Back to Journals » Vascular Health and Risk Management » Volume 13
Efficacy of noninvasive cardiac imaging tests in diagnosis and management of stable coronary artery disease
Authors Mordi IR, Badar AA , Irving RJ, Weir-McCall JR, Houston JG , Lang CC
Received 5 July 2017
Accepted for publication 7 October 2017
Published 21 November 2017 Volume 2017:13 Pages 427—437
DOI https://doi.org/10.2147/VHRM.S106838
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Naga Venkata Amarnath Kommuri
Ify R Mordi,1,2 Athar A Badar,2 R John Irving,2 Jonathan R Weir-McCall,1 J Graeme Houston,1 Chim C Lang1,2
1Division of Molecular and Clinical Medicine, University of Dundee, Dundee, UK; 2Department of Cardiology, Ninewells Hospital and Medical School, Dundee, UK
Abstract: The aim of this review was to discuss the current literature regarding the utility of noninvasive imaging in diagnosis and management of stable coronary artery disease (CAD) including recent data from large randomized trials assessing diagnosis and prognosis. Current guidelines recommend revascularization in patients with refractory angina and in those with potential prognostic benefit. Appropriate risk stratification through noninvasive assessment is important in ensuring patients are not exposed to unnecessary invasive coronary angiograms. The past 20 years have seen an unprecedented expansion in noninvasive imaging modalities for the assessment of stable CAD, with cardiovascular magnetic resonance and computed tomography complementing established techniques such as myocardial perfusion imaging, echocardiography and exercise electrocardiogram. In this review, we examine the current state-of-the-art in noninvasive imaging to provide an up-to-date analysis of current investigation and management options.
Keywords: angina, noninvasive imaging, SPECT, stress echo, cardiovascular magnetic resonance, CT coronary angiography
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, and despite advances in diagnosis and treatment, coronary artery disease (CAD) remains the number 1 cardiovascular (CV) cause of mortality and morbidity.1 Given the increasing burden of CAD worldwide, diagnostic techniques for identification of CAD are particularly important. In routine clinical practice, the definitive diagnosis of significant CAD is typically made during invasive coronary angiography (ICA), with significant disease being historically defined as ≥50% stenosis in the left main stem or ≥70% stenosis in other coronary arteries.2 The past 20 years have seen a rapid expansion in the number of different noninvasive imaging modalities used for the assessment of stable CAD. In addition to established noninvasive techniques such as the exercise electrocardiogram (exECG), myocardial nuclear perfusion imaging (MPI – most commonly single-photon emission computed tomography [SPECT]) and stress echocardiography, newer techniques such as CV magnetic resonance (CMR) and computed tomography (CT) are likely to have a significant clinical utility in the future. The aim of this review was to discuss the current literature on the efficacy of noninvasive imaging tests compared to ECG and MPI in the diagnosis of stable CAD.
Current recommendations for diagnostic testing in stable CAD
Current European and American guidelines for the assessment of stable CAD2,3 recommend a common approach. First, an assessment of the pretest probability (PTP) of CAD is made on the basis of clinical history, examination and basic tests such as the ECG. Following this assessment of PTP, a decision on further investigation should be taken. In patients with high PTP (>85%), no further noninvasive testing is required to make the diagnosis of CAD, and ICA can be performed for risk stratification. Conversely, in patients at low risk (PTP < 15%), the likelihood of CAD is so low that consideration should be directed toward non-CAD causes of chest pain. If CAD still needs to be excluded in patients with low PTP, CT coronary angiography (CTCA) has a high negative predictive value and is useful in this group.
Further noninvasive testing for CAD is particularly required in the intermediate-risk group (PTP 15–85%). This group comprises the majority of patients referred to the clinic for evaluation of chest pain, and therefore, imaging plays a key role in risk stratification and management. The main aim of noninvasive testing is to select patients likely to benefit from ICA and revascularization. The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial demonstrated that although percutaneous coronary intervention (PCI) in addition to optimal medical therapy (OMT) reduced frequency of angina compared to OMT alone, there was no reduction in death, nonfatal myocardial infarction [MI] or hospitalization for acute coronary syndrome (ACS).4 This trial, along with evidence from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI-2D) trial and from meta-analyses,5–7 has led to the general guideline recommendation that invasive angiography revascularization with PCI should only be performed in patients whose symptoms are refractory to OMT, or in those in whom there is likely to be prognostically significant disease. In addition, however, ICA can also be performed for “softer” reasons, in particular, to provide diagnostic clarity and reassurance.
