Back to Journals » Drug Design, Development and Therapy » Volume 15
Efficacy of Pre-Treatment with Remimazolam on Prevention of Propofol-Induced Injection Pain in Patients Undergoing Abortion or Curettage: A Prospective, Double-Blinded, Randomized and Placebo-Controlled Clinical Trial
Authors Guan X , Jiao Z, Gong X, Cao H, Liu S, Lan H, Huang X, Tan Y, Xu B, Lin C
Received 13 August 2021
Accepted for publication 22 October 2021
Published 4 November 2021 Volume 2021:15 Pages 4551—4558
DOI https://doi.org/10.2147/DDDT.S334100
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Xuehai Guan,1,* Ziyin Jiao,1,* Xiaofang Gong,1 Huiyu Cao,1 Susu Liu,1 Hongmeng Lan,1 Xiaofang Huang,1 Yanmeng Tan,1 Bing Xu,2 Chengxin Lin1
1Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 2Department of Rehabilitation, The People`s Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuehai Guan; Chengxin Lin
Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, 6 Shuangyong Road, Nanning, Guangxi, 530021, People’s Republic of China
Tel/Fax +86-771-5356250
Email [email protected]; [email protected]
Background: Propofol-induced injection pain (PIP) is a well-known problem in general anesthesia. We hypothesized that pre-treatment with remimazolam prevents PIP in patients undergoing abortion or curettage.
Materials and Methods: In this prospective, single-center, double-blinded, randomized, placebo-controlled clinical trial, adult patients aged 18 to 60 undergoing abortion or curettage were randomly assigned to three groups. Group Lido received system lidocaine (a bolus of 0.5 mg kg− 1, iv). Group Remi received remimazolam (a bolus of 0.1 mg kg− 1, iv). Group NS received identical volumes of 0.9% normal saline. Sixty seconds after the injection of lidocaine, remimazolam or saline, patients were injected with propofol at a rate of 12 mL/min until the loss of consciousness. The primary outcome was the incidence of PIP at the time of induction using 4-point scale. Secondary outcomes included propofol-induced injection pain, vital signs, the characteristics of anesthesia and surgery, and adverse events.
Results: The incidence of patients with PIP was higher in group NS than that in group Lido and group Remi (75.7, 44.3, and 42.9%, respectively, p < 0.001). The percentages of patients with moderate PIP were higher in group NS than that in group Lido and group Remi (20.0, 2.9, and 1.4%, respectively, p < 0.001). Moreover, the consumption of propofol and the incidence of adverse event (hypoxemia and chin lifting) in group Remi were lower than that in group NS and Lido, and less patients got physical movement and cough in group Remi. The recovery time in group NS was longer than that in group Lido and Remi.
Conclusion: Our findings indicate that pre-treatment with remimazolam reduced the incidence and intensity of PIP in abortion or curettage patients, equivalent to that of lidocaine without severe adverse effects.
Trial Registration: Chinese Clinical Trial Registry (identifier: ChiCTR2100041805).
Keywords: lidocaine, remimazolam, propofol injection pain, abortion, curettage
Introduction
Due to its rapid onset and recovery, propofol is popularly used during general anesthesia. However, the intravenous injection of propofol induces local pain and discomforts. The incidence of propofol-induced injection pain (PIP) varies from approximately 28 to 90%.1–3 To reduce the incidence of PIP, many techniques have been developed, including pre-treatment or mixed use with medium-chain and long-chain triglycerides,4 pre-treatment or mixed use with lidocaine,5–9 nonsteroidal anti-inflammatory drugs,10 magnesium sulfate,6,11 dexmedetomidine,12 opioids,13–16 or ketamine.11,17 Although these strategies relieved PIP in varying degrees, the adverse event of these drugs such as emergence agitation,18 laryngospasm,19 pulmonary embolism,20 gastrointestinal ulcer,10 lengthy onset21 or tinnitus and dizziness22 limit their widespread clinical use. More patients complained of tinnitus or dizziness after the injection of lidocaine,22 and the addition of lidocaine may disrupt the stability of propofol emulsions and may cause pulmonary embolism.19 These drawbacks limited the use of lidocaine for preventing PIP. Consequently, there is a need for finding news drugs to decrease the incidence of PIP.
