhang W, Chen X, Huang Y
Received 31 August 2020
Accepted for publication 6 November 2020
Published 20 November 2020 Volume 2020:12 Pages 11921—11932
DOI https://doi.org/10.2147/CMAR.S279782
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Ce Zhu,* Xiang Wang,* Sian Chen,* Xinxin Yang, Jing Sun, Bujian Pan, Weiteng Zhang, Xiaodong Chen, Yingpeng Huang
Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingpeng Huang Department of Gastrointestinal Surgery
The Second Affiliated Hospital of Wenzhou Medical University, No. 109 West College Road, Wenzhou, Zhejiang Province People’s Republic of China
Tel +86 13587616686
Email [email protected]
Purpose: The liver function index can predict the prognosis of hepatocellular carcinoma and many other non-neoplastic diseases. We aimed to determine whether the preoperative albumin–bilirubin (ALBI) grade could predict the prognosis of patients with gastric cancer (GC).
Patients and Methods: Data of 243 patients with GC who underwent radical resection were collected retrospectively. Patients were divided into the high ALBI (>− 2.34) and low ALBI (≤− 2.34) grade groups. Overall survival was analyzed between the two groups using the Kaplan–Meier curves. Univariate and multivariate analyses identified the independent factors associated with postoperative complications and overall survival.
Results: The postoperative complication rates were higher in the high ALBI grade group than in the low ALBI grade group (P=0.005). The high ALBI grade group also had worse overall survival (P< 0.001), especially TNM stage II–III patients (stage II, P=0.043; stage III, P< 0.001). In the high ALBI grade group, patients with TNM stage III not undergoing chemotherapy had significantly worse survival times (P=0.001). High ALBI grade (P=0.032), Charlson score of 1– 2 (P=0.007), and laparotomy surgery (P=0.045) were independent risk factors for postoperative complications. High ALBI grade (P=0.005), age ≥ 70 years (P=0.002), nutritional risk screening score 2002 score of 5– 6 (P=0.019), tumor located in the cardia (P=0.020), diffuse tumor (P< 0.001), and TNM stage III (P< 0.001) were independent risk factors for overall survival.
Conclusion: Preoperative ALBI grade could predict postoperative complications and overall survival of patients with GC, especially those with TNM stages II–III. This grading method has the advantages of preoperative availability, simplicity, and objectivity and aids in improving preoperative prognosis prediction and in achieving better outcomes of postoperative chemotherapy.
Keywords: gastric cancer, albumin–bilirubin, liver function, prognosis, postoperative complications
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide. In 2018, >1,000,000 new patients were diagnosed with GC and approximately 780,000 deaths occurred.1 Asia has the highest incidence of GC in the world.1 Only two countries in Asia, Japan and South Korea, have screening programs for high-risk groups to detect early-stage GC,2 but the clinical evidence of endoscopic screening is not sufficient to recommend its use on a global scale.3 Many patients are often diagnosed only when there is local invasion of the tumor or distant metastases because early-stage GC has atypical symptoms.2
At present, surgical-based comprehensive treatment is still the main treatment of advanced GC.4 The prognosis of postoperative GC is often one of the most concerning issues among patients and clinicians. Studies have reported that risk factors, such as preoperative serum albumin (ALB), preoperative electrolytes, and preoperative nutritional status, predict the prognosis of GC.5–7 However, there is still no simple, effective, and objective indicator to predict the prognosis after a gastrectomy.
The albumin–bilirubin (ALBI) grade was originally developed to assess the liver function in patients with hepatocellular carcinoma (HCC).8 Further studies have found that the ALBI grade could predict the prognosis of patients with HCC.9,10 Using only two objective indexes, ie ALB and total bilirubin (TB), which can reflect the synthesis and metabolic function of the liver, the ALBI grading method has the advantage of being low cost, easily implemented, and objective.8 It is reported that nutritional status could affect the prognosis of GC patients after radical resection.11,12 The liver is the main site for the synthesis and metabolism of nutrients, and an abnormal liver function will affect the nutritional status of the body. In addition, liver function is closely related to the tolerance for postoperative chemotherapy.13 Therefore, we want to know whether the preoperative ALBI grade can affect the prognosis of patients with GC. A previous study has found that ALBI grade can predict the long-term prognosis of GC.14 However, this study only found that ALBI grade can predict the long-term prognosis of GC, and whether ALBI grade can predict postoperative complications in patients with GC is still unclear. The aim of this study was to comprehensively analyze whether the preoperative ALBI grade had a significant influence on the short-term and long-term prognoses of patients with GC who underwent radical resection, and to provide an effective preoperative assessment tool for clinicians.