In light of these studies, the role of noninvasive imaging in guiding selection of patients for ICA has become increasingly important. We now discuss each of these noninvasive techniques in turn. Table 1 summarizes all discussed modalities.
  | Table 1 Summary of non-invasive imaging modalities for assessment of suspected stable angina Note: Median ionizing radiation dose for invasive coronary angiography is 7.4 mSv.78 Abbreviations: CMR, cardiovascular magnetic resonance; CT, computed tomography; CTCA, CT coronary angiography; exECG, exercise echocardiography; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; LBBB, left bundle branch block; LGE, late gadolinium enhancement; LV, left ventricular; MPS, myocardial perfusion scintigraphy; MR, magnetic resonance; PET, positron emission tomography; SPECT, single-photon emission computed tomography. |
exECG
The exECG is one of the most widely studied and utilized techniques for the identification of ischemia. Exercise can be performed using either treadmill or exercise bicycle (supine or upright). The most commonly conducted technique for the exECG is the Bruce protocol.8 This involves exercise increased incrementally at 3-minute intervals until the patient reaches a desired target (most commonly heart rate of 85% of predicted heart rate for age) or until the patient can no longer continue. Continuous 12-lead ECG monitoring and noninvasive blood pressure are measured throughout. Typical end points suggestive of the presence of ischemia include ST segment elevation, ST segment depression (downsloping or flat and >2 mm), development of ventricular arrhythmias or reproduction of angina symptoms. exECG has the advantage of being cheap and relatively easy to perform and interpret as well as providing rapidly available clinically useful information which can be used to direct further investigation or reassure patients.
One of the main limitations of exECG is the presence of resting ECG abnormalities such as left bundle branch block (LBBB). Lack of physical fitness also may provide inconclusive results. In addition, there is the potential for induction of ventricular arrhythmias.
exECG performs moderately for the diagnosis of significant CAD. A meta-analysis of 34 studies including more than 3,000 patients by Banerjee et al8 reported that a negative test still leads to a diagnosis of CAD in 37% of men and 18% of women. exECG also has a significant amount of prognostic data, with a negative, low-risk test conferring a very good prognosis.10,11 In addition to the assessment of ischemia, functional capacity on exercise testing also provides important prognostic information, with patients able to exercise to 10 metabolic equivalents (METs) having an extremely low risk of major adverse CV events independent of the amount of ischemia.12–14
Despite these limitations and its downgrading in recent guidelines, exECG continues to be used in many centers due to its easy availability and clinicians’ confidence with its performance. It is also still important in other settings such as in exercise-induced arrhythmias.
Nuclear imaging
Although encompassing a number of techniques, the most commonly used nuclear imaging technique is SPECT. This technique can be performed using either exercise or pharmacological methods (using vasodilators such as dipyridamole) and relies upon the uptake of radioactive tracers into the myocardium at rest and stress. The most commonly used tracers are thallium and technetium. The heart is scanned using a gamma camera to assess the perfusion of the tracer into the myocardium. A positive test for inducible ischemia is obtained when there is a relative reduction in uptake of the tracer at stress due to the reduction in blood flow to the ischemic territory compared to normal tissue which becomes hyperemic. SPECT is also able to provide information on viability as areas of previous MI which are nonviable will display a fixed perfusion defect with a reduction in tracer uptake at both rest and stress. SPECT is also able to provide an estimate of left ventricular function.
One limitation of SPECT is in the assessment of “balanced ischemia.”15 Because interpretation of the study relies on comparison of ischemic areas to normal areas, in patients (such as those with significant triple vessel disease) where there is widespread ischemia throughout the myocardium, there is the possibility of incorrectly reporting a scan as showing no inducible ischemia when in fact all areas are relatively underperfused during stress, thus causing a false-negative study.
A further limitation is caused by the relatively high radiation dose, which most recently has been reported as around 7 mSv.16 Furthermore, many of the commonly used tracers take some time to leave the myocardium. Given this, many protocols involve the initial acquisition of stress images, with rest images taken at a later interval (depending on the tracer this can be up to 1 week later), thus meaning that results can be delayed as well as the study involving two visits for the patient. SPECT is also affected by LBBB. Because the septum is dyskinetic and contracts at a slightly different time to the lateral wall, the septum may not thicken at the time of image acquisition. Because the septum appears thinner, tracer uptake in the area may appear less, giving the impression of left anterior descending artery (LAD) territory ischemia. This is less of an issue using vasodilators rather than exercise however.17
Despite these limitations, there is a wealth of diagnostic and prognostic information on the use of SPECT for the assessment of stable CAD.
One important study on the prognosis of stable CAD patients using SPECT was the COURAGE nuclear substudy.18 This was an observational substudy of the COURAGE trial in which 314 patients underwent SPECT imaging at baseline and at 6–18 months post randomization. The study found that patients who underwent PCI + OMT had a greater reduction in ischemia than those who had OMT alone. There was also a trend to improved outcomes, with patients who had no residual ischemia having no clinical events. This substudy was underpowered for clinical events, however, and so the question of ischemia burden and using PCI to improve prognosis is still uncertain.
