Back to Journals » Infection and Drug Resistance » Volume 14
Efficacy of Low-Dose Trimethoprim/Sulfamethoxazole for the Treatment of Pneumocystis jirovecii Pneumonia in Deceased Donor Kidney Recipients
Authors Ji J, Wang Q, Huang T, Wang Z , He P, Guo C, Xu W, Cao Y, Dong Z, Wang H
Received 18 September 2021
Accepted for publication 17 November 2021
Published 24 November 2021 Volume 2021:14 Pages 4913—4920
DOI https://doi.org/10.2147/IDR.S339622
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jianlei Ji,* Qinghai Wang,* Tao Huang, Ziyu Wang, Pingli He, Chen Guo, Weijia Xu, Yanwei Cao, Zhen Dong, Hongyang Wang
Department of Kidney Transplantation, the Affiliated Hospital of Qingdao University, Qingdao, 266000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhen Dong; Hongyang Wang
Department of Kidney Transplantation, the Affiliated Hospital of Qingdao University, No. 59 Haier Road, Qingdao, 266000, People’s Republic of China
Tel +8613455263336
; +8618661803752
Email [email protected]; [email protected]
Background: Trimethoprim/sulfamethoxazole (TMP-SMX) is considered the first-choice treatment for Pneumocystis jirovecii pneumonia (PJP) in recipients of solid organ transplantation. However, this treatment is associated with various severe adverse events that might not be tolerable for some renal transplant recipients, and the optimal dose remains elusive. The present study assessed the efficacy of low-dose TMP-SMX in recipients of a deceased donor kidney.
Methods: A total of 37 adult deceased donor kidney recipients who suffered PJP between January 2015 and June 2020 were included. The survival rates of the patients and grafts, the rate of invasive ventilation, and adverse events, including gastrointestinal discomfort, hematologic side effects, hyperkalemia, and renal function impairments, were assessed.
Results: The patient and graft survival rates were both 100%. Two patients (5.4%) required invasive ventilation. Eight patients (21.6%) reported gastrointestinal discomfort, but none required dose reduction or discontinued treatment. The frequencies of hematologic side effects, hyperkalemia and impaired kidney function were 5.4% (2/37), 2.7% (1/37), and 2.7% (1/37), respectively.
Conclusion: Optimization of TMP-SMX dose may reduce the risk of adverse events without compromising efficacy for the treatment of PJP in deceased donor kidney recipients.
Keywords: efficacy, low dose, trimethoprim/sulfamethoxazole, Pneumocystis jirovecii pneumonia, deceased donor kidney recipients
Introduction
Pneumocystis jirovecii pneumonia (PJP) is a life-threatening complication in recipients of solid organ transplantation with a reported incidence of 0.6%–15%. The mortality rate for PJP can reach 50% despite aggressive antibiotic therapy.1–3
Trimethoprim/sulfamethoxazole (TMP-SMX) is considered the first-choice treatment for PJP in renal transplant recipients.4 The standard dosage is 15–20 mg/kg of TMP and 75–100 mg/kg of SMX per day administered 3–4 times a day intravenously or orally.5 This dose is unfortunately associated with severe adverse effects, such as cytopenias (leucopenia and thrombocytopenia), increased serum creatinine, hyperkalemia, and gastrointestinal symptoms. These adverse events are not always well-tolerated and sometimes result in discontinuation of treatment.6,7 In addition, the minimum inhibitory concentration (MIC) of TMP-SMX against P. jirovecii infection has not been standardized.8
Some reports have suggested that dose reduction of TMP-SMX might reduce the risk of adverse events while retaining efficacy in the treatment of PJP. Li et al reduced the dose of TMP-SMX for renal transplant recipients with PJP that experienced intolerable gastrointestinal side effects and still achieved a high recovery rate of 83%.9 Similarly, Tu et al successfully treated three cases of severe PJP in renal transplant recipients with combination caspofungin and low-dose TMP-SMX (480 mg q8h).10
In the absence of randomized controlled trials, the optimal dosage of TMP-SMX in PJP remains unknown, and the existing literature reports conflicting results. The present study aimed to assess the efficacy of low-dose TMP-SMX for PJP treatment in recipients of a deceased donor kidney.