Materials and Methods
Inclusion and Exclusion Criteria
Data of GC patients who underwent radical resection and perigastric lymph node dissection according to the Japanese Gastric Cancer Treatment Guidelines in the 2nd Affiliated Hospital of Wenzhou Medical University between December 2014 and December 2016 were collected retrospectively in this analysis.15 After undergoing radical resection, GC patients are recommended to undergo adjuvant S-1 monotherapy or S-1- based combined chemotherapy.16,17 The exclusion criteria for selecting the study participants are as follows: (1) Patients who had undergone palliative surgery; (2) those with liver infiltration; (3) those who had received neoadjuvant chemotherapy before the operation (patients with tumor, node, metastasis (TNM) stage IV GC were not included in this study); (4) those with serious organic liver diseases or other tumors; and (5) those who were lost to follow-up and followed for less than 1 year. Outpatient or telephone follow-ups were carried out every 3 months after hospital discharge.
Clinical Parameters and Laboratory Results
The following data were obtained from the medical records: (1) clinical parameters, including age, sex, American Association of Anesthesiologists (ASA) classification, body mass index (BMI), nutritional risk screening 2002 (NRS-2002) score;18 (2) operation record, including combined organ resection, laparoscopic assisted surgery, the number of positive lymph nodes dissected; (3) postoperative outcomes, including postoperative complications (Clavien-Dindo grade ≥II considered as postoperative complications),19 hospitalization cost, and postoperative hospital stay; (4) laboratory results, including biochemical data (TB and ALB); and (5) follow-up data (all patients were followed until October 2019 or death).
Blood samples from all enrolled patients were collected within 7 days before the operation. The ALBI grade was calculated as follows: ALBI = (log10 TB concentration × 0.66) + (ALB concentration × −0.085), (the unit of TB is μmol/L; the unit of ALB concentration is g/L).8 Two researchers evaluated any complications that occurred within 30 days after the operation according to the postoperative complication grading system of Clavien-Dindo,19 and a third researcher reassessed the differences in the classification of complications.
Statistical Method
X-tile software (version 3.6.1, Yale University, USA) was used to determine the optimal cut-off value for the ALBI grade,20 which was set at −2.34. We divided the GC patients into two groups based on their ALBI grade (low ALBI grade group, ≤−2.34 and high ALBI grade group, >−2.34). Continuous data were expressed as mean (n) ± interquartile range (IQR) or mean ± standard deviation (SD) depending on their normality. Categorical variables were expressed as the number of patients (percentage). Independent sample student's t-test or non-parametric test was used to analyze the continuous data. Chi-square test or Fisher’s exact test (theoretical number less than 5) was used to analyze the categorical variables. Univariate and multivariate analyses of various clinicopathological factors for postoperative complications were carried out using binary logistic regression analysis. Overall survival (OS) was calculated from the date of surgery to the date of death or the last available follow-up. The survival curve of the high ALBI grade group versus the low ALBI grade group was compared using the Kaplan–Meier method, and the Log rank test was used to analyze the significance. Univariate and multivariate analyses for OS were carried out using Cox regression analysis. The possible risk factors that were significant in the univariate analysis were included in the multivariate Cox proportional hazards model, and a forward stepwise selection: LR was performed. All tests were considered statistically significant at P<0.05 (two-sided), and all statistical analyses were performed using SPSS software (version 22.0 IBM Corp., Armonk, USA).
Results
Clinicopathological Characteristics of Patients
At the final follow-up in October 2019, among the 272 patients initially enrolled in this study, 77 patients had died, 27 patients were excluded because they were lost to follow-up or were followed up for less than 1 year, and 2 patients had died within the first month. Finally, we analyzed a total of 243 patients. The median follow-up period was 1077 (706–1553) days. Of the 243 patients, 141 (58.0%) had a high ALBI grade and 102 (42%) had a low ALBI grade. The clinicopathological features of these patients with GC are shown in Table 1. The high ALBI grade group was older (P<0.001) and had a higher NRS-2002 score than the low ALBI grade group (P=0.018). The high ALBI grade group had a lower BMI than the low ALBI grade group (P=0.015). There were no significant differences in sex, hypertension, diabetes, history of abdominal surgery, TNM stage, tumor differentiation, and tumor location between the two groups.