Remimazolam is an ultrashort-acting benzodiazepine, acting on GABA receptors to induce sedation. It is developed for procedural sedation.23–25 Unlike midazolam, remimazolam differs from all other benzodiazepines by its carboxylic ester linkage, metabolized by tissue esterase rapidly to inactive metabolites only.23–25 Remimazolam provided adequate procedural sedation for endoscopy, and faster recovery than midazolam.26–29 Data on the influence of remimazolam on PIP during abortion or curettage have not been published. Therefore, we designed this prospective, single-center, double-blinded, randomized, placebo-controlled clinical trial to investigate the efficacy of remimazolam 0.1 mg/kg and lidocaine 0.5 mg/kg compared to placebo in the prevention of PIP during abortion or curettage.
Materials and Methods
Patients
The randomization schedule was computer-generated by using Epical 2000 soft. According to the randomization schedule, all 210 patients were randomly divided into three groups (n = 70 in each group) in a 1:1:1 group allocation to receive either lidocaine (Group Lido, received system lidocaine, a bolus of 0.5 mg kg-1, iv), remimazolam (Group Remi, received remimazolam, a bolus of 0.1 mg kg-1, iv), or normal saline (Group NS, received equivalent volume of 0.9% normal saline). Sealed envelopes were used for concealment of study group allocation until the pretreatment drug was prepared. An assistor who did not participate in anesthesia induction prepared all drugs. Both patients and investigators were blinded to the randomized grouping allocation and the drugs.
Study Protocol
After obtaining approval from the Ethics Committee of the first affiliated hospital of Guangxi Medical University and written consent from the selected patients, we enrolled two hundred ten ASA physical status 1 and 2, aged 18–60 years, who were scheduled for elective abortion or curettage procedures with general anesthesia. Patients with liver and kidney dysfunction, drug allergy, nervous system or cardiovascular disease, obesity, difficult airway were excluded. Patients receiving analgesics were also excluded. The study was registered in the Chinese Clinical Trial Registry (ChiCTR2100041805, Principal investigator: Xuehai Guan, Date of registration: 2021-1-6). This study was conducted in accordance with the Declaration of Helsinki. All participants were informed about the purpose of the trial.
Patients were fasted for 6 hours. Only clear liquids were allowed up to 2 hours before the induction of anesthesia. No sedative premedication was given before induction. A 22-gauge cannula was inserted into the vein on the dorsum of the left hand without local anesthetics, at least 10 min before the induction of anesthesia, and an infusion of Ringer’s Lactate (2 mL/kg/h) was started to maintain its patency. The infusion of Ringer’s lactate was closed during the induction period.
After entering the operation room, patients were given routine nasal catheter oxygen inhalation of 2L/min, and the noninvasive blood pressure, electrocardiogram, and peripheral capillary oxygen saturation (SpO2) were monitored. The pre-treating drugs were prepared in a 10-mL syringe with either 10mL of normal saline, 0.5 mg/kg of lidocaine (Shanghai Harvest Pharmaceutical CO., China), or 0.1 mg/kg of remimazolam (Jiangsu Hengrui Medicine Co., China; diluted with normal saline to 10 mL) according to the group allocation by an assistor who did not participate in anesthesia induction. Both patients and investigators were blinded to the randomized grouping allocation and the drugs. All drugs were prepared and stored at room temperature and used within 10 mins. All patients were injected with a mixture of fentanyl (Yichang Humanwell Pharmaceutical Co., China) and atropine (1 ug/kg and 5 ug/kg, respectively). Fifteen seconds later, patients in Group Lido received system lidocaine (a bolus of 0.5 mg kg-1, iv), in Group Remi received remimazolam (a bolus of 0.1 mg kg-1, iv), and in Group NS received equivalent volume of 0.9% normal saline. Sixty seconds after the injection of lidocaine, remimazolam or saline, all patients received propofol (Guangdong JiaBo Pharmaceutical Co., China) at a rate of 12 mL/min until loss of consciousness. The sedation was monitored by using the Modified Observer’s Assessment Alertness/Sedation Scale (OAA/S; 5: responds readily to their name spoken in a normal tone, 4: lethargic response to their name spoken in a normal tone, 3: response only after their name is called loudly and/or repeatedly; 2: response only after name spoken with mild prodding or shaking; 1: unresponsive to mild prodding or shaking; 0: unresponsive to noxious stimuli)30 with 1 min interval. If an OAA/S score of 0 was not achieved, infusions of propofol continued until it reached 0.