Materials and Methods
Enrollment of Participants
The present retrospective, single-center cohort study enrolled adult deceased donor kidney recipients at the Affiliated Hospital of Qingdao University (Qingdao, China) between January 2015 and June 2020. Rabbit anti-human thymocyte immunoglobulin (rATG), basiliximab, or ATG-F-Fresenius (ATG-F) were used to prevent immediate transplant rejection, and tacrolimus, mycophenolic acid (MPA), and prednisone were used to maintain immunosuppression thereafter. The inclusion criteria according to the institutional protocol were as follows: i) Chinese ethnicity; ii) received kidney transplant from a deceased donor; iii) experienced PJP after transplantation; and iv) treated with TMP-SMX. Patients who received a simultaneous non-renal transplant, who were lost to follow up, or who used any experimental medications were excluded.
The donated organs were obtained with full informed consent from the next of kin of the donor. Kidney donations were conducted in accordance with the Declaration of Istanbul. This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Qingdao, China; no. QYFYWZLL 26483). All procedures involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethics standards. Written informed consent was obtained from all individual participants included in this study.
Immunization and Prophylaxis Program
The induction regimen consisted of five doses of rATG and two doses of basiliximab or ATG-F. If rATG was administered, then 50 mg was administered on post-operative day (POD) 0 and 25 mg on PODs 1, 2 and 3. If basiliximab was administered, then it was administered on PODs 0 and 4. ATG-F was administered as described previously.11 Maintenance therapy with oral tacrolimus, MPA, corticosteroids, and prophylaxis for P. jiroveci and cytomegalovirus (CMV) also was administered as described previously.11
Diagnosis of PJP
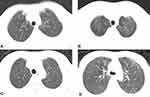
The diagnosis of PJP was made when Pneumocystis cysts or trophozoites were detected in bronchoalveolar lavage (BAL) fluid or sputum samples using Gomori’s methenamine silver staining (GMS) and patients’ computed tomography (CT) scans showed diffuse, patchy, and cloud-like density shadows with ground-glass-like morphology that were symmetrical and apically distributed in both lungs (Figure 1).
Treatment of PJP
All patients included in the present study were treated with oral TMP–SMX at dosages of 480/2400 mg per day divided into 3 doses, or at dosages of 240/1200 mg per day divided into 3 doses as previously reported10 when the serum creatinine levels exceed 2 mg/dl. The antifungal medication caspofungin (50 mg) was administered once a day. Carbapenems or third generation cephalosporin was used as prophylaxis against bacterial infection. Methylprednisolone was intravenously administered at a dose of 20 mg q12h, and at a dose of 40 mg q12h in critically ill patients with respiratory failure. Calcineurin inhibitor was continuously infused, and the concentration was maintained at around 5 ng/mL. Depending on the severity of the disease, mycophenolic acid was reduced or discontinued. Oxygen was delivered via nasal cannula or oxygen mask, or even invasive ventilation if necessary. CT scanning was carried out every 5–7 days to assess the progression of the pulmonary lesions.
Endpoint Definitions
The primary endpoints were the rates of patient and graft survival. Secondary endpoints included the rates of invasive ventilation and adverse effects, such as cytopenia (leucopenia and thrombocytopenia), increased serum creatinine, hyperkalemia, and gastrointestinal discomfort.
Statistical Analysis
Data for continuous variables are expressed as mean ± standard deviation if normally distributed or as median (range) if non-normally distributed. All statistical analyses were performed using SPSS 23 (IBM Corp., Armonk, NY).
Results
Clinical Characteristics of Study Participants
Thirty-seven of the 1098 deceased donor kidney recipients screened between January 2015 and June 2018 were included in the final analysis. The demographic characteristics of these patients are presented in Table 1. The average age of the included patients was 45.9±10.1 years (range, 27–64 years). Out of the 37 patients, 26 were male (70.3%) and 11 were female (29.7%).
