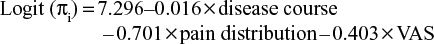Back to Journals » Journal of Pain Research » Volume 9
Efficacy of stereotactic gamma knife surgery and microvascular decompression in the treatment of primary trigeminal neuralgia: a retrospective study of 220 cases from a single center
Authors Dai Z , Huang Q, Liu H, Zhang W
Received 8 April 2016
Accepted for publication 26 May 2016
Published 26 July 2016 Volume 2016:9 Pages 535—542
DOI https://doi.org/10.2147/JPR.S110161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael Schatman
Zi-Feng Dai, Qi-Lin Huang, Hai-Peng Liu, Wei Zhang
Department of Neurosurgery, Xinqiao Hospital, The Third Military Medical University, Chongqing, People’s Republic of China
Objectives: A retrospective study was undertaken to compare the efficacy of stereotactic gamma knife surgery (GKS) and microvascular decompression (MVD) in the treatment of primary trigeminal neuralgia (TN) at a single center. The study included the evaluation of clinical outcomes of pain relief and pain recurrence and complications associated with GKS and MVD.
Methods: The study included 202 patients with primary TN and was conducted between January 2013 and December 2014; about 115 patients were treated with GKS and 87 patients were treated with MVD. TN pain was evaluated using the Barrow Neurological Institute and the visual analog scale scoring systems. Preoperative magnetic resonance tomographic angiography was performed for all patients. Microscope-assisted MVD used the suboccipital retrosigmoid sinus approach. GKS targeted the trigeminal nerve root entry zone with a margin radiation dose of 59.5 Gy, and brainstem dose <12 Gy. Posttreatment follow-up was for 2 years.
Results: Postoperative Barrow Neurological Institute scores for patients treated with GKS and MVD were significantly improved compared with preoperative scores (P<0.01). Reduction in postoperative pain following MVD (95.4% patients) was significantly greater than that following GKS (88.7% patients) (P<0.01). Postoperative visual analog scale scores of the MVD group were significantly reduced compared with those of patients treated with GKS at the same postoperative time points (P<0.01). Patients treated with GKS had a significantly increased rate of loss of corneal reflex compared with patients treated with MVD (P=0.002).
Conclusion: Both GKS and MVD are safe and effective first-line and adjunctive treatment options for patients with TN. The clinical outcomes of pain relief and reduction of pain recurrence were better with MVD. For GKS, this study showed that the optimal radiation therapeutic dose range was 70–90 Gy, but brainstem radiation protection is recommended.
Keywords: gamma knife surgery, microvascular decompression, stereotactic radiosurgery, trigeminal neuralgia
Introduction
Trigeminal neuralgia (TN) is characterized by unilateral, paroxysmal, and painful “shocks” or pain along the anatomical areas innervated by the trigeminal nerve.1,2 Washing, hair brushing, and chewing are the most common causes of TN-induced pain. Neurovascular compression is the most accepted hypothesis for explaining the etiology of TN,3 but in 3.1%−17% of patients, there is no evidence of vascular compression.4,5 Other causes of TN include demyelinating nerve disorders, because of the reported association between TN and multiple sclerosis.6–9
In 2008 and 2009, the American Academy of Neurology and the European Federation of Neurological Societies published guidelines on neuropathic pain assessment and management.10,11 In 2013, the International Headache Society has defined strict clinical criteria for the diagnosis of TN.12 Recently, advances in the diagnosis and treatment of TN have been reviewed.13 Because most cases of idiopathic TN are believed to be due to vascular compression of the trigeminal nerve (fifth cranial nerve), cranial magnetic resonance tomographic angiography (MRTA) is a common diagnostic method.14,15
Pharmacological treatment with drugs including oxcarbazepine can provide pain relief in about 25% of patients. However, most patients seek surgical treatment because their condition is refractory to drug treatment, or because they experience adverse drug reactions.4
The two main forms of surgical treatment for TN are microvascular decompression (MVD) and stereotactic gamma knife surgery (GKS). MVD can, in some cases, remove the cause of primary TN and protect the structural integrity of the trigeminal nerve, with few postoperative complications.16 GKS is a noninvasive approach to treat TN and can be used as the initial treatment for drug-refractory TN or as an adjunctive treatment when other treatments fail. GKS involves destruction of the trigeminal nerve, which can provide pain control. In GKS, which uses radiation, the root entry zone of the trigeminal nerve, situated at 2–3 mm from the brainstem surface, is usually used as a target, with the radiation dose protocols ranging from 70 to 100 Gy.17–21