Measurements
The primary outcome of this study was the incidence of PIP. Secondary outcomes included the intensity of PIP, vital signs, and adverse events, including hypotension, bradycardia (<50 beats/min), hypoxemia (SpO2 < 90%), chin lifting, physical movement and cough. Investigators who were blinded to the groups location evaluated the severity of propofol injection pain according to the 4-point pain scale every 5 seconds during anesthesia induction: grade 0, no pain; grade 1, mild pain but no physical movement; grade 2, moderate pain, pain accompanied by physical activity when the anesthetist asked, or during the injection; and grade 3, severe pain, accompanied by facial pain, painful expression, or strong vocal response, arms retracted, or tears.31 The characteristics of anesthesia and surgery, and adverse events were recorded too.
Sample Size
Our preliminary study revealed that the incidence of PIP was about 50% in our department which was among the previous study between 28 and 90%. We hypothesized a 50% reduction in the incidence of pain after propofol administration based on an alpha of 0.05 and a power of 80%. Under these assumptions, 57 patients were included in each group to detect a significant difference. Considering potential loss (20%) to follow-up, we increased the sample size to 70 in each group.
Statistical Methods
All data are expressed as numbers (%) or the mean ± SD. Continuous data of patients among the three groups were compared by one-way analysis of variance or two-way analysis of variance where appropriate. Categorical data were compared by  2 test or Fisher`s test, as appropriate. All statistical analyses were performed with IBM SPSS 25.0 statistical software. A p-values or corrected p-values of 0.05 were defined as statistically significant.
2 test or Fisher`s test, as appropriate. All statistical analyses were performed with IBM SPSS 25.0 statistical software. A p-values or corrected p-values of 0.05 were defined as statistically significant.
Results
All 210 patients enrolled in the study were evaluable (Figure 1; from 2021-1-6 to 2021-5-6). There were no significant differences among the three groups in demographics – age, height, weight, ASA score rate, or Mallampati score rate (Table 1).
 |
Table 1 Demographic Data of the Patients (n = 70 in Each Group) |
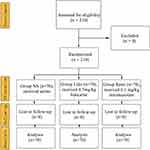 |
Figure 1 CONSORT flow of clinical procedures. Abbreviations: NS, normal saline; Lido, lidocaine; Remi, remimazolam. |
The overall incidence and intensity of PIP during propofol injection in the three groups were showed in Table 2. The incidence of PIP was significantly less in group Lido (44.3%) and Remi (42.9%) than those in group NS (75.7%) (p < 0.001). There was no significant difference between group Lido and group Remi in incidence of PIP (p > 0.05). No significant difference was found in the percentages with mild pain among the three groups. The percentage of patients with moderate pain was significantly less in group Lido (2.9%) and group Remi (1.4%) than those in group NS (20.0%) (p < 0.001). No significant difference was found in the percentages of patients with moderate pain between group Lido and group Remi. Although there was no significant difference among the three groups in the percentages of patients suffering from severe pain (p > 0.05), 2 cases and 1 case were found in group NS and group Lido, respectively.