Another nuclear imaging modality that has recently been studied for use in stable CAD is positron emission tomography (PET). One particular advantage of this technique is that it gives the ability to measure absolute myocardial blood flow, thus avoiding problems with balanced ischemia.19 Coronary flow reserve is then calculated from the difference in blood flow in stress and rest.20 In addition, it can be combined with CT to provide both functional and anatomical assessment of CAD. The most common PET tracers are rubidium-82 and 13N-labeled ammonia, although various other tracers can be used. A meta-analysis by Mc Ardle et al20 reported an increased diagnostic accuracy compared to SPECT for diagnosis in patients with suspected CAD, with a sensitivity of 90% and specificity of 95%. There have also been some recent data on prognosis using PET, with several studies reporting a significant increase in adverse events in patients with reduced coronary flow reserve.22–24 PET using rubidium-82 has been shown to provide incremental prognostic value above clinical variables in a series of 1,432 patients undergoing scans, with an increasing burden of ischemia being associated with worse outcome.25
Despite these benefits, PET remains predominantly a research tool, with availability and expertise the predominant limitations to more widespread clinical use.
Stress echocardiography
Alongside SPECT, stress echocardiography is the most commonly used technique for the assessment of stable CAD. It can be performed using either exercise (treadmill or bicycle) or using pharmacological stress with dobutamine. Bicycle exercise has the advantage of allowing acquisition of images at peak exercise if performed while supine, whereas cycling in the upright position or on a treadmill requires the patient to be repositioned to an optimal position to obtain adequate echo images. Continuous 12-lead ECG monitoring is usually also performed. Of course, echocardiography remains the “workhorse” of cardiology, providing rapid assessment of ventricular volumes, function and assessment of valves. During stress echo, in ischemic segments, the wall motion abnormalities develop at peak exercise which are not present at rest (with wall motion abnormalities present at rest which do not improve on exercise suggestive of nonviable tissue). In addition, hibernating myocardium is associated with an improvement in wall motion on exercise. Exercise echocardiograph also provides a physiologically accurate assessment of the effects of exercise on cardiac function. This may be particularly important in the setting of associated dyspnea, which may be caused by exercise-induced or exacerbated mitral regurgitation, left ventricular outflow tract obstruction or pulmonary hypertension.
Dobutamine stress echocardiography (DSE) can also be performed. This has the advantage of being able to be used in patients unable to exercise. Unfortunately, some of the physiological assessment benefits (such as in exercise-induced pulmonary hypertension) are more difficult to interpret when dobutamine is used. Typical protocols involve the use of “high-dose” dobutamine (as opposed to “low dose” in patients with aortic stenosis), with increasing increments of dobutamine up to a usual maximum of 40 mg/kg/min, with the additional use of atropine if target heart rate is not reached. One advantage of DSE is the assessment of viability, which is more validated than with exercise echo. This relies on a bimodal change in wall motion, with ischemic but viable segments of myocardium typically initially improving in function at low doses of dobutamine (10–20 mg/kg/min) before function worsens again at high doses. This is because at low doses dobutamine initially acts as an inotropic agent, improving myocardial contractility, whereas at high doses its chronotropic and vasodilator effects are more predominant. In contrast, in nonviable tissue, wall motion remains impaired at all doses.26
Stress echo performs reasonably for the diagnosis of CAD, with all techniques performing similarly. Sensitivities are 85%, 80% and 78% and specificities are 77%, 86% and 91% for exercise, dobutamine and dipyridamole stress, respectively.27 While these values are not too different to SPECT,28 they are significantly better than exECG.29 In terms of prognosis, a normal stress echo has been associated with a mortality rate of around 1% per year in several large observational studies.30–33
Of course, there are limitations with stress echocardiography. First, as with resting echocardiography, scan quality can be limited by the patient body habitus and inability to obtain suitable windows. This difficulty is often exacerbated at the high heart rates caused by exercise. Endocardial border definition can be improved by the addition of left ventricular contrast, and many centers use this routinely. Contrast can also be used to provide an assessment of myocardial perfusion, although this is not typically used in standard clinical practice.34–36 Nevertheless, in some patients, despite considerable time and effort, adequate images are not available. A further limitation with the use of dobutamine is the potential for ventricular arrhythmias; thus, DSE is usually performed with a clinician and resuscitation facilities available.
Another limitation with stress echocardiography is that analysis is often qualitative, with a reviewer making a judgment call as to the presence and severity of any wall motion abnormality. Quantitative measures of wall motion using speckle tracking have been tested; however, their utility is still to be confirmed, particularly in the posterior circulation. Overall, speckle tracking does not appear to improve diagnostic accuracy sufficiently to mandate its incorporation into routine clinical practice.37,38
Three-dimensional (3D) echocardiography has some theoretical benefits, including faster acquisition (ability to obtain multiple planes in one scan) and more accurate assessment of ventricular volumes and function. 3D echo may be more accurate than 2D, particularly in the LAD territory.39,40 Again, there are no large trials to strongly support its routine use, and it still remains predominantly a technique used in specialist centers.
