Back to Journals » Clinical and Experimental Gastroenterology » Volume 12
Efficacy of the four weeks treatment of omeprazole plus mosapride combination therapy compared with that of omeprazole monotherapy in patients with proton pump inhibitor-refractory gastroesophageal reflux disease: a randomized controlled trial
Authors Sirinawasatien A , Kantathavorn N
Received 6 May 2019
Accepted for publication 16 July 2019
Published 26 July 2019 Volume 2019:12 Pages 337—347
DOI https://doi.org/10.2147/CEG.S214677
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Everson L.A. Artifon
Apichet Sirinawasatien, Nontapat Kantathavorn
Division of Gastroenterology, Department of Medicine, Rajavithi Hospital, College of Medicine, Rangsit University, Bangkok 10400, Thailand
Purpose: The aim of this study was to compare the effect of omeprazole plus mosapride combination therapy with that of omeprazole monotherapy in proton pump inhibitor (PPI) refractory gastroesophageal reflux disease (GERD) patients.
Patients and methods: Patients were eligible to participate in this study if they had experienced symptoms of heartburn and/or regurgitation more than twice weekly and were unresponsive to at least 8 weeks of a standard dose of PPI. A total of 44 consecutive patients were randomized to receive omeprazole 20 mg once daily plus either mosapride 5 mg or placebo three times daily for 4 weeks. We evaluated their clinical symptoms by means of frequency scale for symptoms of GERD (FSSG) questionnaires completed at the beginning and the end of the study. The primary outcome was to compare changes in FSSG scores between treatment groups during the study period.
Results: Most of the study population had non-erosive reflux disease (91.0% in the combination group and 81.8% in the control group). The minority of patients had Los Angeles grade A or B erosive esophagitis (9% in the combination group and 18.2% in the control group). None of the patients had Los Angeles grade C or D erosive esophagitis. FSSG total scores significantly decreased both in the combination group and the control group, with no significant differences in improvement between the groups (−8.00±7.18 for the combination group versus −5.68±6.29 for the control group, p=0.129). As a secondary outcome, our data showed that the effect of combination therapy on a number of symptom-free days (heartburn-free days, regurgitation-free days, and night-time heartburn-free days) was not superior to PPI monotherapy.
Conclusion: Combining mosapride for four weeks with a standard dose of PPI is not more effective than PPI alone in patients with PPI-refractory GERD.
Keywords: mosapride, proton pump inhibitors, gastroesophageal reflux
Background
Gastroesophageal reflux disease (GERD) comprises a spectrum of clinical presentations in which gastric content refluxes into the esophagus cause troublesome symptoms with or without visible damage to the esophageal mucosa.1 It is a common clinical disorder with an estimated prevalence of 9–28% in Europe and North America, and 5–18% in Asia.2,3 Proton pump inhibitor (PPI) is highly efficacious in providing symptomatic relief, healing erosions and improving quality of life in patients with GERD,4 but there are still unmet clinical needs. The recent study has shown that prolonging PPI therapy from 4 weeks to 8 weeks does not increase the symptom response rate, however, reduces symptom relapse in patients with Los Angeles grade A or B erosive esophagitis.5
PPI-refractory GERD refers to patients with symptoms of GERD who do not respond, or only partially respond, to therapy. The definition of refractory GERD is controversial, however, according to the Asia-Pacific consensus on the management of GERD, it may be defined as persistent and troublesome GERD symptoms unresponsive to at least 8 weeks of a standard dose of PPI.6 Several mechanisms have been proposed for the pathogenesis of refractory GERD, including weakly acidic reflux, delayed gastric emptying and concomitant functional bowel disorders.7
The prokinetic agent cisapride, which is a 5HT-4 receptor agonist, was previously shown to have a synergistic effect with PPI on maintenance therapy for reflux esophagitis,8 but it has been found to be associated with potentially fatal heart arrhythmia. However, mosapride, which is also a 5-HT4 receptor agonist, is an alternative prokinetic agent that can be safely used in patients with various gastrointestinal disorders.9,10 It acts by increasing acetylcholine release from parasympathetic nerve endings and stimulating esophageal motility as well as gastric emptying.11,12 A previous study reported that mosapride with pantoprazole combination therapy was more effective than pantoprazole monotherapy in providing symptomatic relief to patients with erosive GERD, but that it offered no benefit over pantoprazole monotherapy in non-erosive reflux disease (NERD) patients.13 Another study of PPI-refractory patients found that administration of mosapride in addition to omeprazole improved gastroesophageal reflux symptoms and gastric emptying in PPI-refractory NERD patients with delayed gastric emptying, determined by the13C-acetate breath test.12
A recent systematic review aimed at assessing the potential benefits of mosapride plus PPI in the treatment of GERD found that mosapride combined therapy is no more effective than PPI alone as a first-line therapy. Whether it is effective in PPI-refractory patients still remains to be determined;14 therefore, in this study, we aimed to investigate whether omeprazole plus mosapride combination therapy was more effective than omeprazole monotherapy in achieving symptom relief in PPI-refractory GERD patients using the frequency scale for symptoms of GERD (FSSG) questionnaire.