 |
Table 2 Incidence of Propofol Induced Injection Pain (n = 70 in Each Group) |
The incidence of adverse event is shown in Table 3. There were no differences in the proportion of patients developed hypotension and bradycardia among the three groups. The proportion of patients with hypoxemia in group Remi (11.4%) was lower than that in group NS (34.3%) and group Lido (38.6%) (p < 0.001). The proportion of patients needing chin lift in group Remi (4.3%) was lower than that in group NS (34.3%) and group Lido (38.6%) (p < 0.001). Although there was no significant difference among the three groups in the percentages of patients with physical movement (p > 0.05), 5 cases, 7 case and 1 case were found in group NS, group Lido and group Remi respectively. Although there was no significant difference among the three groups in the percentages of patients with cough (p > 0.05), 1 case and 4 cases were found in group NS and group Lido respectively.
 |
Table 3 Incidence of Adverse Event Between Groups (n = 70 in Each Group) |
The characteristic of anesthesia and surgery is shown in Table 4. The overall consumption of propofol in group Remi (119.4 ± 25.33 mg) was lower than those in group NS (134.6 ± 28.37 mg) and group Lido (140.1 ± 37.16 mg) (p < 0.001). There was no difference in the length of anesthesia and surgery among three groups. The recovery time was shorter in group Lido (2.20 ± 1.73 min) and group Remi (2.09 ± 3.08 min) than that in group NS (3.74 ± 1.81 min) (p < 0.001). But there was no difference in recovery time between group Lido and group Remi.
 |
Table 4 Characteristics of Anesthesia and Surgery (n = 70 in Each Group) |
There were no differences in the systolic blood pressure, diastolic blood pressure, mean blood pressure, heart rate and SpO2 at any time point among the three groups (Figure 2).
Discussion
To the best of our knowledge, this was the first findings revealed that pre-treatment with remimazolam (0.1 mg/kg, iv) effectively reduced the incidence and intensity of PIP in abortion or curettage patients, equivalent to that of lidocaine without severe adverse effects. Moreover, pret-reatment with remimazolam could reduce the consumption of propofol and the incidence of adverse event, and shorter the recovery time.
Propofol has become a popular sedative agent. Due to its rapid onset and recovery, propofol was widely used in endoscopy, abortion and curettage. However, PIP is a common adverse event.32,33 The incidence of PIP varies from approximately 28 to 90%.1–3 But the definite pathophysiological mechanism of PIP is still unknown. Many factors are related to PIP, such as concentration of free propofol in aqueous solution, type of preparation, oil and solvent, injection technology (injection site,34 injection speed,35 intravenous infusion, puncture technology, syringe material), blood buffering, filtration treatment, age, sex, and so on. To reduce the incidence of PIP, many techniques have been developed, including medium-chain and long-chain triglycerides, pre-treatment or mixed use with lidocaine, nonsteroidal anti-inflammatory drugs, magnesium sulfate, dexmedetomidine, opioids, or ketamine. These various strategies relieved PIP in varying degrees. Lidocaine is a common local anesthetic, reversibly blocking peripheral pathway. Premedication with lidocaine 0.5 mg/kg before the injection of propofol reduced the incidence of PIP significantly, which was consistent with previous study.21,36 A dosage of 40 mg lidocaine is an appropriate dosage to alleviate PIP within the same vein through a local anesthetic effect. Lidocaine reduces PIP through a central analgesic effect and a local anesthetic effect when the dosage reaches 1.5 mg/kg.22 In our study, we believe that lidocaine prevented PIP through a local anesthetic effect under the use of 0.5 mg/kg.