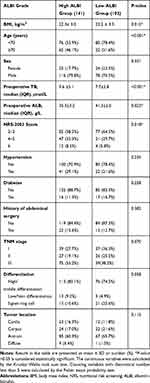 |
Table 1 Clinical Data Table of ALBI Grade |
Short-Term Postoperative Complications
As shown in Table 2, 63 (25.9%) patients had postoperative complications. The complication and severe complication (Clavien-Dindo grade ≥III considered as severe complications) rates were higher in the high ALBI grade group than in the low ALBI grade group (32.6% versus 16.7%, P=0.005; 11.3% versus 3.9%, P=0.038, respectively). For the surgical and medical complications, the surgical complication rate was significantly higher in the high ALBI grade group than in the low ALBI grade group (27.0% versus 15.7%, P=0.035). The high ALBI grade group also had a higher medical complication rate than the low ALBI grade group (15.6% versus 11.8%), but the difference was not statistically significant (P=0.395).
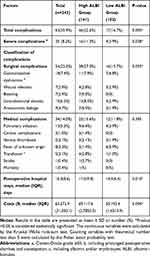 |
Table 2 Postoperative Outcomes |
The results of the univariate and multivariate analyses for postoperative complications are shown in Table 3. The univariate analysis revealed that a high ALBI grade (odds ratio [OR] 2.4, P=0.006), ASA score of 3–4 (OR 2.4, P=0.009), Charlson score of 1–2 (OR 2.7, P=0.002), Billroth II anastomosis (OR 2.7, P=0.029), Roux-en-Y anastomosis (OR 2.5, P=0.009), TNM stage III (OR 2.8, P=0.006), and laparotomy surgery (OR 2.7, P=0.015) were associated with postoperative complications. In the multivariate analysis, a high ALBI grade (OR 2.0, 95% confidence interval [CI]: 1.1–3.9, P=0.032), Charlson score of 1–2 (OR 2.3, 95% CI 1.3–4.4, P=0.007), and laparotomy surgery (OR 2.3, 95% CI: 1.0–5.4, P=0.045) were independent risk factors for postoperative complications.
 |
Table 3 Univariate/Multivariate Analysis Table of Postoperative Complications |
Postoperative Overall Survival
As shown in Figure 1, the high ALBI grade group had a shorter OS than the low ALBI grade group (median survival time of 33.8 versus 39.8 months, P<0.001). When staging by TNM, the survival time was significantly worse in the high ALBI grade patients with TNM stages II and III than in the low ALBI grade patients with the same stages (stage II, median survival time in high ALBI grade versus low ALBI grade was 33.9 months versus 41.9 months, P=0.043; stage III, median survival time in high ALBI grade versus low ALBI grade was 24.1 months versus 40.4 months, P<0.001). Among patients with TNM stage I, there was no significant difference in OS between the high and low ALBI grade groups (median survival time was 41.2 months versus 38.7 months, P=0.900).
Univariate analysis demonstrated that a high ALBI grade (hazard ratio [HR] 3.2, P<0.001), age≥70 years (HR 2.9, P<0.001), NRS-2002 score of 5–6 (HR 4.3, P<0.001), ASA score of 3–4 (HR 2.6, P<0.001), Charlson comorbidity index of 1–2 (HR 1.9, P=0.007), Charlson comorbidity index of 3–6 (HR 3.1, P=0.011), diffuse tumors (HR 9.1, P<0.001), and TNM stage III (HR 9.3, P<0.001) are associated with a worse prognosis. In the multivariate analysis, a high ALBI grade (HR 2.3, 95% CI: 1.3–4.1, P=0.005), age ≥70 years (HR 2.2, 95% CI: 1.3–3.6, P=0.002), NRS-2002 score of 5–6 (HR 2.3, 95% CI: 1.1–4.6, P=0.019), tumor located in the cardia (HR 2.1, 95% CI: 1.1–3.8, P=0.020), diffuse tumors (HR 3.0, 95% CI: 2.8–13.2, P<0.001), and TNM stage III (HR 9.5, 95% CI: 4.0–22.1, P<0.001) were independent risk factors for OS (Table 4).