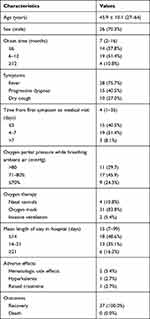 |
Table 1 Demographic Characteristics, Symptoms, Treatments and Outcomes |
Clinical Presentations of Kidney Transplant Recipients
The majority of the kidney transplant recipients (86.5%, 950/1098) received PJP prophylaxis with TMP-SMX for 6 months, while 13.5% (148/1098) did not receive or discontinued TMP-SMX within 3 months after transplantation due to intolerable adverse effects such as an increased serum creatinine level or severe leukopenia. None of the included patients were receiving TMP-SMX at the time when PJP occurred. The median time to occurrence of PJP after renal transplantation among the included patients was 7 months (range, 2–16 months). Onset of PJP occurred within 6 months after renal transplantation in 37.8% (14/37) of the patients, within 6–12 months in 51.4% (19/37) of the patients, and after more than 1 year in 10.8% (4/37) of the patients. PJP occurred within 1 month after immunosuppressant treatment in 5.4% (2/37) of patients. The median time from onset of symptoms to treatment was 4 days (range, 1–35 days). The most common symptom was fever (28/37, 75.7%), followed by progressive dyspnea (15/37, 40.5%), and dry cough (10/37, 27.0%; Table 1).
Imaging Features of PJP in Kidney Transplant Recipients Treated with Low-Dose TMP-SMX
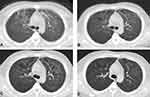
During the initial phases of PJP, the patients’ lung imaging changes were as described above. Air bronchogram was present in 16.2% (6/37) of the patients, and 8.1% (3/37) presented with multiple nodules in the mediastinum (Table 2). Pulmonary lesions were improved on CT scans after 5–6 days of treatment and significantly improved after 10 days in most patients. Complete absorption of the lesions or residual fibrosis was seen on CT scans during follow-up at 1–2 months after treatment (Figure 2).
 |
Table 2 Laboratory and Radiological Findings |
Sputum smears were carried out in all included patients at the time of admission, but PJP was only detected in 5.4% (2/37) of the patients. The detection rate was significantly higher when induced sputum or BAL was performed. Cysts and trophozoites were detected by GMS in 76.5% (13/17) of the patients subjected to induced sputum testing, and 80.0% (24/30) of the patients that underwent BAL (Table 2).
Laboratory Test Results in Kidney Transplant Recipients with PJP Treated Using Low-Dose TMP-SMX
Elevated 1,3-β-D-glucan levels were seen in 83.8% (31/37) of the patients and the median level was 353 pg/mL (<10–1288 pg/mL). Increased lactate dehydrogenase levels, with a median level of 260 mmol/L (107–449 mmol/L) were found in 59.5% (22/37) of the patients. Decreased lymphocyte counts with a mean value of 0.83±0.44 ×109/L were present in 81.1% (30/37) of the patients. Increased white blood cell counts were found in only 16.2% (6/37) of the patients, and the mean value was 7.03±2.76 × 109/L. CMV was detected in 16.2% (6/37) of the patients, and 5.4% (2/37) of the patients were positive for BK virus (Table 2).
Clinical Outcomes and Adverse Effects of Low-Dose TMP-SMX Treatment for PJP in Kidney Transplant Recipients
The survival rates of patients and grafts were both 100%. The median length of hospital stay was 15 days (7–99 days; Table 1); 48.6% (18/37) of the patients were discharged within 2 weeks; and 35.1% (13/37) of the patients were discharged after 2–3 weeks (Table 1). Only 5.4% (2/37) of the patients required invasive ventilation (Table 1). Gastrointestinal discomfort was experienced by 21.6% (8/37) of the patients, and the symptoms improved following usage of proton pump inhibitors and mucosal protective agents. The frequencies of hematologic side effects, hyperkalemia, and transient impaired kidney function were 5.4% (2/37), 2.7% (1/37), and 2.7% (1/37), respectively (Table 1). No cases required dose reduction of TMP-SMX or treatment discontinuation due to adverse effects.