The incidence and intensity of PIP was significantly less in group Lido and group Remi than those in group NS. γ-aminobutyric acid (GABA), a central inhibitory neurotransmitter acting on GABAA receptor and benzodiazepines can enhance the synaptic inhibitory effect of GABAergic neurotransmission. A lot of research showed that benzodiazepine has analgesic effect. For example, intravenous midazolam-a classic benzodiazepine drug administered in conscious sedation doses was found to significantly reduce the affective and motivational component of the pain experience.37 Midazolam intervention was revealed to substantially reduce the pain scores and analgesic consumption after knee arthroscopy.38 Midazolam was effective in decreasing pain after nasogastric tube insertion.39 Remimazolam is an ultrashort-acting benzodiazepine, acting on GABA receptors to induce sedation.23 Based on this, we infer that the first underling mechanism remimazolam preventing PIP may be its role by acting on GABA receptors to enhance the synaptic inhibitory effect of GABAergic neurotransmission. Propofol acts on the vein endothelial tissue, and then stimulates the kallikrein kinin system to produce bradykinin, which makes the blood vessel dilate and increase the permeability, and causes the free propofol to contact with the nerve endings on the inner wall of the blood vessel to cause pain. A recent study revealed that treating with remimazolam intraperitoneally alleviated pain behaviors induced by injecting complete Freund`s adjuvant in hind paw via regulating bradykinin receptors B1.40 Therefore, we deduced that the treatment of remimazolam intravenously alleviated PIP by blocking bradykinin signal too.
The consumption of propofol in group Remi was lower than that in group NS and group Lido. The recovery time in group NS was longer than that in group Lido and group Remi. As mention about, remimazolam can act on GABA receptors to enhance the synaptic inhibitory effect of GABAergic neurotransmission, which may result in less consumption of propofol and faster recovery from sedation in the present study. The incidence of adverse event (hypoxemia, chin lifting, physical movement and cough) in group Remi were lower than that in group NS and group Lido, which was in consistent with previous results that the adverse event was positively correlated with the dosages of propofol.21
There were some limitations. First, our study was only conducted in single center. Additionally, we just investigated one dose of remimazolam on the incidence of PIP. In the future, we will coordinate with multi center to evaluate the effect of remimazolam on the prevention of PIP.
In conclusion, our current findings indicate that pre-treatment with remimazolam reduced the incidence and intensity of PIP in abortion or curettage patients, equivalent to that of lidocaine without severe adverse effects. Moreover, pre-treatment with remimazolam 0.1 mg/kg can reduce the consumption of propofol and the incidence of adverse event, and shorter the recovery time.
Data Sharing Statement
The data generated during the current study are available from the corresponding author (Xuehai Guan) on reasonable request. The study protocol, statistical analysis plan and clinical study report will also be available.
Acknowledgments
We are grateful to Juanjuan Wang and Xinxin Luo for their kind assistance. This work was supported by the Natural Science Foundation of Guangxi Zhuang Autonomous Region (Nos. 2018GXNSFAA281049 and 2015GXNSFBA139133) and the National Natural Science Foundation of China (Nos. 81660200 and 81460176).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wrench IJ, Girling KJ, Hobbs GJ. Alfentanil-mediated analgesia during propofol injection: no evidence for a peripheral action. Br J Anaesth. 1996;77(2):162–164. doi:10.1093/bja/77.2.162
