Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 10
Efficacy of low-dose cinacalcet on alternate days for the treatment of secondary hyperparathyroidism in hemodialysis patients: a single-center study
Authors Gojaseni P , Pattarathitinan D, Chittinandana A
Received 18 October 2016
Accepted for publication 15 December 2016
Published 7 February 2017 Volume 2017:10 Pages 47—53
DOI https://doi.org/10.2147/IJNRD.S124844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Pongsathorn Gojaseni, Dolnapa Pattarathitinan, Anutra Chittinandana
Division of Nephrology, Department of Medicine, Bhumibol Adulyadej Hospital, Directorate of Medical Services, Royal Thai Air Force, Bangkok, Thailand
Introduction: Cinacalcet is effective in reducing serum parathyroid hormone (PTH) in patients with secondary hyperparathyroidism (HPT). This study focused on testing whether a prescription of low-dose cinacalcet on alternate days could be an option for treatment of secondary HPT.
Materials and methods: A retrospective clinical study was conducted on chronic maintenance hemodialysis patients. Patients with secondary HPT who received cinacalcet at a starting dose of 25 mg on alternate days were reviewed (low-dose group). Patients who were being treated with a standard dose of cinacalcet in the same period of time were selected as the control group. The primary outcome was difference in the percentage of patients achieving >30% reduction of intact parathyroid hormone (iPTH) levels at 16 weeks. The changes of serum iPTH and other biochemical data were also tested.
Results: A total of 30 patients (16 low doses and 14 controls) took part in the study. Baseline iPTH levels in the low-dose and control group were 1,065.9±477.7 and 1,214.1±497.6 pg/mL, respectively (p=0.413). The analysis showed that the percentage of patients who achieved the primary outcome showed little or no difference (33.3% in the low-dose group compared with 38.5% in the control group, p=1.0). Serum iPTH reduction during 16 weeks of study period in the low-dose and control group was 253.5±316.1 and 243.4±561.3 pg/mL, respectively (p=0.957). There was no difference in the adverse events between both groups.
Conclusion: Among patients with secondary HPT, initial treatment with cinacalcet 25 mg on alternate days can decrease serum PTH levels. The role of low-dose cinacalcet in secondary HPT should be further determined in large-scale, randomized controlled trials.
Keywords: cinacalcet, secondary hyperparathyroidism, hemodialysis
Introduction
Secondary hyperparathyroidism (HPT) is a common condition in hemodialysis patients and has been associated with a high incidence of bone fractures, cardiovascular (CV) disease, and mortality.1–5 Standard treatment for this condition includes the treating of hyperphosphatemia, correcting 1,25-hydroxy vitamin D deficiency, and surgery.6,7 Although parathyroidectomy can effectively lower parathyroid hormone (PTH) levels, it is associated with major complications such as postoperative hypocalcemia and recurrent laryngeal nerve paralysis.8,9 Cinacalcet is a modulator of calcium-sensing receptor. It acts by increasing the sensitivity to extracellular calcium10,11 and effectively reducing serum PTH in clinical studies.12–19 Moreover, treatment with cinacalcet decreases the incidence of vascular calcification20 and bone fractures.21 In Thailand, however, the use of this medication as a treatment is limited by its cost because cinacalcet is not covered under the universal health coverage scheme. According to data obtained from westernized countries, the mean dose of cinacalcet in clinical trials ranges from 44 to 64 mg/d and about 40% of patients can be effectively maintained on 25 mg/d.18 There is no clinical data available for the use of cinacalcet at starting dosages lower than 25 mg daily, and cinacalcet has a long-elimination half-life of more than 30 hours.22 We, therefore, conducted a pilot retrospective clinical study to test whether a prescription of low-dose cinacalcet on alternate days could be an option for the treatment of secondary HPT.
Materials and methods
Study design and population
This study was a single-center retrospective clinical study conducted at the Department of Medicine at Bhumibol Adulyadej Hospital, Royal Thai Air Force, Bangkok. The medical records of chronic maintenance hemodialysis patients treated with low-dose cinacalcet on alternate days from July 1, 2014 to October 31, 2015 were reviewed. The major inclusion criteria for this study were: patients with 18 years or older, hemodialysis treatment for at least 6 months, and the baseline intact PTH (iPTH) levels were more than the current recommendation from the Kidney Disease Improving Global Outcome (KDIGO) guidelines (>9 times the upper normal limit for the assay, ie, 585 pg/mL).7 The exclusion criteria were: those with previous parathyroidectomy, a baseline corrected serum calcium <8.4 mg/dL, diagnosed with tertiary HPT and having other causes of hypercalcemia and hypocalcemia. Hemodialysis patients who were being treated with standard-dose cinacalcet during the same period of time were selected as the control group.