Discussion
There is no consensus on the optimal dose of TMP-SMX for the treatment of PJP in renal transplant recipients. TMP-SMX is associated with various severe adverse events that are not well-tolerated by some renal transplant recipients. Finding the lowest possible effective dose with the best adverse event profile is therefore of clinical importance. The present study presents evidence that low-dose TMP-SMX can provide adequate protection against PJP with a low incidence of adverse effects in deceased donor kidney recipients.
According to previous reports, the incidence of PJP was highest within 6 months after renal transplantation after intensifying immunosuppression.5,9 The Kidney Disease Improving Global Outcomes (KDIGO) and Kidney Health Australia Caring for Australians with Renal Impairment (KHA-CARI) guidelines recommend administering TMP-SMX for 3–6 months after kidney transplantation to prevent PJP.12,13 In the present study, all recipients received TMP-SMX as PJP prophylaxis for 6–12 months (86.5% of the patients received TMP-SMX for 6 months) unless severe leukopenia or kidney dysfunction (serum creatinine level exceeding 2 mg/dl) were present. This might be why 62.2% (23/37) of the patients developed PJP more than 6 months after transplantation. When TMP-SMX (80 mg/400 mg) was administered for 12 months after transplantation, the median time to onset of PJP was 17 months after transplantation and only 23.1% (3/13) recipients developed PJP within 1 year.14 It seems that the time from transplantation to PJP onset varies depending on the prevention program of each center and is delayed when the prophylaxis time is extended.
Elevated 1,3-β-D-glucan levels were seen in 83.8% (31/37) of the patients. Beta-D-glucan is a main structural component of P. jirovecii and the detection of beta-D-glucan has a high diagnostic value in HIV-infected patients.15 Elevated serum beta-D-glucan concentrations were also confirmed to be a good noninvasive indicator of P. jirovecii infection in renal transplant patients according to a previous study.16 It cannot be ignored that 1,3-β-D-glucan levels were not elevated in 6 of the patients in the present study. Although the sensitivity and specificity of beta-D-glucan vary according to differences in diagnostic criteria, detection time and cut-off value, these patients might be just colonized and not truly infected with P. jirovecii. The clinical relevance of beta-D-glucan needs to be explored in larger studies in renal transplant patients.
Cytomegalovirus (CMV) infection is considered to be an independent non-immunological risk factor for PJP.17 It has been reported that almost half of the kidney transplant recipients (46%) that suffer from PJP have experienced a previous or simultaneous CMV infection.14 In the present study, 16.2% (6/37) of the patients experienced a simultaneous CMV infection. CMV alters host immune responses through a variety of mechanisms, ultimately suppressing the functions of helper T and antigen-presenting cells.18 Therefore, one opportunistic infection in a transplant recipient should trigger an extensive search for another one.3
TMP-SMX is the first-line treatment for PJP both in HIV patients and non-HIV patients.4,19 Oral TMP–SMX therapy has excellent bioavailability and outstanding effectiveness and is widely used in clinical practice, but intravenous administration of TMP–SMX has also been reported in patients with mild to severe PJP.20 The recommended dosage and period of TMP-SMX treatment is TMP 15–20 mg/kg/day + SMX 75–100 mg/kg/day for 21 days for PJP in patients with HIV8 and 2–3 weeks for PJP in patients with solid organ transplantation.19 However, evidence from randomized controlled trials supporting this dosage regime is lacking. Thomas et al suggests that low dosage of TMP 10 mg/kg/day + SMX 50 mg/kg/day has comparable efficacy in HIV PJP.21 Tu et al also attempted to treat PJP using low-dose SMX-TMP.10 We treated all of our renal transplantation recipients that acquired PJP with low-dose TMP-SMX for 2–3 weeks and achieved good outcomes. The patient and graft survival rates were both 100% among the 37 patients in the present study.