2. Mangar D, Holak EJ. Tourniquet at 50 mm Hg followed by intravenous lidocaine diminishes hand pain associated with propofol injection. Anesth Analg. 1992;74(2):250–252. doi:10.1213/00000539-199202000-00014
3. Picard P, Tramer MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000;90(4):963–969. doi:10.1097/00000539-200004000-00035
4. Singla B, Malde AD. A prospective observational study of injection pain in children with medium plus long chain triglyceride and long chain triglyceride propofol premixed with lignocaine. Indian J Anaesth. 2018;62(3):214–218. doi:10.4103/ija.IJA_506_17
5. Zirak N, Bameshki A, Yazdani M, et al. Lipid composition and lidocaine effect on immediate and delayed injection pain following propofol administration. Anesth Essays Res. 2016;10(1):29–32. doi:10.4103/0259-1162.164728
6. Sun J, Zhou R, Lin W, et al. Magnesium sulfate plus lidocaine reduces propofol injection pain: a double-blind, randomized study. Clin Ther. 2016;38(1):31–38. doi:10.1016/j.clinthera.2015.10.011
7. Jeong M, Yoon H. Comparison of the effects of lidocaine pre-administration and local warming of the intravenous access site on propofol injection pain: randomized, double-blind controlled trial. Int J Nurs Stud. 2016;61:209–218. doi:10.1016/j.ijnurstu.2016.06.012
8. Hong JM, Lee HJ, Cho AR, et al. Pretreatmet with 5% lidocaine patch reduces cannula-induced and propofol-induced pain: a randomized, double-blind, placebo-controlled study. Korean J Anesthesiol. 2016;69(5):468–473. doi:10.4097/kjae.2016.69.5.468
9. Euasobhon P, Dej-Arkom S, Siriussawakul A, et al. Lidocaine for reducing propofol-induced pain on induction of anaesthesia in adults. Cochrane Database Syst Rev. 2016;2:CD007874. doi:10.1002/14651858.CD007874.pub2
10. Madan HK, Singh R, Sodhi GS. Comparison of intravenous lignocaine, tramadol and ketorolac for attenuation of propofol injection pain. J Clin Diagn Res. 2016;10(7):UC05–08. doi:10.7860/JCDR/2016/20444.8118
11. Akbari H, Nasiri E, Nikkhah A, et al. Analgesic effects of ketamine, magnesium sulfate, and sodium-thiopental on propofol injection pain: a single-blind randomized clinical trial. Tanaffos. 2018;17(1):22–28.
12. Yu J, Zhang Y, Lu Y, Dong C. Preemptive dexmedetomidine to prevent propofol injection pain in children. Ir J Med Sci. 2015;184(2):375–378. doi:10.1007/s11845-014-1122-3
13. Lee SH, Lee SE, Chung S, et al. Impact of time interval between remifentanil and propofol on propofol injection pain. J Clin Anesth. 2016;34:510–515. doi:10.1016/j.jclinane.2016.06.029
14. Singh A, Sharma G, Gupta R, et al. Efficacy of tramadol and butorphanol pretreatment in reducing pain on propofol injection: a placebo-controlled randomized study. J Anaesthesiol Clin Pharmacol. 2016;32(1):89–93. doi:10.4103/0970-9185.175703
15. Lee M, Kwon T, Kim S, et al. Comparative evaluation of the effect of remifentanil and 2 different doses of esmolol on pain during propofol injection: a double-blind, randomized clinical consort study. Medicine (Baltimore). 2017;96(10):e6288. doi:10.1097/MD.0000000000006288
16. Kizilcik N, Menda F, Bilgen S, et al. Effects of a fentanyl-propofol mixture on propofol injection pain: a randomized clinical trial. Korean J Anesthesiol. 2015;68(6):556–560. doi:10.4097/kjae.2015.68.6.556
17. Cheng D, Liu L, Hu Z. Prevention of anesthesia-induced injection pain of propofol in pediatric anesthesia. Pak J Med Sci. 2017;33(3):752–756. doi:10.12669/pjms.333.12026
18. Sinha PK, Neema PK, Rathod RC. Effect of nitrous oxide in reducing pain of propofol injection in adult patients. Anaesth Intensive Care. 2005;33(2):235–238. doi:10.1177/0310057X0503300213
19. Kaabachi O, Chettaoui O, Ouezini R, et al. A ketamine-propofol admixture does not reduce the pain on injection compared with a lidocaine-propofol admixture. Paediatr Anaesth. 2007;17(8):734–737. doi:10.1111/j.1460-9592.2007.02242.x