 |  |  |
Table 4 Univariate/Multivariate Analysis Table of Overall Survival |
Influence of Chemotherapy Outcome
As shown in Figure 2, patients with TNM stage III had significantly worse survival times. The median survival time was 40.7 months in patients with a low ALBI grade who were undergoing chemotherapy, 39.6 months in patients with a low ALBI grade who were not undergoing chemotherapy (P=0.760); 27.4 months in patients with a high ALBI grade who were undergoing chemotherapy, and 12.5 months in patients with a high ALBI grade who were not undergoing chemotherapy (P=0.001). Among the patients with TNM stage I, the OS was not significantly different between patients with a low ALBI grade with and without chemotherapy (median survival time: 42.0 versus 36.5 months P=0.320), and between patients with a high ALBI grade with and without chemotherapy (37.4 versus 41.3 months, P=0.991). Among the patients with TNM stage II, the OS was 42.7, 34.3, 39.3 and 32.3 months in patients with a low ALBI grade with chemotherapy, those with a low ALBI grade without chemotherapy (versus patients with a low ALBI grade with chemotherapy, P=0.409), those with a high ALBI grade with chemotherapy, and those with a high ALBI grade without chemotherapy (versus patients with a high ALBI grade with chemotherapy, P=0.200).
Discussion
The preoperative ALBI grading system, as a new method for assessing liver function, has been validated in many retrospective studies.8,21 A large number of studies have shown that the preoperative ALBI grade could predict the prognosis of HCC.9,22 Apart from HCC cases, a study also showed that the ALBI grade had a great ability to predict recurrences after radical gastrectomy in patients with pT2-4 GC.14 In the current study, we first tried to comprehensively analyze the ability of preoperative ALBI grade to predict the long- and short-term prognoses of patients with GC. As expected, we found that the preoperative ALBI grade can also predict the long- and short-term prognoses of these patients. The preoperative ALBI grading method has the advantages of preoperative accessibility, convenience, and low cost; thus, it is expected to be a good biomarker for predicting the prognosis of advanced GC.
In this study, the incidence of postoperative complications, especially the surgical complications and severe complications, length of postoperative hospital stay, and hospitalization cost was higher in the high ALBI grade group than in the low ALBI grade group. These findings are similar to the results of a previous study reporting that ALBI grade is associated with bile leakage and liver failure after hepatectomy.23 Furthermore, a previous study showed that preoperative hypoproteinemia was an independent risk factor for incision infection after gastrointestinal surgery and was associated with longer hospital stay.24 In contrast, Mitsuro Kanda et al reported that preoperative ALBI grades did not predict the postoperative complications in patients with pT2-4 GC.14 The reason for this result may be due to the different definition of postoperative complications used in their study. The mechanism of the influence of preoperative ALBI on postoperative complications may be related to the decreased ability of liver albumin synthesis in patients with a high ALBI grade, which results in malnutrition. In our study, we found that the high ALBI group had lower BMI and higher NRS-2000 score than the low ALBI group. Malnutrition can lead to increased susceptibility to infection, damaged blood coagulation, vascular wall fragility, prolonged wound healing, and increased risk for postoperative complications.25,26 In addition, patients with a high ALBI grade are generally older, which is one of the reasons for the high incidence of postoperative complications in this patient group.
Another concern is the effect of ALBI on the long-term prognosis of patients with GC. In this study, we analyzed the influence of the ALBI grade on OS in different TNM stages, and we found that a high ALBI grade had a significant effect on the OS of GC patients with TNM stages II and III. While in TNM stage I GC, no significant association was found between ALBI and OS. This may be because the long-term prognosis of GC patients with TNM stage I is good and, in our data-set, the number of patients with TNM stage I is relatively small, with only six deaths occurring in this stage. Owing to the insufficient sample size of GC patients with stage I, the existing data can-not support the correlation between ALBI and OS. Or ALBI grade itself lacks prognostic value in patients with early stage of gastric cancer, which should be validated in further multicenter and large-sample studies. A previous study also reported similar results and concluded that preoperative ALBI grade could affect the long-term survival of patients with pT2-4 GC.14 The mechanism of how the ALBI grade affects the long-term survival of patients with GC can be explained from two aspects. The first one is the long-term damage caused by malnutrition. As mentioned previously, a high ALBI grade is usually accompanied by malnutrition. Malnutrition is the main cause of secondary immunodeficiency.27 Malnutrition affects T cell-mediated immune response, cytokines production and the ability of lymphocytes to respond appropriately to cytokines.28,29 Protein and energy malnutrition not only can cause muscle mass and weight loss but also can lead to the decrease in interleukin (IL)-2, IL-3, and helper T cells, which leads to the decline of the immune function, thereby accelerating tumor progression.30–32 Some related studies have also proved that preoperative malnutrition has an adverse effect on the long-term prognosis of GC patients.11,12 The second one is the effect of high ALBI grade on chemotherapy tolerance. A previous study has found that malnutrition and liver dysfunction are the main factors leading to adverse reactions to chemotherapy.13 Postoperative adjuvant chemotherapy has survival benefits to patients with advanced GC.33,34 However, due to the intolerance for chemotherapy in patients with a high ALBI grade, they refuse to undergo postoperative adjuvant chemotherapy or discontinue their chemotherapy halfway, resulting in a low survival rate among patients with a high ALBI grade.14,35 This is consistent with the results of our study showing that patients with stage III GC with a high ALBI grade had the worst prognosis, but they could gain more benefits from chemotherapy. In addition, older age may also affect the long-term survival of GC patients with a high ALBI grade.