The most common adverse events following SMX-TMP treatment are hematological side effects, renal or hepatic dysfunction, and gastrointestinal discomfort.9 Tu et al treated two cases of PJP in renal transplant recipients with TMP-SMX at the initial dose of 240/1200 mg q8h, but the dose was reduced to 80/400 mg tid in one case because of hepatic dysfunction and in the other case due to leukopenia.10 Similarly, when Li et al gave patients oral TMP–SMX therapy at a dose of 1–6 g/kg divided over three administrations, the dosage of SMX was later reduced due to adverse reactions in some patients.9 Some published studies reported poor tolerability and high discontinuation rates even when SMX-TMP was used at 1 single-strength tablet daily as PJP prophylaxis in kidney transplant recipients.22–24 In the present study, all the eligible patients were treated with TMP–SMX 480/2400mg or 240/1200mg per day divided into three doses, and only 21.6% (8/37) of the patients experienced gastrointestinal symptoms, 5.4% (2/37) suffered hematologic side effects, 2.7% (1/37) developed hyperkalemia, and 2.7% (1/37) experienced transient impairment in kidney function. TMP–SMX was not reduced or discontinued for any patient as a consequence of the adverse effects. Therefore, the dose of TMP-SMX used against PJP in deceased donor kidney recipients in the present study provided adequate protection with a low incidence of adverse events.
TMP-SMX treatment alone can have an inadequate effect against some cases of PJP, as it is less effective against the cystic form of P. jirovecii. Although trophic forms constitute 90–95% of all Pneumocystis life cycle stages, mature cysts can be detected in the bronchial lumen.25,26 Caspofungin targets the synthesis of β-1,3-glucan, a major component of the P. jirovecii cell wall and thereby is effective for the treatment of PJP.10,27 Combination of low-dose TMP-SMX and caspofungin therefore has a theoretical advantage of being able to target all forms of P. jirovecii. Previous case reports have shown this combination to be effective in the treatment of severe PJP and to enable the dose of TMP-SMX to be lowered, thereby decreasing the incidence of TMP-SMX–related adverse events.10,28 In the present study, all 37 PJP patients were treated with low-dose TMP-SMX and caspofungin, and the combination demonstrated satisfactory results, which is in line with previous case reports.
There is currently no consensus on the efficacy of adjunctive glucocorticoid therapy in the treatment of PJP in solid organ transplant recipients. The optimal dose and duration are also elusive in cases of renal transplantation. According to the American Society of Transplantation, glucocorticoids are recommended to be administered within 72 hours if PaO2 drops below 70 mmHg.20 However, in clinical practice, adjunctive glucocorticoids are often administered as soon as possible in most cases with an abrupt onset and poor prognosis.9,10 In the present study, all 37 PJP patients were treated with methylprednisolone 20–40 mg twice daily during the early stage of the disease, instead of waiting until severe hypoxia. The results were good, and only 5.4% (2/37) of the patients later required invasive ventilation.
Although it is well known that immunosuppression is the main reason for PJP, the optimal strategy for immunosuppression is still unclear. Most reports encourage the reduction of immunosuppression as a general measure during the treatment of PJP.29 Tacrolimus and mycophenolate mofetil were discontinued in the three cases of severe PJP in renal transplant recipients reported by Tu et al10 and Goto et al8 initially only discontinued mycophenolate mofetil for 2–3 weeks but went on to discontinue both tacrolimus and mycophenolate mofetil temporarily when PJP seriously threatened the life of the patient. In our protocol, tacrolimus was continued and the trough concentration was maintained at 5 ng/mL unless the medication had to be stopped to save the patient’s life. Mycophenolate mofetil was reduced or discontinued depending on the severity of the illness. Only 2.7% (1/37) of the patients discontinued tacrolimus and 5.4% (2/37) discontinued mycophenolate mofetil in the present study. The grafts from deceased donors maintained good function, and no rejection occurred during treatment.