Treatment protocol
Subjects included in this study received cinacalcet at the starting dose of 25 mg on alternate days. After 8 weeks of treatment, the dose of cinacalcet could be increased to 25 mg once daily if the serum PTH levels were not achieving the KDIGO target. However, the dose would not be increased or may even be withheld at the doctor’s discretion in the case of adverse events or significant decreases in serum calcium levels. No restrictions were imposed on the use of active vitamin D and its analogs or phosphate binders. In the control group, the dose of cinacalcet was started at the standard recommended dosage of 25 mg, once daily, without a specific treatment algorithm provided. Patient compliance was evaluated at each clinic visit as standard practice. All patients in this study were managed according to the current KDIGO guidelines for Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD).7 The duration of the evaluation period in this study was 16 weeks. The study protocol was approved by Bhumibol Adulyadej Hospital’s Ethics Committee and was conducted in compliance with recommendations of the 18th World Health Congress (Helsinki, 1964). All participants provided written informed consent including their data to be used for this study.
Laboratory methods
Laboratory tests were performed at the baseline for all subjects. Subsequently, follow-up tests were performed at 8 and 16 weeks after the start of the medication as per standard practice without additional clinic visits. We compared the levels of iPTH, phosphate, calcium, and alkaline phosphatase at these time points. Blood was analyzed using a modular analytics cobas 8000 analyzer series (Roche Diagnostics, Mannheim, Germany). The total serum calcium level was corrected according to albumin level as follows: corrected Ca = Ca + 0.8 × (4.4 – albumin) g/dL.23 All patients in our study also had lateral X-ray of the thoracic and lumbar spines to detect abdominal aortic calcification before receiving cinacalcet.24
Study outcomes
The primary outcome was the percentage of patients achieving >30% reduction of iPTH from the baseline to 16 weeks. Secondary outcomes included changes in serum iPTH, calcium, phosphate, and alkaline phosphatase. The efficacy outcomes analyses included all subjects who completed their treatment regimens and laboratory data during the study period. In terms of safety analyses, all subjects who received at least one dose of cinacalcet were included.
Statistical analysis
Categorical variables were summarized using frequency and percentages, while continuous variables were summarized using mean ± standard deviations unless otherwise indicated. Differences in the percentage of patients achieving primary outcome were performed using Fisher’s exact test. Mean values for intact PTH, calcium, phosphate, and alkaline phosphatase during the study period were used to evaluate the secondary outcomes. Differences in the changes of these parameters between both groups were performed using two sided student’s t test (Mann–Whitney U test for non-normally distributed variables). The relationship between primary outcome and covariates was assessed using a simple logistic regression model and reported as crude odds ratios (ORs) with 95% confident intervals (CIs). Differences in adverse events rates were performed using the χ2 and Fisher’s exact test. p-values were two sided, and p<0.05 was considered to indicate statistical significance. All analyses were performed using SPSS statistical package version 20.0 (IBM, Armonk, NY, USA).
Results
A total of 16 patients who were treated with a low dose of cinacalcet on alternate days were enrolled (low-dose group). In addition, 14 patients who received a standard dose of cinacalcet were recruited as the control group. Baseline characteristics of the study patients are summarized in Table 1. Demographic data of the patients between both groups were not statistically significant except for the ages of the patients in the control group were older. The majority of the patients had been on renal replacement therapy for more than 5 years and were being treated with hemodialysis thrice weekly. The baseline biochemical data of the patients from both groups were comparable, except that serum phosphate and albumin were found to be higher in the low-dose group. The mean value for vascular calcification scores was numerically lower in the low-dose group compared with the control group. Four patients discontinued cinacalcet (three patients with adverse events and one patient underwent a deceased donor kidney transplantation) before the end of the study period. One patient had no final laboratory measurements due to personal reasons. Therefore, 25 patients were entered into the efficacy outcomes analyses.