CMR
CMR has become increasingly utilized in clinical cardiology over the past 2 decades. CMR has a particular advantage in that it is able to offer noninvasive imaging in any plane without any limitations due to lungs or ribs and avoiding nonionizing radiation. The most useful technique for the assessment of stable CAD is stress CMR. Although there have been reports of exercise stress,41,42 stress CMR is traditionally performed using pharmacological stress. Both vasodilator (typically using adenosine or regadenoson) and dobutamine stress have been used. Vasodilator methods involve the administration of intravenous gadolinium contrast during vasodilator infusion, with the presence of perfusion defects (seen as subendocardial hypointense areas) in ischemic territories during peak hyperemia, typically defined as a 15 mmHg drop in systolic blood pressure and an increase in heart rate. Dobutamine stress CMR, on the other hand, uses a similar principle to that of DSE, relying on the demonstration of wall motion abnormalities in the presence of ischemia.43 Imaging of perfusion is commonly performed in three short-axis planes to obtain a 16-segment model, while wall motion abnormalities using dobutamine can also be imaged in long-axis planes.
In routine clinical practice, the assessment of perfusion defects by CMR is most commonly qualitative, with visual assessment by the operator of the presence and extent of ischemia. More recently, several groups have conducted research on quantitative measures of perfusion. These are either semiquantitative, using the difference in signal intensity between areas of the myocardium, or fully quantitative, measuring absolute blood flow using mathematical modeling.44 The use of qualitative measures appears to improve diagnostic accuracy; however, it does require further post-processing which makes its routine clinical use less attractive currently.45
CMR stress perfusion appears to have better diagnostic accuracy than stress echo and SPECT with a meta-analysis by Jaarsma et al46 reporting a sensitivity of 89% and specificity of 76% compared to ICA with visual assessment and a more recent meta-analysis by Takx et al47 reporting a sensitivity of 89% and specificity of 87% at the per-patient level compared to fractional flow reserve (FFR). There have also been a number of randomized controlled trials performed comparing CMR and SPECT. The Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial (MR-IMPACT) trial compared adenosine stress CMR vs. SPECT in 234 patients in 18 centers. The authors found that CMR performed better than SPECT when an optimal gadolinium dose (0.1 mmol/kg) was used, suggesting at least that it was a viable alternative to SPECT.48
The CMR and single-photon emission CT for the diagnosis of coronary heart disease (CE-MARC trial) by Greenwood et al48 was a trial conducted in two UK centers to determine the diagnostic accuracy of CMR (including adenosine stress, magnetic resonance [MR] angiography and late gadolinium enhancement [LGE] imaging at 1.5 T) vs. SPECT in the diagnosis of stable angina with a gold standard of ICA. In this trial, 752 patients judged to be at intermediate risk of CAD were randomized to CMR or SPECT. The authors again found that CMR had superior diagnostic accuracy to SPECT (CMR: sensitivity 86.5%, specificity 83.4%; SPECT: sensitivity 66.5%, specificity 82.6%), although they did find that MR angiography did not add any significant diagnostic benefit. These results were replicated in the multicenter, multi-vendor MR-IMPACT II, which again showed that adenosine stress CMR was superior to SPECT.50
While these studies suggested that CMR was at least as good, if not better than SPECT, there was still some doubt as to whether the cost and expertise required were worth investing in. The CE-MARC 2 trial, which randomized patients to a CMR-guided strategy, SPECT or UK National Institute for Health and Care Excellence guideline recommendation found that CMR was equivalent to SPECT and that both strategies reduced unnecessary angiography, the primary outcome of the trial.51
One important factor in these large randomized trials was the use of ICA as the “gold standard.” As discussed earlier, FFR assessment of ischemia is now accepted as the optimal measure of coronary lesion significance. The MR-INFORM trial was a multicenter trial comparing adenosine stress CMR at 1.5 T vs. FFR in patients with suspected stable angina.52 The study showed that CMR was non-inferior to FFR, with both conferring ≤4% risk of major adverse cardiovascular events within 1 year, providing some prognostic data. In addition, only 37 out of 454 patients (8.1%) in the CMR-guided arm had a negative invasive angiogram (48.8% had a negative CMR and did not proceed to invasive testing). The full results of the MR-INFORM study are yet to be reported; however, these preliminary results suggest that adenosine stress CMR could well be a useful technique in the setting of stable angina, with some strong prognostic data also.
Beyond the improvement in imaging quality compared to echocardiography,53 CMR has several other advantages. First, CMR is currently accepted as the noninvasive gold standard for the assessment of cardiac structure and function. This allows clearer assessment of wall motion abnormalities as well as providing further diagnostic information. Second, the use of gadolinium contrast also allows the assessment of myocardial fibrosis and scarring. Imaging performed 10–15 minutes after intravenous contrast injection, known as LGE imaging, can demonstrate areas of myocardial infarct scar. The presence of an infarct-type pattern can help in the differentiation between ischemic and non-ischemic cardiomyopathy, while the transmural extent of the infarct scar can be used to determine myocardial viability.54
Nevertheless, CMR has some limitations. The technique itself is relatively time-consuming, expensive and not readily available in all centers. In addition, not all patients are able to tolerate the scan, either due to claustrophobia or inability to breath hold adequately. Gadolinium contrast should be used with caution and only when strictly necessary in patients with an estimated glomerular filtration rate of <30 mL/min due to the risk of nephrogenic systemic fibrosis. Finally, image quality may be degraded by arrhythmia, although techniques such as real-time imaging can be used to mitigate this.