Conclusions
In conclusion, the present study is a retrospective and non-randomized single-center study with no control group. We observed good outcomes with a comprehensive preemptive treatment regime including low-dose TMP–SMX and caspofungin as antipathogenic therapy, third generation cephalosporin or carbapenems to prevent bacterial infection, and glucocorticoids as anti-inflammatories. Moreover, immunosuppression was reduced when necessary and oxygen inhalation or inventive ventilation was applied to improve hypoxemia. The results suggest that low-dose TMP-SMX in the treatment of PJP in deceased donor kidney recipients is feasible when given as a part of this comprehensive treatment regime.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author Hongyang Wang on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Qingdao, China; no. QYFYWZLL 26483). All procedures involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethics standards. Written informed consent was obtained from all individual participants included in this study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Struijk GH, Gijsen AF, Yong SL, et al. Risk of Pneumocystis jiroveci pneumonia in patients long after renal transplantation. Nephrol Dial Transplant. 2011;26(10):3391–3398. doi:10.1093/ndt/gfr048
2. Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev. 2004;17(4):770–782, table of contents. doi:10.1128/cmr.17.4.770-782.2004
3. Neff RT, Jindal RM, Yoo DY, Hurst FP, Agodoa LY, Abbott KC. Analysis of USRDS: incidence and risk factors for Pneumocystis jiroveci pneumonia. Transplantation. 2009;88(1):135–141. doi:10.1097/TP.0b013e3181aad256
4. Eitner F, Hauser IA, Rettkowski O, et al. Risk factors for Pneumocystis jiroveci pneumonia (PcP) in renal transplant recipients. Nephrol Dial Transplant. 2011;26(6):2013–2017. doi:10.1093/ndt/gfq689
5. Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–2498. doi:10.1056/NEJMra032588
6. Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2014;2014(10):Cd005590. doi:10.1002/14651858.CD005590.pub3
7. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.7.1 late infections. Pneumocystis carinii pneumonia. Nephrol Dial Transplant. 2002;17(Suppl 4):36–39. doi:10.1093/ndt/17.suppl_4.36-a
8. Goto N, Futamura K, Okada M, et al. Management of Pneumocystis jirovecii pneumonia in kidney transplantation to prevent further outbreak. Clin Med Insights Circ Respir Pulm Med. 2015;9(Suppl 1):81–90. doi:10.4137/ccrpm.s23317
9. Li T, Shi J, Xu F, Xu X. Clinical characteristics of pneumocystis pneumonia after parental renal transplantation. Infect Drug Resist. 2020;13:81–88. doi:10.2147/idr.s234039
10. Tu GW, Ju MJ, Xu M, et al. Combination of caspofungin and low-dose trimethoprim/sulfamethoxazole for the treatment of severe Pneumocystis jirovecii pneumonia in renal transplant recipients. Nephrology (Carlton). 2013;18(11):736–742. doi:10.1111/nep.12133
11. Chai YX, Ji JL, Li SJ, et al. Efficacy of anti-T-lymphocyte globulin-Fresenius as an induction agent in deceased-donor renal transplantation: a cohort study. Exp Ther Med. 2020;19(3):2384–2390. doi:10.3892/etm.2020.8451
12. Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299–311. doi:10.1038/ki.2009.377
13. Chadban SJ, Barraclough KA, Campbell SB, et al. KHA-CARI guideline: KHA-CARI adaptation of the KDIGO clinical practice guideline for the care of kidney transplant recipients. Nephrology (Carlton). 2012;17(3):204–214. doi:10.1111/j.1440-1797.2011.01559.x