  | Table 1 Patient characteristics at initiation of cinacalcet Note: Data presented as mean±SD and percentage. Abbreviation: iPTH, intact parathyroid hormone; SD, standard deviation. |
Primary outcome: percent reduction of iPTH
For patients who included in the efficacy outcomes analyses (12 low doses and 13 controls), the baseline iPTH in the low-dose and control group was 949.3±400.7 and 1,195.3±512.7 pg/mL, respectively (p=0.157). Patients received cinacalcet and maintained the initial doses for 8 weeks, and then the dose could be adjusted to achieve iPTH levels. The mean dose of cinacalcet during the second half of the study in the low-dose group was 21.88±10.83 mg/d, while the control group had a mean dose of 34.62±12.66 mg/d (p=0.008). Following 8 weeks of treatment, the percentage reduction of iPTH in the low-dose and control group was 16.16±36.34% and 9.76±69.55%, respectively (p=0.870). At the end of the study period, percentage of iPTH reduction during the 16 weeks of the study period in both groups was comparable (22.45±31.85% and 21.86±52.97%, respectively, p=0.974). In terms of primary outcome, it has been shown that the percentage of patients who had iPTH reduction by more than 30% was not significantly different (33.33% in the low-dose group compared with 38.46% in the control group, p=1.0) (Table 2).
  | Table 2 Percent reduction in serum iPTH during study period Note: Data presented as mean±SD. Abbreviation: iPTH, intact parathyroid hormone; SD, standard deviation. |
Secondary outcome: changes in biochemical markers during the study period
The key biochemical data in all patients are summarized in Figure 1A–D. The mean serum iPTH, calcium, and phosphate significantly decreased from the baseline in both groups. Serum iPTH reduction during the 16 weeks of the study period in both groups was comparable; –253.5±316.1 pg/mL in the low-dose group and –243.4±561.3 pg/mL in the control group (p=0.957). Serum calcium reduction was numerically greater in the control group compared with the low-dose group, but the difference was not statistically significant (–0.94±1.0 vs –0.34±0.68, p=0.095). For serum phosphate, there was a trend for a higher reduction in the low-dose group compared with the control group (–1.17±1.17 vs –0.66±0.90, p=0.238). However, it is important to note that the baseline serum phosphate was significantly higher in the low-dose group. In terms of serum alkaline phosphatase, there was a small increase in both groups during the study period.
Factors associated with percent reduction of iPTH
Because of the retrospective nature of the study which led to differences in baseline characteristics, we investigated whether these factors were associated with response rate to cinacalcet use. OR of achieving >30% reduction of iPTH at 16 weeks was estimated for various variables (ie, age, gender, years in dialysis, frequency of dialysis, baseline biochemical data, dose of elemental calcium and vitamin D, type of phosphate binders, and aortic calcification score) using regression analysis (Table 3). It has been found that none of those variables was significantly associated with percent reduction of iPTH. However, there was a trend for a higher number of patients achieving >30% reduction of iPTH in patients with lower serum phosphate (OR =3.11, 95% CI: 0.47–20.53) and male gender (OR =2.17, 95% CI: 0.69–6.79).
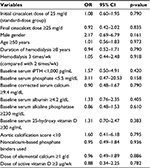  | Table 3 OR and 95% CI for achieving >30% reduction of iPTH at 16 weeks after initiation of cinacalcet Abbreviations: iPTH, intact parathyroid hormone; OR, odds ratio; CI, confidence interval. |
Adverse events
All adverse events in this study are summarized in Table 4. Adverse events occurred in 9 of the 16 (56.3%) subjects in the low-dose group and in 9 of the 14 (64.3%) subjects in the control group. Adverse events ascribed to the treatment with cinacalcet included gastrointestinal symptoms (43.8% in the low-dose group vs 7.1% in the control group; p=0.103), numbness (0% in the low-dose group vs 7.1% in the control group; p=0.946), and hypocalcemia (18.8% in the low-dose group vs 28.6% in the control group; p=0.590). Serious adverse events causing discontinuation of cinacalcet were reported in two subjects in the low-dose group (12.6%) and in one subject in the control group (7.1%). After careful review, only one serious event was attributed to cinacalcet (patient in the control group who developed severe gastrointestinal symptoms). There were no deaths during the study period.