This study has some limitations. First, this is a single-center retrospective study. We did not validate our risk model externally or internally. Second, the current analysis only includes preoperative ALBI levels, and the dynamic changes of ALBI levels during treatment may affect the prognosis of patients with GC. In the future, we will study the significance of dynamic ALBI changes in the treatment of gastric cancer. Intervention of preoperative ALB and TB to improve chemotherapy tolerance and long-term efficacy requires prospective clinical trials.
Conclusion
The preoperative ALBI grade could predict postoperative complications and OS of patients with GC after operation, especially those with TNM stages II–III. Patients with different ALBI grade can be divided into either a poor or a good prognosis group. The results of the multivariate analysis showed that a high ALBI grade was an independent risk factor for postoperative complications and OS. The ALBI grading method has the advantages of preoperative availability, simplicity, and objectivity and aids in improving preoperative prognosis prediction and in making better treatment plans after surgery.
Abbreviations
ALBI, albumin–bilirubin; GC, gastric cancer; ALB, albumin; TB, total bilirubin; HCC, hepatocellular carcinoma; TNM, tumor-node-metastasis; ASA, American Association of Anesthesiologists; BMI, body mass index; IQR, interquartile range; SD, standard deviation; OS, overall survival.
Ethics Approval and Informed Consent
This study was carried out in accordance with the guidelines stipulated in the Declaration of Helsinki. Patient consent was provided by all patients. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University.
Acknowledgments
This study was funded by the Zhejiang Province Medical and Health Science and Technology Project, a Study on the Mechanism of Nuclear-located UBR5 Promoting the Expression and Regulation of PYK2 in Straight Cancer Cells (No.2019317606), Study on the Effect and Mechanism of SCFA Butyrate-GPR109A Axis in Promoting Apoptosis of Gastric Cancer in Wenzhou Science and Technology Bureau (No.Y20190060), and Zhejiang Province commonwealth Technology Research Program/Social Development Project, a Study on the Mechanism of GPR109A-mediated Probiotic Metabolite Butyric Acid Promoting Autophagy-induced Apoptosis of Gastric Cancer Cells (No. LGF20H070003). We would also like to thank Editage for English language editing.
Disclosure
The authors declare that they have no competing interests related to this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Shen L, Shan Y-S, Hu H-M, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14(12):e535–e547. doi:10.1016/S1470-2045(13)70436-4
3. Kamangar F, Dores G, Anderson W. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J clin oncol. 2006;24(14):2137–2150. doi:10.1200/JCO.2005.05.2308
4. f0001gProgress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39(7):1010428317714626.
5. Takama T, Okano K, Kondo A, et al. Predictors of postoperative complications in elderly and oldest old patients with gastric cancer. Gastric Cancer. 2015;18(3):653–661. doi:10.1007/s10120-014-0387-6
6. Xu J, Chen X, Wang X, et al. Preoperative hyponatremia and hypocalcemia predict poor prognosis in elderly gastric cancer patients. Cancer Manag Res. 2019;11:8765–8780.
7. SL W, CL Z, DD H, et al. Sarcopenia adversely impacts postoperative clinical outcomes following gastrectomy in patients with gastric cancer: a prospective study. Ann Surg Oncol. 2016;23(2):556–564. doi:10.1245/s10434-015-4887-3
8. PJ J. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J clin oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
9. YY W, JH Z, ZY S, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(6):725–734. doi:10.1002/bjs.10095
10. Ma XL, Zhou JY, Gao XH, et al. Application of the albumin-bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinoma. Clin Chim Acta. 2016;462:15–22.