The Barrow Neurological Institute (BNI) in the USA has developed a pain scale, which can be adapted as a questionnaire for use by patients with TN before and after treatment.22 The visual analog scale (VAS) has been used for more than 30 years to evaluate pain levels in acute and chronic conditions.23
In 2004, Lin and Ayiku24 undertook a systematic review of the clinical efficacy and safety of stereotactic GKS in the treatment of TN, on behalf of the National Institute for Clinical Excellence. The findings were that, due to the limited number of studies, which were presented mainly as case studies, there were no reliable data to support the use of GKS instead of other surgical techniques.24 However, the findings of this systematic review suggested that, in typical TN, GKS offered similar clinical efficacy, in terms of initial short-term pain relief, as MVD, percutaneous glycerol rhizolysis, percutaneous radiofrequency thermocoagulation rhizotomy, and percutaneous balloon compression.24 These authors found that the safety of GKS could not be determined from available published evidence at the time. However, in studies included up to 2004, GKS showed a low risk of complications when compared with other surgical techniques, with a zero rate of operative mortality and a reported zero rate of stroke, particularly when compared with MVD.24
More recently, in 2015, Montano et al13 reviewed the current status of recent advances in the diagnosis and surgical treatment of TN. These authors highlighted the complex pathogenesis of TN and the need for surgical methods that can be adapted for each patient, with endoscopic and neuronavigation techniques to improve both MVD and GKS and improved definition required for GKS targets.13
This retrospective study was undertaken to compare the efficacy of stereotactic GKS and MVD in the treatment of primary TN at a single center. The study included evaluation of clinical outcomes of pain relief and pain recurrence and complications associated with these two treatments.
Methods
Patients and informed consent
The study was approved by the Ethics Committee of the Third Military Medical University, Chongqing, People’s Republic of China. Written informed consent was obtained from all the patients prior to surgery, and all the patients consented to the submission of this report for publication.
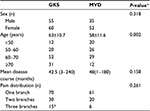
This study included 202 patients who were diagnosed with primary TN at our hospital between January 2013 and December 2014. All the patients were informed about the treatment procedures and were allowed to choose their own treatment; 115 patients were included in the stereotactic GKS treatment group, which consisted of 60 women and 55 men, with ages ranging from 26 to 89 years (mean ± standard deviation: 63±10.7 years). The remaining 87 patients were included in the MVD treatment group, which consisted of 52 women and 35 men, with ages ranging from 36 to 77 years (mean ± standard deviation: 58±11.6 years). Characteristics of the patient population are shown in Table 1.
Preoperative MRTA
Preoperative MRTA was performed in all the patients to exclude the presence of intracranial lesions, including neoplasms, and to confirm the neurovascular relationships. MRTA was performed on a 3.0-Tesla imaging system (GE Signa HDX3.0, Milwaukee, WI, USA) and the parameters used were: repetition time 17.0 milliseconds, echo time 5.7 milliseconds, slice thickness 0.5 mm, interslice gap 1.0 mm, and matrix 512×512.
Microscope-assisted MVD
For MVD treatment, the suboccipital retrosigmoid sinus approach was used. We made a ∼6 cm straight incision along the hairline, incised the skin, subcutaneous tissue, muscle, and periostium, and then drilled a 3×3 cm bone window to expose the superior border of the transverse sinus and the inner border of sigmoid sinus. A “Y” incision was made in the dura mater, cerebrospinal fluid from the cisterna magna was released, and the cerebellopontine angle area was exposed. Finally, the fifth cranial nerve and a compromising vessel were observed. After separating the fifth cranial nerve and any vessel, a Teflon patch was placed between them. The fifth, seventh, and eighth cranial nerves were monitored continuously by electromyography and brainstem auditory-evoked potentials.