Methods
Study design
This was a prospective, randomized, double-blind, placebo-controlled trial conducted from January 2016 to January 2018 at the out-patient clinic of the Department of Medicine at Rajavithi Hospital, a tertiary referral center in Bangkok, Thailand. It was performed in accordance with the clinical principles laid down in the Declaration of Helsinki and informed consent was obtained from all the patients before their enrollment. The study protocol was reviewed and approved by the ethics committee of Rajavithi Hospital (clinicaltrials.in.th number, TCTR20190418003) and all participants provided written informed consent.
Participants
The inclusion criteria were patients who (i) were aged more than 18 years; (ii) were diagnosed as having GERD by the presence at least twice a week of heartburn, defined as a burning sensation in the retrosternal area, and/or regurgitation, defined as the perception of flow of refluxed gastric content into the mouth or hypopharynx;1 and (iii) had received a standard dose of PPI for at least 8 weeks and still had FSSG total score (FSSG-TS) of eight points or more (Table S1), which is defined as having refractory GERD with substantial symptoms.15
We excluded patients who (i) had a previous history of esophageal or gastrointestinal surgery apart from appendectomy and cholecystectomy; (ii) had severe cardiac or pulmonary disease, renal failure, liver cirrhosis or severe systemic illness; (iii) had an active Helicobacter pylori infection, determined by both the rapid urease test and histological identification, or upper gastrointestinal diseases such as malignancy or peptic ulcers; (iv) were in a state of pregnancy or lactating; or (v) were hypersensitive or allergic to mosapride or omeprazole. Concomitant medications such as anti-hypertensive drugs were allowed if the dosage was not changed during the study period. Medications that affect esophageal motility or gastric acid secretion, eg prokinetics, PPIs, H2 receptor antagonists, cholinergic and⁄or anti-cholinergic agents, were discontinued 1 week before the start of the study.
The sample size was calculated using two independent means formula (two-tailed test), based on data from a previous report16 in which significant improvement in the FSSG-TS was observed after the addition of prokinetics for 4 weeks in PPI non-responsive NERD patients (FSSG-TS; initial 14.6±6.0 vs after 4 weeks of PPI plus prokinetics 7.7±5.2, p<0.0001). The minimum required number of participants was calculated at 11 in each group in order to detect a significant association with 80% power (β =0.20) and a 5% probability of type I error (2-sided) (α =0.05).