20. Davies AF, Vadodaria B, Hopwood B, et al. Efficacy of microfiltration in decreasing propofol-induced pain. Anaesthesia. 2002;57(6):557–561. doi:10.1046/j.1365-2044.2002.02602.x
21. Wang J, Duan J, Xie C, et al. Comparison between intravenous nalbuphine and lidocaine in reducing propofol-induced injection pain during gastroscopy: a randomized controlled trial. Pain Ther. 2020;9(2):563–571. doi:10.1007/s40122-020-00188-y
22. Xing J, Liang L, Zhou S, et al. Intravenous lidocaine alleviates the pain of propofol injection by local anesthetic and central analgesic effects. Pain Med. 2018;19(3):598–607. doi:10.1093/pm/pnx070
23. Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. doi:10.1016/j.jclinane.2020.109899
24. Wang F, Zhou Q, Shen M, et al. Efficacy and safety of remimazolam in procedural sedation and analgesia: a protocol for systematic review and meta analysis. Medicine (Baltimore). 2020;99(27):e20765. doi:10.1097/MD.0000000000020765
25. Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33(4):506–511. doi:10.1097/ACO.0000000000000877
26. Schuttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi:10.1097/ALN.0000000000003103
27. Schippers F, Pesic M, Saunders R, et al. Randomized crossover trial to compare abuse liability of intravenous remimazolam versus intravenous midazolam and placebo in recreational central nervous system depressant users. J Clin Pharmacol. 2020;60(9):1189–1197. doi:10.1002/jcph.1614
28. Eisenried A, Schuttler J, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II. Pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020;132(4):652–666. doi:10.1097/ALN.0000000000003102
29. Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi:10.1007/s00540-020-02788-6
30. Bae JY, Choi DY, Woo CH, et al. The BIS and hemodynamic changes in major burn patients according to a slow infusion of propofol for induction. Korean J Anesthesiol. 2011;60(3):161–166. doi:10.4097/kjae.2011.60.3.161
31. Kwak K, Kim J, Park S, et al. Reduction of pain on injection of propofol: combination of pretreatment of remifentanil and premixture of lidocaine with propofol. Eur J Anaesthesiol. 2007;24(9):746–750. doi:10.1017/S026502150600233X
32. Salman AE, Salman MA, Saricaoglu F, et al. Pain on injection of propofol: a comparison of methylene blue and lidocaine. J Clin Anesth. 2011;23(4):270–274. doi:10.1016/j.jclinane.2010.09.008
33. Angst MS, Mackey SC, Zupfer GH, et al. Reduction of propofol injection pain with a double lumen i.v. set. J Clin Anesth. 1997;9(6):462–466. doi:10.1016/s0952-8180(97)00101-3
34. McCulloch MJ, Lees NW. Assessment and modification of pain on induction with propofol (Diprivan). Anaesthesia. 1985;40(11):1117–1120. doi:10.1111/j.1365-2044.1985.tb10615.x
35. Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988;43(6):492–494. doi:10.1111/j.1365-2044.1988.tb06641.x
36. Turan A, Memis D, Kaya G, Karamanlioglu B. The prevention of pain from injection of propofol by dexmedetomidine and comparison with lidocaine. Can J Anaesth. 2005;52(5):548–549. doi:10.1007/BF03016541
37. Coulthard P, Rood JP. An investigation of the effect of midazolam on the pain experience. Br J Oral Maxillofac Surg. 1992;30(4):248–251. doi:10.1016/0266-4356(92)90268-n
38. Chen X, Mou X, He Z, Zhu Y. The effect of midazolam on pain control after knee arthroscopy: a systematic review and meta-analysis. J Orthop Surg Res. 2017;12(1):179. doi:10.1186/s13018-017-0682-0
39. Rouhi AJ, Zeraatchi A, Rahmani F, et al. Effect of oral midazolam in pain relief of patients need nasogastric tube insertion: a clinical trial study. J Res Pharm Pract. 2020;9(2):112–117. doi:10.4103/jrpp.JRPP_19_80
40. Xie H, Lu F, Liu W, et al. Remimazolam alleviates neuropathic pain via regulating bradykinin receptor B1 and autophagy. J Pharm Pharmacol. 2021;rgab080. doi:10.1093/jpp/rgab080
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