14. Borstnar S, Lindic J, Tomazic J, et al. Pneumocystis jirovecii pneumonia in renal transplant recipients: a national center experience. Transplant Proc. 2013;45(4):1614–1617. doi:10.1016/j.transproceed.2013.02.107
15. Costa JM, Botterel F, Cabaret O, Foulet F, Cordonnier C, Bretagne S. Association between circulating DNA, serum (1->3)-β-D-glucan, and pulmonary fungal burden in Pneumocystis pneumonia. Clin Infect Dis. 2012;55(2):e5–8. doi:10.1093/cid/cis412
16. Corsi-Vasquez G, Ostrosky-Zeichner L, Pilkington EF, Sax PE, Caliendo AM. Point-counterpoint: should serum β-d glucan testing be used for the diagnosis of Pneumocystis jirovecii pneumonia? J Clin Microbiol. 2019;58(1). doi:10.1128/jcm.01340-19
17. de Boer MG, Kroon FP, le Cessie S, de Fijter JW, van Dissel JT. Risk factors for Pneumocystis jirovecii pneumonia in kidney transplant recipients and appraisal of strategies for selective use of chemoprophylaxis. Transpl Infect Dis. 2011;13(6):559–569. doi:10.1111/j.1399-3062.2011.00645.x
18. Muhammad Iqbal AH, Lim SK, Ng KP, Tan LP, Chong YB, Keng TC. Pneumocystis jirovecii pneumonia 13 years post renal transplant following a recurrent cytomegalovirus infection. Transpl Infect Dis. 2012;14(4):E23–E26. doi:10.1111/j.1399-3062.2012.00738.x
19. Martin SI, Fishman JA. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):272–279. doi:10.1111/ajt.12119
20. White PL, Price JS, Backx M. Therapy and management of Pneumocystis jirovecii infection. J Fungi (Basel). 2018;4(4). doi:10.3390/jof4040127
21. Thomas M, Rupali P, Woodhouse A, Ellis-Pegler R. Good outcome with trimethoprim 10 mg/kg/day-sulfamethoxazole 50 mg/kg/day for Pneumocystis jirovecii pneumonia in HIV infected patients. Scand J Infect Dis. 2009;41(11–12):862–868. doi:10.3109/00365540903214256
22. Gabardi S, Millen P, Hurwitz S, Martin S, Roberts K, Chandraker A. Atovaquone versus trimethoprim-sulfamethoxazole as Pneumocystis jirovecii pneumonia prophylaxis following renal transplantation. Clin Transplant. 2012;26(3):E184–190. doi:10.1111/j.1399-0012.2012.01624.x
23. Giullian JA, Cavanaugh K, Schaefer H. Lower risk of urinary tract infection with low-dose trimethoprim/sulfamethoxazole compared to dapsone prophylaxis in older renal transplant patients on a rapid steroid-withdrawal immunosuppression regimen. Clin Transplant. 2010;24(5):636–642. doi:10.1111/j.1399-0012.2009.01129.x
24. Mitsides N, Greenan K, Green D, et al. Complications and outcomes of trimethoprim-sulphamethoxazole as chemoprophylaxis for pneumocystis pneumonia in renal transplant recipients. Nephrology (Carlton). 2014;19(3):157–163. doi:10.1111/nep.12201
25. Aliouat-Denis CM, Martinez A, Aliouat El M, Pottier M, Gantois N, Dei-Cas E. The Pneumocystis life cycle. Mem Inst Oswaldo Cruz. 2009;104(3):419–426. doi:10.1590/s0074-02762009000300004
26. Dei-Cas E. Pneumocystis infections: the iceberg? Med Mycol. 2000;38(Suppl 1):23–32. doi:10.1080/mmy.38.s1.23.32
27. Armstrong-James D, Stebbing J, John L, et al. A trial of caspofungin salvage treatment in PCP pneumonia. Thorax. 2011;66(6):537–538. doi:10.1136/thx.2010.135350
28. Koshy R, Chen T. Combination therapy with trimethoprim-sulfamethoxazole and caspofungin in a case of severe pneumocystis pneumonia. IDCases. 2019;15:e00496. doi:10.1016/j.idcr.2019.e00496
29. Kasiske B, Zeier M, Craig J, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi:10.1111/j.1600-6143.2009.02834.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.