Stereotactic GKS
A Leksell model “G” frame was fixed to the patient’s head under local anesthesia. We then transferred a gadolinium-enhanced MRTA image onto the gamma knife computer workstation. Leksell gamma knife software (ELEKTA, Stockholm, Sweden) was used to acquire the radiosurgical target. The treatment procedure was performed with two isocenters, where one target was the nearby Gasserian ganglion, and the other target was about 2−4 mm away from the brainstem, via a 4 mm collimator helmet that targeted the trigeminal nerve root entry zone. The margin dose was 59.5 Gy, and a 70% isodose line enclosed the target margin in all patients. The brainstem dose was <12 Gy.
Follow-up and treatment outcome assessments
Follow-up was conducted for all the patients via telephone or in the Outpatient Department. The BNI pain intensity scale was used as follows: BNI I: no pain, no medication; BNI II: occasional pain, not requiring medication; BNI III: some pain, adequately controlled with medication; BIN IV: some pain, not adequately controlled with medication; BNI V: severe pain or on pain relief. The VAS was also used to assess pain relief. Operative complications were also recorded.
Statistical analysis
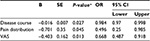
Continuous data were described as mean ± standard deviation. The independent samples t-test was used to analyze continuous data. The χ2 test and the Mann−Whitney U test were used for comparing the categorical data. Binary logistic regression (Forward: LR) was performed to analyze how preoperative factors (sex, age, disease course, pain distribution, and VAS scores) influenced postoperative pain relief. SPSS version 19.0 (IBM Corporation, Armonk, NY, USA) was used for performing these analyses. Kaplan−Meier survival curves were used to analyze the relationship between pain relief and follow-up time by log-rank tests, using GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was assumed as P<0.05.
Results
Vascular involvement using preoperative MRTA
We found that 80.7% of the involved vessels were arteries, while 19.3% of the involved vessels were veins, of which 82% were large petrosal veins.
Comparison of pre- and postoperative BNI scores
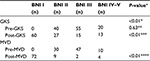
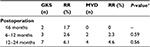
The distribution of the preoperative BNI scores was similar between the GKS and MVD groups (P=0.63). Postoperative GKS and MVD BNI scores were both significantly improved compared with their respective preoperative BNI scores (P<0.01). After 2 years of follow-up, 102 patients treated with GKS attained pain relief (BNI pain score I−III), compared to 83 patients in the MVD group; there was, therefore, a significant difference in the total pain remission rate between GKS (88.7%) and MVD (95.4%), with P<0.01. The detailed distribution of the BNI scores is shown in Table 2.
Pre- and postoperative VAS scores
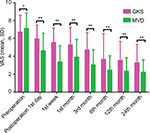
The preoperative VAS score of the GKS group (mean ± SD: 6.71±1.88) was not significantly different from that of the MVD group (7.10±1.73; P=0.132). The postoperative VAS scores of the MVD group were superior to those of the GKS group at different time points (all P<0.01). The details of the VAS score distribution are shown in Figure 1.
Postoperative pain relief over time
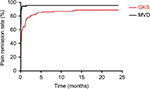
Figure 2 shows the pain remission rate at different time points. The pain remission rate on the first day after surgery was 24.3% in the GKS group and 69% in the MVD group. The pain remission rate on the first day after surgery of the MVD group was significantly greater than that of the GKS group (P<0.01).
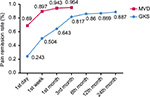  | Figure 2 The postoperative remission rate at different follow-up time points. Abbreviations: GKS, gamma knife radiosurgery; MVD, microvascular decompression. |
Within the first week after MVD, 89.7% patients experienced pain relief. Within 3 months after the operation, the pain remission rate of the GKS and MVD groups were significantly different (P=0.004) at 81.7% (n=94) and 95.4% (n=83), respectively. Kaplan−Meier survival analysis showed that the time-related pain relief rate of MVD was superior to that of GKS (P<0.01; Figure 3).