Cardiac CT (CCT)
Although CCT is a noninvasive imaging technique, it differs from all of the previously discussed techniques as it is a predominantly anatomical technique. CCT commonly refers to two different modalities – calcium scoring and CTCA. Both are carried out using ECG gating to minimize cardiac motion artifact, with images usually acquired in diastole where the heart is at its most still. In addition, breath holding is used to minimize respiratory motion artifact.
CT calcium scoring is performed without contrast. With modern 64-slice scanners, imaging is usually acquired in one breath hold. The noncontrast study is analyzed using rapid post-processing software to measure the amount of coronary calcium. The most commonly used scoring method is the Agatston score which takes into account the size and radiographic density of the plaque to provide a score which reflects the coronary calcified plaque burden.55 The presence of coronary artery calcification does reflect the burden of CV risk; however, not all significant obstructive coronary lesions are calcified; in contrast, heavily calcified coronary arteries can often be non-obstructed, hence calcium scoring alone cannot be used as a diagnostic tool in stable angina. Indeed, calcium scoring has fallen out of favor in recent guidelines.2
Currently, CTCA is the preferred method of CCT imaging. Current-generation 64-slice CT scanners have reduced concerns about radiation dose – mean radiation dose for one CCT is 1–2 mSv with appropriate scan optimization.56 Attaining these doses requires the patients to have slow (<60 bpm), regular heart rates. This can be achieved by oral and intravenous beta-blockade if necessary (calcium channel blockers and ivabradine can also be used). A bolus of contrast is injected intravenously, and using ECG gating, a coronary angiogram is obtained. CTCA provides excellent anatomical detail and has shown excellent diagnostic accuracy in comparison to ICA. CTCA is recommended for use in patients with low-to-intermediate risk of CAD. This is predominantly because of its high sensitivity (95–99%) and negative predictive value (97–99%) but low specificity (64–83%).57,58
Beyond its low specificity, ionizing radiation dose and scan preparation, CTCA does have some other limitations. Scan accuracy is reduced in the presence of heavy calcification, which can result in “blooming” artifact, which can make it difficult to interpret the study.59 In addition, CTCA is not as accurate in the diagnosis of in-stent restenosis, particularly in stents <3 mm.60 Because of this, CTCA is currently only recommended in low–intermediate-risk patients in the current guidelines, predominantly as a “rule-out” technique. Finally, CTCA is an anatomical technique, providing no functional information on stenosis severity. In the context of what is known about ICA, this does pose an issue. The Fractional Flow Reserve vs. Angiography for Guiding Percutaneous Coronary Intervention (FAME) trial demonstrated that use of FFR, an invasive measure of ischemia, leads to superior outcomes compared to visual stenosis assessment for guiding intervention.61 This, in combination with the COURAGE trial, has led to an acceptance within cardiology that revascularization in stable angina should preferentially be performed in functionally significant lesions only.62
Outcome data are also now emerging. The recent Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial was designed to compare outcomes in patients with CT vs. functional testing.63 In this trial of 10,003 intermediate-risk patients, CTCA was associated with significantly fewer invasive angiograms with no significant CAD; however, patients in the CTCA arm were more likely to undergo ICA within 90 days. Clinical outcomes were not different between the two cohorts. The SCOT-HEART trial, which randomized 4,146 patients with suspected angina to CTCA or standard care, revealed that CTCA changed management in many cases; however, it did not have any impact on readmission rates.64 There was also a reduction in MI with CTCA (26 vs. 42, p = 0.053).65 Several observational studies have also reported the prognostic significance of CAD identified on CTCA.66–68
Recently, two more novel techniques have been used to add functional assessment to CTCA and overcome this limitation. The first is CT perfusion imaging. This uses a similar principle to CMR perfusion, relying on the passage of (iodinated rather than gadolinium) contrast from the myocardial blood pool into the myocardium. Areas of infarction or ischemia appear hypointense compared to normal myocardium. Again, imaging can be performed at rest and stress to determine the functional significance of lesions.69 CT perfusion requires optimal timing, as continuous imaging is not performed to reduce radiation dose, unlike in CMR. Diagnostic accuracy appears to be reasonable; however, due to limited availability and radiation dose concerns it has not yet been adopted into routine clinical practice.47
Another promising technique that is under investigation is CT fractional flow reserve (CTFFR). This technique uses computational fluid dynamics to provide a prediction of the invasive FFR. One advantage of this technique is that it requires no further imaging beyond a standard CTCA and also does not involve administration of adenosine to induce hyperemia. In the most studied technique, CT scans are sent to a CTFFR company, which provides an FFR in all major vessels at any point in the coronary tree within 24 hours. The methods for measuring CTFFR are however proprietary and somewhat time-consuming, requiring intense computational fluid dynamics and computer modeling. In addition, there may be some reservations regarding sending studies to a separate company. Several companies however are working on CTFFR software which will be available on workstations to all clinicians. CTFFR has shown good reproducibility and diagnostic accuracy in several randomized trials. The Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial was the first proof of concept trial using a CTFFR in patients with known or suspected angina, reporting a diagnostic accuracy of 84.3% per vessel, and 87.4% per patient, with invasive FFR as the gold standard. Importantly, these were significantly improved compared to standard CTCA (58.5% and 61.2%, respectively).70 These promising results were also replicated in two further trials.71,72 The PLATFORM trial used CTFFR as part of a strategy comparing CTCA with standard care and found that CTCA led to reduced referrals for ICA and also had similar clinical outcomes at 1 year and lower cost than usual care.73,74 Further data are required however for this technique to reach routine clinical use.