Endoscopic assessment
Upper GI endoscopies had been conducted on all the patients within the 3-month period prior to their enrollment by two endoscopists (NK & AS). Endoscopic findings were graded using the Los Angeles (LA) classification, with mucosal breaks classified as erosive GERD of LA grades A-D.17
Grade A: One (or more) mucosal break no longer than 5 mm that does not extend between the tops of two mucosal folds
Grade B: One (or more) mucosal break more than 5 mm long that does not extend between the tops of two mucosal folds
Grade C: One (or more) mucosal break that is continuous between the tops of two or more mucosal folds but which involves less than 75% of the esophageal circumference
Grade D: One (or more) mucosal break which involves at least 75% of the esophageal circumference
Patients found to have no esophageal mucosal breaks were classified as having NERD. Sliding hiatal hernia was diagnosed when the apparent separation between the squamocolumnar junction and the diaphragmatic impression was greater than 2 cm as measured using the hash marks on the endoscope relative to the incisors.18
Symptom assessment
Patients were evaluated for severity of symptoms using the FSSG questionnaire (Table S1). This is a validated instrument consisting of 12 questions developed specifically to evaluate GERD symptoms and their response to medical treatment, and it has been widely used in Japan and other countries.15,19,20 All patients were asked to supply answers to the questionnaire via a single investigator (NK) in order to control inter-observer variability in questioning consistency. Each question was rated using a five-point Likert scale, indicating the frequency of their symptoms as follows: never (0%) =0; occasionally (around 30% of the time) =1; sometimes (50%) =2; often (70%) =3; and always (100%) =4. The investigator (NK) was blinded to the type of medication that patients received while assessing their symptoms before, and 4 weeks after, treatment.
All patients were given a diary (Table S2) in which to record their symptoms every day. The diary comprised three questions regarding symptoms of: (i) heartburn; (ii) regurgitation; and (iii) night-time heartburn. The patients were instructed to record their symptoms, or lack of them, in the diary each day.
Randomization and study protocol
After informed consent had been obtained, eligible patients were randomized into two treatment groups to receive omeprazole 20 mg (Miracid®, Berlin Pharmaceutical Industry Co. Ltd., Bangkok, Thailand) once daily plus either one tablet of mosapride 5 mg (Gasmotin®, Dainippon Sumitomo Pharmaceutical Co. Ltd., Osaka, Japan) or a placebo of similar appearance three times daily for 4 weeks. Randomization was performed using a computer-generated list of random numbers. An independent assistant gave mosapride or placebo to participants according to consecutive numbers kept in sealed envelopes. Use of drugs which could affect gastrointestinal motility and gastric acid secretion was not allowed during the study period.
The enrolled patients were evaluated using FSSG questionnaires at the beginning of the study and were given a diary in which to record their symptoms every day. After completing 4 weeks of medical therapy, a second FSSG questionnaire was completed, and any reported adverse events, including severity and duration of symptoms, were checked at this visit (Figure 1).
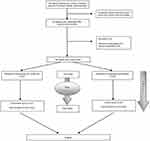 |
Figure 1 Participants flow diagram. |
Outcomes
Clinical symptoms were evaluated using FSSG-TS, FSSG-RS, and FSSG-DS before, and 4 weeks after, treatment. The primary outcome was to evaluate the difference in changes in FSSG scores between the two groups. The secondary outcome was to determine the number of symptom-free days (heartburn, regurgitation, and night-time heartburn) experienced by the patients during the study period, analyzed from their diary reports.
Statistical analysis
Statistical analyses were performed using the SPSS software version 17.0 (SPSS Inc., Chicago, IL). Demographic data and baseline characteristics were analyzed using descriptive statistics, and categorical variables were compared using Chi-square test or Fisher exact test as appropriate. Continuous variables were compared using the Independent t-test or the Mann-Whitney U test. As the FSSG questionnaire was considered as an interval scale, FSSG scores were expressed as mean ± S.D. or median (min-max). All statistical examinations were two-tailed with a p-value <0.05 defined as statistically significant.
Results
Baseline characteristics of patients
A total of 44 patients were enrolled in the study. Twenty-two were randomized into the omeprazole plus mosapride group (combination group) and the other 22 into the omeprazole plus placebo group (control group). All patients completed follow up and finally, 44 patients (n=44) were analyzed at the end of the study.
Demographic data and clinical characteristics of the two groups are shown in Table 1. Age, gender, body mass index (BMI) and comorbid diseases were similar in the two groups. Lifestyle factors, including smoking, alcohol drinking and coffee consumption in the two groups were also comparable. Most of the subjects had NERD (91.0% in the combination group and 81.8% in the control group). The duration of symptoms in the combination group was 35.77 months (SD 21.25) and in the control group 25.68 months (SD 18.62). There were no significant differences in clinical characteristics or pre-treatment medication in the two groups.