In the binary logistic regression (Forward: LR), the Wald χ2 test for each regression coefficient showed that the preoperative disease course, pain distribution, and VAS scores were significantly associated with postoperative pain relief (all P<0.05), while sex (P=0.496) and age (P=0.055) were not significantly associated with postoperative pain relief for two treatments. The results of logistic regression analysis are summarized in Table 3. The logistic regression model was calculated as:
|
|
Pain recurrence
Twelve GKS-treated patients experienced pain recurrence between 6 months and 2 years after the procedure (Table 4). Among the twelve patients, three patients were subsequently re-treated using GKS, two patients with avulsion of the supraorbital branch of the trigeminal nerve, two patients received radiofrequency therapy, and five patients received drug treatment. Six patients suffered pain recurrence after MVD. Four of these patients demonstrated new neurovascular compression. One patient was treated with GKS, three patients received radiofrequency therapy, and two patients received drug treatment. The pain of all 18 of these patients was well controlled. The pain recurrence rates were not significantly different at different time points between patients treated with GKS and MVD (P>0.05).
Posttreatment complications
Twenty-five patients treated with GKS had a significantly increased rate of loss of corneal reflex, compared with five patients treated with MVD (P=0.002). Twenty patients treated with GKS and 13 patients treated with MVD experienced facial numbness (P=0.704). Herpes zoster around the mouth, facial paralysis, and hearing loss were complications specific to MVD. The characteristics of the postoperative complications are shown in Table 5.
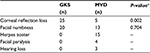  | Table 5 The characteristics postoperative complication of GKS and MVD Note: +Obtained by chi-square test. Abbreviations: GKS, gamma knife surgery; MVD, microvascular decompression; n, number. |
Discussion
The findings of this retrospective study in a single center have shown that stereotactic GKS and MVD are safe and effective first-line and adjunctive treatment options for patients with TN. This study has demonstrated some findings that are supported by those of other studies, but also some distinctive findings. It has previously been reported that the annual incidence of TN ranges from 4.7 to 12.6 per 100,000 people and about 1%−28.2% of such patients have bilateral TN.25–29 In our study, 1.49% of patients (three of 202 patients) suffered bilateral TN. About 83% of patients with primary TN have been reported to have vascular compromise of the trigeminal nerve (fifth cranial nerve), with 63.8%−80% of such vessels found to be superior cerebellar arteries and 9%−35% being veins.5,30–33
Previous studies have shown that success rates of MVD for the treatment of TN have ranged from 75% to 100%.34–38 Theoretically, pain should be relieved or immediately ablated following treatment with MVD, but in our study, 5.7% of cases (five patients) did not achieve pain relief until after 1 month following treatment. We believe that the following factors may have contributed to delay in response in these cases. First, not all the compromising vessels were detached from the trigeminal nerve during the treatment. Second, not all branches of the compromising vessels were detached from the nerve during the treatment. Third, long-term vascular compression resulted in demyelination of the trigeminal nerve. Finally, it is possible that with a longer disease course and higher VAS scores in patients before treatment, these patients became refractory to these forms of treatment.
In this study, in the MVD group, 6.9% of patients experienced pain recurrence at 6 months following surgery, which is supported by the pain recurrence rate of 1%−19.4% found in other studies.2,4,39,40 Potential factors related to post-MVD pain recurrence were inaccurate placement of the Teflon patch, a newly formed neurovascular compression, formation of granulation tissue around the Teflon patch, or arachnoid adhesions around the trigeminal nerve. It is of interest to note that Granit et al7 have reported that pain in recurrent TN could be effectively relieved by re-surgery.
In a study by Apfelbaum,30 the rate of moderate to severe facial paralysis was about 0.5%; 14 patients had hearing loss, in which in nine patients, it was permanent. After reviewing the surgical records of three patients who had experienced hearing loss, it was found that the eighth cranial nerve had been traumatized during surgery, while exposing the fifth cranial nerve. It is believed that both skilled surgery and continuous monitoring of the brainstem auditory-evoked potential could decrease such hearing loss.31 In this study, 15 patients in the MVD group developed herpes zoster around the mouth, which may indicate that latent herpes zoster in the trigeminal ganglia was activated by the surgery. In this study, only functional paralysis of the trigeminal nerve occurred after treatment with MVD, indicating that the only complication of this treatment procedure was reversible one.