Conclusion
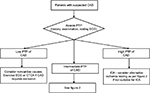
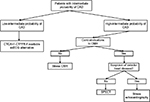
Overall, a recent meta-analysis by Takx et al47 suggested that in comparison to FFR, CMR perfusion, PET and CT perfusion were better techniques for diagnosing CAD at both vessel and patient level than SPECT and stress echocardiography. While SPECT and stress echo are both relatively cheap and widely available, as expertise is gained and more prognostic data become available, CMR, CT and PET may become more attractive for routine clinical practice. CT is quick and particularly useful in ruling out obstructive CAD, particularly in patients with low PTP; however, CMR and PET provide more information on myocardial structure and function. Figures 1 and 2 show a proposed management algorithm for the investigation of stable angina. This of course would need to be tailored dependent on the patient and the expertise available in the hospital. The ongoing ISCHEMIA trial (NCT01471522), which in contrast to both COURAGE and BARI-2D, will randomize patients to OMT or an invasive strategy prior to ICA, but after noninvasive imaging has been performed will also provide further insights into the role of noninvasive imaging in stable CAD. While none of these techniques is likely to be the sole one used, in correctly identified appropriate patients, they will undoubtedly provide useful information with which to guide management.
Disclosure
IRM is supported by an NHS Education for Scotland/Chief Scientist Office Post-Doctoral Clinical Lectureship (PCL/17/07). The authors report no other conflicts of interest in this work.
References
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. | ||
Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. | ||
Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. | ||
Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. | ||
Bangalore S, Pursnani S, Kumar S, Bagos PG. Percutaneous coronary intervention versus optimal medical therapy for prevention of spontaneous myocardial infarction in subjects with stable ischemic heart disease. Circulation. 2013;127(7):769–781. | ||
Pursnani S, Korley F, Gopaul R, et al. Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: a systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv. 2012;5(4):476–490. | ||
The BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–2515. | ||
Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res. 1971;3(6):323–332. | ||
Banerjee A, Newman DR, Van den Bruel A, Heneghan C. Diagnostic accuracy of exercise stress testing for coronary artery disease: a systematic review and meta-analysis of prospective studies. Int J Clin Pract. 2012;66(5):477–492. | ||
Roger VL, Jacobsen SJ, Pellikka PA, Miller TD, Bailey KR, Gersh BJ. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation. 1998;98(25):2836–2841. | ||
Mark DB, Shaw L, Harrell FE Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325(12):849–853. | ||
Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of > or = 10 metabolic equivalents predicts a very low risk of inducible ischemia: does myocardial perfusion imaging have a role? J Am Coll Cardiol. 2009;54(6):538–545. | ||
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. | ||
Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. J Am Coll Cardiol. 1997;30(3):641–648. | ||
Aarnoudse WH, Botman KJ, Pijls NH. False-negative myocardial scintigraphy in balanced three-vessel disease, revealed by coronary pressure measurement. Int J Cardiovasc Intervent. 2003;5(2):67–71. | ||
Rausch I, Fuchsel FG, Kuderer C, Hentschel M, Beyer T. Radiation exposure levels of routine SPECT/CT imaging protocols. Eur J Radiol. 2016;85(9):1627–1636. | ||
Lebtahi NE, Stauffer JC, Delaloye AB. Left bundle branch block and coronary artery disease: accuracy of dipyridamole thallium-201 single-photon emission computed tomography in patients with exercise anteroseptal perfusion defects. J Nucl Cardiol. 1997;4(4):266–273. | ||
Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–1291. | ||
Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. JACC Cardiovasc Imaging. 2009;2(6):751–758. | ||
deKemp RA, Yoshinaga K, Beanlands RS. Will 3-dimensional PET-CT enable the routine quantification of myocardial blood flow? J Nucl Cardiol. 2007;14(3):380–397. | ||
Mc Ardle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease? A systematic review and meta-analysis. J Am Coll Cardiol. 2012;60(18):1828–1837. | ||
Fukushima K, Javadi MS, Higuchi T, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med. 2011;52(5):726–732. | ||
Yamamoto M, Kamata Y, Iida T, et al. Quantification of activated and total caspase-14 with newly developed ELISA systems in normal and atopic skin. J Dermatol Sci. 2011;61(2):110–117. | ||
Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. | ||
Dorbala S, Hachamovitch R, Curillova Z, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. 2009;2(7):846–854. | ||
Senior R, Lahiri A. Dobutamine echocardiography predicts functional outcome after revascularisation in patients with dysfunctional myocardium irrespective of the perfusion pattern on resting thallium-201 imaging. Heart. 1999;82(6):668–673. | ||
Senior R, Monaghan M, Becher H, Mayet J, Nihoyannopoulos P; British Society of Echocardiography. Stress echocardiography for the diagnosis and risk stratification of patients with suspected or known coronary artery disease: a critical appraisal. Supported by the British Society of Echocardiography. Heart. 2005;91(4):427–436. | ||
Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS. Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA. 1998;280(10):913–920. | ||
Geleijnse ML, Elhendy A. Can stress echocardiography compete with perfusion scintigraphy in the detection of coronary artery disease and cardiac risk assessment? Eur J Echocardiogr. 2000;1(1):12–21. | ||
Marwick TH, Case C, Vasey C, Allen S, Short L, Thomas JD. Prediction of mortality by exercise echocardiography: a strategy for combination with the duke treadmill score. Circulation. 2001;103(21):2566–2571. | ||
McCully RB, Roger VL, Mahoney DW, et al. Outcome after normal exercise echocardiography and predictors of subsequent cardiac events: follow-up of 1,325 patients. J Am Coll Cardiol. 1998;31(1):144–149. | ||
Sicari R, Pasanisi E, Venneri L, Landi P, Cortigiani L, Picano E; Echo Persantine International Cooperative (EPIC) Study Group; Echo Dobutamine International Cooperative (EDIC) Study Group. Stress echo results predict mortality: a large-scale multicenter prospective international study. J Am Coll Cardiol. 2003;41(4):589–595. | ||
Chung G, Krishnamani R, Senior R. Prognostic value of normal stress echocardiogram in patients with suspected coronary artery disease – a British general hospital experience. Int J Cardiol. 2004;94(2–3):181–186. | ||
Dijkmans PA, Senior R, Becher H, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol. 2006;48(11):2168–2177. | ||
Gaibazzi N, Rigo F, Reverberi C. Detection of coronary artery disease by combined assessment of wall motion, myocardial perfusion and coronary flow reserve: a multiparametric contrast stress-echocardiography study. J Am Soc Echocardiogr. 2010;23(12):1242–1250. | ||
Mattoso AA, Tsutsui JM, Kowatsch I, et al. Prognostic value of dobutamine stress myocardial perfusion echocardiography in patients with known or suspected coronary artery disease and normal left ventricular function. PLoS One. 2017;12(2):e0172280. | ||
Hanekom L, Cho GY, Leano R, Jeffriess L, Marwick TH. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: an angiographic correlation. Eur Heart J. 2007;28(14):1765–1772. | ||
Aggeli C, Lagoudakou S, Felekos I, et al. Two-dimensional speckle tracking for the assessment of coronary artery disease during dobutamine stress echo: clinical tool or merely research method. Cardiovasc Ultrasound. 2015;13:43. | ||
Aggeli C, Giannopoulos G, Misovoulos P, et al. Real-time three-dimensional dobutamine stress echocardiography for coronary artery disease diagnosis: validation with coronary angiography. Heart. 2007;93(6):672–675. | ||
Badano LP, Muraru D, Rigo F, et al. High volume-rate three-dimensional stress echocardiography to assess inducible myocardial ischemia: a feasibility study. J Am Soc Echocardiogr. 2010;23(6):628–635. | ||
Pflugi S, Roujol S, Akcakaya M, et al. Accelerated cardiac MR stress perfusion with radial sampling after physical exercise with an MR-compatible supine bicycle ergometer. Magn Reson Med. 2015;74(2):384–395. | ||
Foster EL, Arnold JW, Jekic M, et al. MR-compatible treadmill for exercise stress cardiac magnetic resonance imaging. Magn Reson Med. 2012;67(3):880–889. | ||
Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. | ||
Patel AR, Antkowiak PF, Nandalur KR, et al. Assessment of advanced coronary artery disease: advantages of quantitative cardiac magnetic resonance perfusion analysis. J Am Coll Cardiol. 2010;56(7):561–569. | ||
Mordini FE, Haddad T, Hsu LY, et al. Diagnostic accuracy of stress perfusion CMR in comparison with quantitative coronary angiography: fully quantitative, semiquantitative, and qualitative assessment. JACC Cardiovasc Imaging. 2014;7(1):14–22. | ||
Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719–1728. | ||
Takx RA, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015;8(1):e002666. | ||
Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29(4):480–489. | ||