 |
Table 1 Baseline characteristics of patients |
Symptom improvement after treatment
Both treatment groups achieved similar significant symptomatic improvement after 4 weeks of therapy (FSSG-RS combination group −3.91±4.59 vs control group −3.45±4.77, P=0.595; FSSG-DS combination group −4.09±4.17 vs control group −2.23±3.13, P=0.109, and FSSG-TS combination group −8.00±7.18 vs control group −5.68±6.29, P=0.129) (Figure 2).
 |
Figure 2 Reduction of the FSSG score after 4 weeks of treatment between the omeprazole monotherapy group and the omeprazole plus mosapride combination therapy group. |
In the combination group, FSSG-TS decreased from 20.36±6.78 at baseline to 12.36±7.45 at week 4 (P<0.001). Similarly, FSSG-TS in the control group decreased from 16.91±7.57 at baseline to 11.23±7.02 at week 4 (P=0.001). FSSG-RS scores in both groups decreased: from 10.36±3.35 to 6.45±3.57 (P<0.001) in the combination group and from 8.91±4.64 to 5.45±3.20 (P=0.003) in the control group. Also, FSSG-DS scores in both groups improved: from 10.00±4.74 to 5.91±5.32 (P=0.001) in the combination group and from 8.00±4.15 to 5.77±4.70 (P=0.005) in the control group (Table 2). There were no statistically significant differences in clinical symptoms of the two groups pre- and post-treatment (Table 2).
 |
Table 2 Clinical symptoms at pre-treatment and post-treatment between the omeprazole monotherapy group and the omeprazole plus mosapride combination therapy group |
The number of symptom-free days
In our study, all patients were given a diary in which to record their symptoms, including heartburn, regurgitation and night-time heartburn. Two patients in the combination group and one in the control group lost their diaries, so only 20 patients in the combination group and 21 patients in the control group were analyzed.
The secondary outcome was to assess the number of days free from heartburn, regurgitation, and night-time heartburn, and these data are shown in Table 3. In the combination group, the patients had more regurgitation-free and night-time heartburn-free days than the patients in the control group, while patients in the control group had more heartburn-free days than their counterparts in the combination group, but these figures were not significantly different.
 |
Table 3 The number of symptom-free days of the patients during the study period |
Safety concerns
No patients who had been treated for 4 weeks reported any serious side effects before discontinuing the use of either mosapride or omeprazole.
Discussion
This randomized controlled trial of the efficacy of combining mosapride or a placebo with a standard dose of PPI in treating PPI-refractory GERD patients showed that GERD symptoms were ameliorated from the baseline with both the combination therapy and PPI monotherapy, as demonstrated by the reduction in FSSG scores (FSSG- RS, FSSG-DS, and FSSG-TS) after 4 weeks of treatment. There was a trend towards greater improvement in the combination group compared with the control group, but the differences were not statistically significant. In addition, we found no significant differences in the number of heartburn-free days, regurgitation-free days or night-time heartburn-free days between the two treatment groups.
The majority of our patients had NERD, and this is similar to other Asia-Pacific countries in which NERD accounts for 78–93% of all reflux disease.21 From a systematic review of the literature, the PPI symptomatic response pooled rate was only 36.7% in NERD patients and 55.5% in those with erosive reflux disease.22 Some investigators have postulated that delayed gastric emptying may be a contributory factor to PPI-refractoriness in NERD patients. Futagami et al12 evaluated gastric emptying in 20 healthy volunteers and in 44 PPI-refractory NERD patients before and after combined therapy of mosapride (15 mg/day) and omeprazole (20 mg/day) for 12 weeks. Gastric emptying in the PPI-refractory NERD patients was significantly higher than that of healthy volunteers, and the administration of mosapride in addition to omeprazole improved reflux symptoms and gastric emptying in PPI-refractory NERD patients with delayed gastric emptying. On the other hand, a prokinetic agent had almost no effect on NERD patients with normal gastric motor function.