GKS is an alternative treatment modality for TN, with a pain relief rate of >80% at the end of the first year in our study, which was higher than the 57%−60% reported in other studies.31–44 In a recent review, Lucas et al45 proposed that the use of different pain scales resulted in variable treatment outcomes for pain relief. It is important to note that patients treated with GKS as a primary form of management for TN achieved better pain relief than those treated with GKS as a secondary form of management.46 Patients who had TN proven to be caused by nerve compromise by blood vessels have less pain relief when treated by GKS than those without blood vessel compression.46 In a study by Shaya and Nanda,44 they did not observe a significant difference in pain relief after treatment of TN by GKS.
Chen and Lee47 have reported that gamma radiation destroys the structural integrity of the trigeminal nerve, for example, by diffuse demyelination, which raises the pain threshold and slows down electroneurographic signal transduction, resulting in pain relief. In our study, 12 patients (10.8%) experienced pain recurrence during the first 2 years after GKS treatment, possibly because the trigeminal nerve was not sufficiently damaged, or had formed new collateral electrical disturbances, or was stimulated by focal inflammation. If patients experienced pain recurrence, medication, repeated GKS/MVD, or trigeminal nerve avulsion may be effective in ameliorating or controlling pain. This view is supported by the findings of a study by Tyler-Kabara et al48 who compared patients treated with 70, 90 (30% isodose line on the brainstem), and 90 Gy (50% isodose line on the brainstem) and found that patients receiving 90 Gy (50% isodose line on the brainstem) experienced the shortest duration of pain relief, the highest pain remission rate (93.2%), and the lowest pain recurrence rate (11.9%), but also the maximum facial numbness. Kondziolka et al18 have reported that a total radiation dose of between 70 Gy and 90 Gy was optimal. The pain relief rate decreased significantly with a radiological dose <70 Gy, but more postoperative complications occurred with a dose >90 Gy.17,28,49 In another study, patients who received 70 Gy as the marginal dose had higher failure rates of pain relief than those who received 80 Gy.44
Limitations
A retrospective study in a single center may be subject to study bias as the investigators who performed the treatment procedures also interpreted the study data. As part of the patient informed consent, they were told the risks and benefits of the treatment procedures and were allowed to choose their treatment, which was not randomized. In this study, we acknowledge that the treatment intervention selection, based on patient personal preference, may have influenced the outcomes. For these reasons, we recommend that further controlled clinical studies be performed in multiple centers, nationally and internationally.
Conclusion
The findings of this retrospective study in a single center have shown that both GKS and MVD are safe and effective first-line and adjunctive treatment options for patients with TN. The clinical outcomes of pain relief and reduction of pain recurrence were better with MVD. For GKS, this study showed that the optimal radiation therapeutic dose range was 70–90 Gy, but brainstem radiation protection is recommended.