Greenwood JP, Motwani M, Maredia N et al. Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial. Circulation. 2014; 11;129(10):1129–1138. | ||
Schwitter J, Wacker CM, Wilke N et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. European Heart Journal. 2013; 34(10):775–781. | ||
Greenwood JP, Ripley DP, Berry C, et al; CE-MARC 2 Investigators. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 randomized clinical trial. JAMA. 2016;316(10):1051–1060. | ||
Hussain ST, Paul M, Plein S, et al. Design and rationale of the MR-INFORM study: stress perfusion cardiovascular magnetic resonance imaging to guide the management of patients with stable coronary artery disease. J Cardiovasc Magn Reson. 2012;14:65. | ||
Mordi I, Stanton T, Carrick D, et al. Comprehensive dobutamine stress CMR versus echocardiography in LBBB and suspected coronary artery disease. JACC Cardiovasc Imaging. 2014;7(5):490–498. | ||
Romero J, Xue X, Gonzalez W, Garcia MJ. CMR imaging assessing viability in patients with chronic ventricular dysfunction due to coronary artery disease: a meta-analysis of prospective trials. JACC Cardiovasc Imaging. 2012;5(5):494–508. | ||
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. | ||
Einstein AJ. Radiation dose reduction in coronary CT angiography: time to buckle down. JACC Cardiovasc Imaging. 2015;8(8):897–899. | ||
Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52(21):1724–1732. | ||
Mowatt G, Cook JA, Hillis GS, et al. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94(11):1386–1393. | ||
Min JK, Swaminathan RV, Vass M, Gallagher S, Weinsaft JW. High-definition multidetector computed tomography for evaluation of coronary artery stents: comparison to standard-definition 64-detector row computed tomography. J Cardiovasc Comput Tomogr. 2009;3(4):246–251. | ||
Andreini D, Pontone G, Bartorelli AL, et al. Comparison of feasibility and diagnostic accuracy of 64-slice multidetector computed tomographic coronary angiography versus invasive coronary angiography versus intravascular ultrasound for evaluation of in-stent restenosis. Am J Cardiol. 2009;103(10):1349–1358. | ||
Tonino PA, De Bruyne B, Pijls NH, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. | ||
Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35(37):2541–2619. | ||
Douglas PS, Hoffmann U, Patel MR, et al; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–1300. | ||
SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385(9985):2383–2391. | ||
Williams MC, Hunter A, Shah AS, et al; SCOT-HEART Investigators. Use of coronary computed tomographic angiography to guide management of patients with coronary disease. J Am Coll Cardiol. 2016;67(15):1759–1768. | ||
Bittencourt MS, Hulten E, Ghoshhajra B, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–291. | ||
Kang SH, Park GM, Lee SW, et al. Long-term prognostic value of coronary CT angiography in asymptomatic type 2 diabetes mellitus. JACC Cardiovasc Imaging. 2016;9(11):1292–1300. | ||
Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 2012;5(7):690–701. | ||
Mordi I, Tzemos N. Incremental value of CT perfusion in the diagnosis of coronary artery disease. Eur Heart J Cardiovasc Imaging. 2013;14(5):504. | ||
Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58(19):1989–1997. | ||
Leipsic J, Yang TH, Thompson A, et al. CT angiography (CTA) and diagnostic performance of noninvasive fractional flow reserve: results from the determination of fractional flow reserve by anatomic CTA (DeFACTO) study. AJR Am J Roentgenol. 2014;202(5):989–994. | ||
Norgaard BL, Leipsic J, Gaur S, et al; NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014;63(12):1145–1155. | ||
Douglas PS, Pontone G, Hlatky MA, et al; PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J. 2015;36(47):3359–3367. | ||
Douglas PS, De Bruyne B, Pontone G, et al. PLATFORM Investigators. 1-year outcomes of FFRCT-guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol. 2016;68(5):435–445. | ||
Thompson RC, O’Keefe JH, McGhie AI, Bybee KA, Thompson EC, Bateman TM. Reduction of SPECT MPI radiation dose using contemporary protocols and technology. JACC Cardiovasc Imaging. Epub 2017 May 2. | ||
Danad I, Szymonifka J, Twisk JWR, et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J. 2017;38(13):991–998. | ||
Doris M, Newby DE. Coronary CT angiography as a diagnostic and prognostic tool: perspectives from the SCOT-HEART trial. Curr Cardiol Rep. 2016;18(2):18. | ||
Carpeggiani C, Picano E, Brambilla M, et al. Variability of radiation doses of cardiac diagnostic imaging tests: The RADIO-EVINCI study (RADIationdOse subproject of the EVINCI study). BMC Cardiovasc Disord. 2017;17:63. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.