11. Kanda M, Mizuno A, Tanaka C, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine. 2016;95(24):e3781. doi:10.1097/MD.0000000000003781
12. Jiang N, Deng J, Ding X, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537–10544. doi:10.3748/wjg.v20.i30.10537
13. Ramadori G, Cameron S. Effects of systemic chemotherapy on the liver. Ann Hepatol. 2010;9(2):133–143. doi:10.1016/S1665-2681(19)31651-5
14. Kanda M, Tanaka C, Kobayashi D, et al. Preoperative albumin-bilirubin grade predicts recurrences after radical gastrectomy in patients with pt2-4 gastric cancer. World J Surg. 2018;42(3):773–781. doi:10.1007/s00268-017-4234-x
15. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19.
16. Kanda M, Murotani K, Kobayashi D, et al. Postoperative adjuvant chemotherapy with S-1 alters recurrence patterns and prognostic factors among patients with stage II/III gastric cancer: A propensity score matching analysis. Surgery. 2015;158(6):1573–1580. doi:10.1016/j.surg.2015.05.017
17. Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized Phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J clin oncol. 2011;29(33):4387–4393. doi:10.1200/JCO.2011.36.5908
18. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clinical Nutrition. 2003;22(3):321–336. doi:10.1016/S0261-5614(02)00214-5
19. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
20. RL C, D-F M, DL R. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clinical Cancer Research. 2004;10(21):7252–7259. doi:10.1158/1078-0432.CCR-04-0713
21. DJ P. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–346. doi:10.1016/j.jhep.2016.09.008
22. Ma X, Zhou J, Gao X, et al. Application of the albumin-bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinoma. Clin Chim Acta. 2016;462:15–22. doi:10.1016/j.cca.2016.08.005
23. Andreatos N, Amini N, Gani F, et al. Albumin-bilirubin score: predicting short-term outcomes including bile leak and post-hepatectomy liver failure following hepatic resection. J Gastrointestinal Surgery. 2017;21(2):238–248. doi:10.1007/s11605-016-3246-4
24. Hennessey D, Burke J, Ni-Dhonochu T, Shields C, Winter D, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. 2010;252(2):325–329. doi:10.1097/SLA.0b013e3181e9819a
25. Ryan A, Reynolds J, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249(3):355–363. doi:10.1097/SLA.0b013e31819a4789
26. Song G, Tian X, Liang H, et al. Role of enteral immunonutrition in patients undergoing surgery for gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2015;94(31):e1311. doi:10.1097/MD.0000000000001311
27. Rodríguez L, Cervantes E, Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health. 2011;8(4):1174–1205. doi:10.3390/ijerph8041174
28. Pelletier DL, Frongillo EA
29. Rodríguez L, González C, Flores L, Jiménez-Zamudio L, Graniel J, Ortiz R. Assessment by flow cytometry of cytokine production in malnourished children. Clin Diagn Lab Immunol. 2005;12(4):502–507. doi:10.1128/CDLI.12.4.502-507.2005
30. González-Torres C, González-Martínez H, Miliar A, et al. Effect of malnutrition on the expression of cytokines involved in Th1 cell differentiation. Nutrients. 2013;5(2):579–593. doi:10.3390/nu5020579
31. Migita K, Takayama T, Saeki K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20(8):2647–2654. doi:10.1245/s10434-013-2926-5
32. Thurnham D. Interactions between nutrition and immune function: using inflammation biomarkers to interpret micronutrient status. Proc Nutr Soc. 2014;73(1):1–8. doi:10.1017/S0029665113003662
33. Kanda M, Kodera Y, Sakamoto J. Updated evidence on adjuvant treatments for gastric cancer. Expert Rev Gastroenterol Hepatol. 2015;9(12):1549–1560. doi:10.1586/17474124.2015.1094373
34. Noh S, Park S, Yang H, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised Phase 3 trial. Lancet Oncol. 2014;15(12):1389–1396. doi:10.1016/S1470-2045(14)70473-5
35. Iizuka A, Kanda M, Ito S, et al. Proposal of a scoring scale to estimate risk of the discontinuation of s-1 adjuvant monotherapy in patients with stage ii to iii gastric cancer: a multi-institutional dataset analysis. World J Surg. 2019;43(8):2016–2024. doi:10.1007/s00268-019-04942-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