Acknowledgments
The authors thank Professor Shenqing Lv who helped formulate the research strategy and edited the manuscript. Doctor Xi Li also edited the manuscript. The authors are grateful to the health care workers of the Neurosurgery Department, Xinqiao Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
Joffroy A, Levivier M, Massager N. Trigeminal neuralgia. Pathophysiology and treatment. Acta Neurol Belg. 2001;101(1):20–25. | ||
Miller JP, Acar F, Burchiel KJ. Classification of trigeminal neuralgia: clinical, therapeutic, and prognostic implications in a series of 144 patients undergoing microvascular decompression. J Neurosurg. 2009;111(6):1231–1234. | ||
Dandy WE. Concerning the cause of trigeminal neuralgia. Am J Surg. 1934;24:447–495. | ||
Fields HL. Treatment of trigeminal neuralgia. N Engl J Med. 1996;334(17):1125–1126. | ||
Ishikawa M, Nishi S, Aoki T, et al. Operative findings in cases of trigeminal neuralgia without vascular compression: proposal of a different mechanism. J Clin Neurosci. 2002;9(2):200–204. | ||
Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(Pt 12):2347–2360. | ||
Granit R, Leksell L, Skoglund CR. Fibre interaction in injured or compressed region of nerve. Brain Dev. 1944;67:125–140. | ||
Howe JF, Calvin WH, Loeser JD. Impulses reflected from dorsal root ganglia and from focal nerve injuries. Brain Res. 1976;116(1):139–144. | ||
Moller AR. Vascular compression of cranial nerves: II: pathophysiology. Neurol Res. 1999; 21(5):439–443. | ||
Cruccu G, Gronseth G, Alksne J, et al; American Academy of Neurology Society; European Federation of Neurological Society. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013–1028. | ||
Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. Eur J Neurol. 2010;17(8):1010–1018. | ||
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. | ||
Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015;11:289–299. | ||
Krafft RM. Trigeminal neuralgia. Am Fam Physician. 2008;77(9):1291–1296. | ||
Han-Bing S, Wei-Guo Z, Jun Z, Ning L, Jian-Kang S, Yu C. Predicting the outcome of microvascular decompression for trigeminal neuralgia using magnetic resonance tomographic angiography. J Neuroimaging. 2010;20(4):345–349. | ||
Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta neurochir (Wien). 2008;150(3):243–255. | ||
Kondziolka D, Lacomis D, Niranjan A, Mori Y, Maesawa S, Fellows W, Lunsford LD. Histological effects of trigeminal nerve radiosurgery in a primate model: implications for trigeminal neuralgia radiosurgery. Neurosurgery. 2000;46:971–976. | ||
Kondziolka D, Lunsford LD, Flickinger JC, et al. Stereotactic radio-surgery for trigeminal neuralgia: a multiinstitutional study using the gamma unit. J Neurosurg. 1996;84(6):940–945. | ||
Kondziolka D, Perez B, Flickinger JC, Habeck M, Lunsford LD. Gamma knife radiosurgery for trigeminal neuralgia: results and expectations. Arch Neurol. 1998;55(12):1524–1529. | ||
Régis J, Métellus P, Lazorthes Y, Porcheron D, Peragut JC. Effect of gamma knife on secondary trigeminal neuralgia. Stereotact Funct Neurosurg. 1998;70(Suppl 1):210–217. | ||
Young RF, Vermulen S, Posewitz A. Gamma knife radiosurgery for the treatment of trigeminal neuralgia. Stereotact Funct Neurosurg. 1998;70(Suppl 1):192–199. | ||
Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia : the initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013–1019. | ||
Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int. 1985 5(4):145–148. | ||
Lin JNW, Ayiku L. The clinical efficacy and safety of stereotactic radiosurgery (gamma knife) in the treatment of trigeminal neuralgia. Review Body for Interventional Procedures (ReBIP) as commissioned by the National Institute for Clinical Excellence (NICE), 2004. Available from: https://www.nice.org.uk/guidance/ipg85/documents/systematic-review-of-the-clinical-efficacy-and-safety-of-stereotactic-radiosurgery-gamma-knife-in-the-treatment-of-trigeminal-neuralgia2. | ||
Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945–1984. Neuroepidemiology. 1991;10(5–6):276–281. | ||
Koopman JS, Dieleman JP, Huygen FJ, de Mos M, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain. 2009;147(1–3):122–127. | ||