The difference between our results and those of the study by Futagami et al12 could be attributable to the different study populations; furthermore, the longer duration of their study protocol (our study lasted just 4 weeks while Futagami et al spanned 12 weeks) could be another factor that enhanced the efficacy of combinations of prokinetics with PPI in the treatment of refractory reflux disease. There is supporting evidence that PPI is unstable at a low pH, retention of PPI in the stomach for a long time may result in an impaired acid-suppressive effect,23 so that rapid transit of PPI to the upper small intestine could be a benefit in PPI therapy. Arai et al23 reported that the addition of mosapride significantly improved the pharmacokinetic parameters of rabeprazole, particularly increased the peak plasma concentration (Cmax) and the area under the time-concentration curve (AUC). They concluded that co-administration of mosapride could have some favorable effect in PPIs-based therapy. Therefore, although it improved the pharmacokinetics of the PPI by prokinetics, it also required a prolonged duration of therapy of more than 4 weeks in refractory GERD patients to achieve a good response.
Another study by Miyamoto et al16 analyzed FSSG-TS, FSSG-RS, and FSSG-DS of PPI-refractory NERD patients, demonstrated that the addition of mosapride to PPI for 4 weeks in PPI-refractory NERD patients led to significantly lower FSSG-TS, FSSG-RS and FSSG-DS at the end of treatment. They also found that younger age, constipation and dysmotility were the predictors of refractoriness to PPI therapy in NERD patients. These results suggest that a subset of patients with significant dysmotility and functional dyspepsia might benefit from the addition of a prokinetic agent to PPI therapy. Although our study outcomes were similar to those of Miyamoto et al, our findings were not statistically significant, and this might be explained by the fact that our patients were more difficult to treat than Miyamoto et al population due to their higher pretreatment FSSG score (FSSG-TS in our study was 20.4 vs 14.6 in that of Miyamoto et al).19
As a secondary outcome, our data showed that the effect of combination therapy on the number of symptom-free days in PPI-refractory GERD patients was not superior to that of PPI monotherapy. Most patients who have PPI failure are likely to originate from the NERD phenotype (86.4% in our study) which represents a heterogeneous population with complex, multifactorial underlying processes,22 compatible with the present study finding that the patients had symptom-free days in less than 50% of the treatment period, whether on standard dose PPI monotherapy or combination therapy.
The strength of the current study is that it was a randomized, double-blind, placebo-controlled trial. It compared the efficacy of omeprazole plus mosapride combination therapy with that of omeprazole monotherapy in PPI-refractory GERD patients, while previous reports12,16 were open-labeled trials with no control group, so that their positive results may be considered biased. Our study had several limitations, including the small number of patients available for sub-analysis to identify subgroups of patients, which conferred an advantage on combination therapy over PPI monotherapy. The duration of 4 weeks of our study may be another disadvantage that weakening the efficacy of combination therapy, the longer duration of combination therapy such as 12 weeks should be repeated study in these PPI-refractory GERD patients. Also, the diagnosis of GERD in the present study relied on the FSSG score and might have included patients prone to dyspepsia/dysmotility symptoms with concomitant functional bowel disorders that may be more difficult to treat than typical GERD patients. Furthermore, our patients were not undergoing esophageal impedance-pH monitoring which recommended in patients with incomplete or lack of response to PPI therapy.24 So that we cannot exclude functional heartburn patients from our study population that may be the one-factor which impact on the study outcome.
Conclusion
In summary, our results do not support the addition of mosapride to a standard dose of PPI in the PPI-refractory GERD patients. An appropriate therapeutic strategy for these patients needs to be further developed.
Data sharing statement
The data sets used and/or analyzed during this study are available from the corresponding author on reasonable request and were received permission for use by the ethics committee of Rajavithi Hospital.
Ethics approval and consent to participate
The experimental protocols were performed after approval and in accordance with the guidelines set by the ethics committee of Rajavithi Hospital (No. 055/2559). Written informed consent was obtained from all participants included in the present study.