Pollack IF, Jannetta PJ, Bissonette DJ. Bilateral trigeminal neuralgia: a 14-year experience with microvascular decompression. J Neurosurg. 1988;68(4):559–565. | ||
Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27(1):89–95. | ||
Bozkurt M, Al-Beyati ES, Ozdemir M, Kahilogullari G, Elhan AH, Savas A, Kanpolat Y. Management of bilateral trigeminal neuralgia with trigeminal radiofrequency rhizotomy: a treatment strategy for the life-long disease. Acta neurochir (Wien). 2012;154(5):785–791. | ||
Apfelbaum RI. Comparison of the long-term results of microvascular decompression and percutaneous trigeminal neurolysis for the treatment of trigeminal neuralgia. Int Congr Ser. 2002;1247:629–643. | ||
Laghmari M, El Ouahabi A, Arkha Y, Derraz S, El Khamlichi A. Are the destructive neurosurgical techniques as effective as microvascular decompression in the management of trigeminal neuralgia? Surg Neurol. 2007;68(5):505–512. | ||
Zhong J, Li ST, Zhu J, et al. A clinical analysis on microvascular decompression surgery in a series of 3000 cases. Clin Neurol Neurosurg. 2012;114(7):846–851. | ||
Setty P, Volkov AA, D’Andrea KP, Pieper DR. Endoscopic vascular decompression for the treatment of trigeminal neuralgia: clinical outcomes and technical note. World Neurosurg. 2014;81(3–4):603–608. | ||
Revuelta-Gutiérrez R, López-González MA, Soto-Hernández JL. Surgical treatment of trigeminal neuralgia without vascular compression: 20 years of experience. Surgical Neurol. 2006;66(1):32–36. | ||
Linskey ME, Jho HD, Jannetta PJ. Microvascular decompression for trigeminal neuralgia caused by vertebrobasilar compression. J Neurosurg. 1994;81(1):1–9. | ||
Apfelbaum RI. A comparison of percutanous radiofrequency rhizotomy and microvascular decompression of the trigeminal nerve for the treatment of tic doloreux. Neurosurgery. 1977;1(1):16–21. | ||
Jo KW, Kong DS, Hong KS, Lee JA, Park K. Long-term prognostic factors for microvascular decompression fortrigeminal neuralgia. J Clin Neurosci. 2013;20(3):440–445. | ||
Olson S, Atkinson L, Weidmann M. Microvascular decompression for trigeminal neuralgia: recurrences and complications. J Clin Neurosci. 2005;12(7): 787–789. | ||
Hardy DG, Rhoton AL Jr. Microsurgical relationships of the superior cerebellar artery and the trigeminal nerve. J Neurosurg. 1978;49(5):669–678. | ||
Lee CC, Liao CH, Lin CF, Yang TF, Hsu SP, Yen YS, Shih YH. Brainstem auditory evoked potential monitoring and neuro-endoscopy: two tools to ensure hearing preservation and surgical success during microvascular decompression. J Chin Med Assoc. 2014;77(6):308–316. | ||
Pollock BE, Phuong LK, Gorman DA, Foote RL, Stafford SL. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2002;97(2):347–353. | ||
Dhople AA, Adams JR, Maggio WW, Naqvi SA, Regine WF, Kwok Y. Long-term outcomes of gamma knife radiosurgery for classic trigeminal neuralgia: implications of treatment and critical review of the literature. Clinical article. J Neurosurg. 2009;111(2):351–358. | ||
Baschnagel AM, Cartier JL, Dreyer J, et al. Trigeminal neuralgia pain relief after gamma knife stereotactic radiosurgery. Clin Neurol Neurosurg. 2014;117:107–111. | ||
Shaya AJM, Nanda A. Gamma knife radiosurgery for trigeminal neuralgia: a study of predictors of success, efficacy, safety, and outcome at LSUHSC. Surg Neurol. 2004;61(6):529–535. | ||
Lucas JT Jr, Nida AM, Isom S, et al. Predictive nomogram for the durability of pain relief from gamma knife radiation surgery in the treatment of trigeminal neuralgia. Int J Radiat Oncol Biol Phys. 2014;89(1):120–126. | ||
Brisman R. Gamma knife radiosurgery for primary management for trigeminal neuralgia. J Neurosurg. 2000;93(Suppl 3):159–161. | ||
Chen JF, Lee ST. Comparison of percutaneous trigeminal ganglion compression and microvascular decompression for the management of trigeminal neuralgia. Clin Neurol Neurosurg. 2003;105(3):203–208. | ||
Tyler-Kabara EC, Kassam AB, Horowitz MH, Urgo L, Hadjipanayis C, Levy EI, Chang YF. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. J Neurosurg. 2002;96(3):527–531. | ||
Nicol B, Regine WF, Courtney C, Meigooni A, Sanders M, Young B. Gamma knife radiosurgery using 90Gy for trigeminal neuralgia. J Neurosurg. 2000;93(Suppl 3):152–154. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.