Acknowledgments
The authors greatly appreciate Dr. Bancha Ovartlarnporn, NKC Institute of Gastroenterology and Hepatology, Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla, Thailand, for his fruitful scientific discussion on this study. The authors would like to thank Ms. Wannakorn Homsuwan, research assistant of Rajavithi Hospital, for her kind help in statistical analysis and our sincere gratitude also goes to all patients in this study.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi:10.1111/j.1572-0241.2006.00630.x
2. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi:10.1136/gutjnl-2012-304269
3. Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogastroenterol Motil. 2011;17:14–27. doi:10.5056/jnm.2011.17.1.14
4. Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367:2086–2100. doi:10.1016/S0140-6736(06)68932-0
5. Hsu PI, Lu CL, Wu DC, et al. Eight weeks of esomeprazole therapy reduces symptom relapse, compared with 4 weeks, in patients with Los Angeles grade A or B erosive esophagitis. Clin Gastroenterol Hepatol. 2015;13:859–866. doi:10.1016/j.cgh.2014.09.033
6. Fock KM, Talley N, Goh KL, et al. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett’s oesophagus. Gut. 2016;65:1402–1415. doi:10.1136/gutjnl-2016-311715
7. Fass R, Gasiorowska A. Refractory GERD: what is it? Curr Gastroenterol Rep. 2008;10:252–257.
8. Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106–1110. doi:10.1056/NEJM199510263331703
9. Carlsson L, Amos GJ, Andersson B, et al. Electrophysiological characterization of the prokinetic agents cisapride and mosapride in vivo and in vitro: implications for proarrhythmic potential? J Pharmacol Exp Ther. 1997;282:220–227.
10. Potet F, Bouyssou T, Escande D, Baró I. Gastrointestinal prokinetic drugs have different affinity for the human cardiac human ether-à-gogo K(+) channel. J Pharmacol Exp Ther. 2001;299:1007–1012.
11. Ruth M, Hamelin B, Röhss K, Lundell L. The effect of mosapride, a novel prokinetic, on acid reflux variables in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1998;12:35–40.
12. Futagami S, Iwakiri K, Shindo T, et al. The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying. J Gastroenterol. 2010;45:413–421. doi:10.1007/s00535-009-0173-0
13. Madan K, Ahuja V, Kashyap PC, Sharma MP. Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastroesophageal reflux disease: a randomized trial. Dis Esophagus. 2004;17:274–278. doi:10.1111/j.1442-2050.2004.00424.x
14. Liu Q, Feng CC, Wang EM, Yan XJ, Chen SL. Efficacy of mosapride plus proton pump inhibitors for treatment of gastroesophageal reflux disease: a systematic review. World J Gastroenterol. 2013;19:9111–9118. doi:10.3748/wjg.v19.i47.9111
15. Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–891. doi:10.1007/s00535-004-1417-7
16. Miyamoto M, Manabe N, Haruma K. Efficacy of the addition of prokinetics for proton pump inhibitor (PPI) resistant non-erosive reflux disease (NERD) patients: significance of frequency scale for the symptom of GERD (FSSG) on decision of treatment strategy. Intern Med. 2010;49:1469–1476. doi:10.2169/internalmedicine.49.3615
17. Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–180. doi:10.1136/gut.45.2.172
18. Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601–616. doi:10.1016/j.bpg.2007.12.007
19. Miyamoto M, Haruma K, Takeuchi K, Kuwabara M. Frequency scale for symptoms of gastroesophageal reflux disease predicts the need for addition of prokinetics to proton pump inhibitor therapy. J Gastroenterol Hepatol. 2008;23:746–751. doi:10.1111/j.1440-1746.2007.05218.x
20. Hsu YC, Yang TH, Hsu WL, et al. Mosapride as an adjunct to lansoprazole for symptom relief of reflux oesophagitis. Br J Clin Pharmacol. 2010;70:171–179. doi:10.1111/j.1365-2125.2010.03696.x
21. Goh KL. Gastroesophageal reflux disease in Asia: a historical perspective and present challenges. J Gastroenterol Hepatol. 2011;26(Suppl 1):2–10. doi:10.1111/j.1440-1746.2010.06534.x
22. Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease-where next? Aliment Pharmacol Ther. 2005;22:79–94. doi:10.1111/j.1365-2036.2005.02531.x
23. Arai K, Takeuchi Y, Watanabe H, et al. Prokinetics influence the pharmacokinetics of rabeprazole. Digestion. 2008;78:67–71. doi:10.1159/000165351
24. Roman S, Gyawali CP, Savarino E, et al. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the Porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil. 2017;29:1–15. doi:10.1111/nmo.13067
Supplementary materials
 |
Table S1 FSSG questionnaire |
 |
Table S2 Diary |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