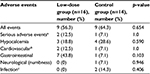  | Table 4 Adverse events during study period Notes: aAdverse events that caused discontinuation of cinacalcet; b,cAdverse events that were not attributed to cinacalcet. |
Discussion
This study evaluated the efficacy of cinacalcet to treat secondary HPT at a dosage lower than the standard recommendation. Despite the differences in some of the baseline clinical characteristics, low-dose cinacalcet showed comparable effectiveness compared to the standard dose. The low-dose cinacalcet achieved the primary outcome (iPTH reduction more than 30% at 16 weeks) in 33.33% of the patients compared with 38.46% in the control group. Patients in the low-dose group had a serum iPTH reduction of 171.6±335.4 pg/mL during the first 8 weeks. It was numerically higher compared to the control group that had a mean reduction of 71.7±733.8 pg/mL during the same period. After a dose adjustment, however, iPTH reduction during the 16 weeks of the study period in both groups was almost the same (253.5±316.1 pg/mL in the low-dose group compared with 243.4±561.3 in the control group, p=0.957). Following the treatment with cinacalcet, serum phosphate and calcium levels were reduced in both groups but at different rates. While the low-dose cinacalcet group had a less reduction in serum calcium, it was associated with a higher reduction in serum phosphate. All patients in both low-dose and control groups received the assigned dose of cinacalcet during the first 8 weeks. After a dose adjustment, the mean dose of cinacalcet in the low-dose and control group was 21.88±10.83 and 34.62±12.66 mg/d, respectively.
The efficacy of low-dose cinacalcet in this study should be interpreted carefully, and these results need to be further confirmed in the future. In terms of pharmacokinetics, peak plasma concentrations of cinacalcet occur within 2–6 hours with a bioavailability of 20%–25%. The elimination half-life is 30–40 hours, and steady-state concentrations are achieved within 7 days. Furthermore, nadir PTH levels occur approximately 2–3 hours after dosing.10,11,22 However, these pharmacokinetic profiles are not the best explanation for the efficacy results because of the wide differences in the efficacy of cinacalcet in this study (both in the low-dose and control groups). Our analyses have been tested to explore the predictor of response to cinacalcet, but the population in the study was too small to determine.
In terms of adverse events, cinacalcet was well tolerated in both groups. Though there were three patients who discontinued medication due to adverse events, only one of them was attributed to cinacalcet and that patient in the control group had severe gastrointestinal side effects when the dose was increased to 50 mg/d. The other patients who discontinued (one from each group) dropped out because they developed CV events (myocardial infarction and pericarditis) during the study, and the patients decided to discontinue cinacalcet. After extensive review, both CV events could not be attributed to the taking of cinacalcet. For adverse events associated with cinacalcet, it was not different between both groups. However, it is important to note that within the low-dose group there was a higher incidence of gastrointestinal side effects, but it was not statistically significant. The explanation for the higher incidence of gastrointestinal symptoms in the low-dose group is unclear, and the compliance issue was less likely explanation since most of the patients in the study were taking cinacalcet as prescribed. However, even with the higher rate, none of the patients in the low-dose group needed to stop the medication because of these symptoms. As for hypocalcemia, the control group’s serum calcium levels decreased more than those in the low-dose group. However, the hypocalcemia events (corrected serum calcium <8.4 mg/dL) throughout the study period showed very little difference between both groups (18.8% vs 28.6%, p=0.590).
This study used a 30% reduction of iPTH as a primary end point which was different from any major previous studies conducted of cinacalcet. Studies in western countries and in Japan used the percentage of patients who achieved iPTH targets of 250–300 pg/mL.12–17 Nonetheless, it is important to note that the objective of this study was to compare the efficacy of different cinacalcet dosages during a short period of time, and where the dosage was not increased to the same ranges as those studies. Considering the differences in the baseline iPTH levels between intervention groups, using percentage reduction as primary outcome would be considered more appropriate than using a recommended target scenario. Nonetheless, we also analyzed the data using KDIGO iPTH target (585 pg/mL) as a primary outcome. The analysis showed that the percentage of patients who achieved the KDIGO PTH target was not significantly different between the two groups (41.7% in the low-dose group compared with 30.8% in the control group, p=0.688). In terms of pharmacoeconomic analysis using the data derived from this study, the low-dose, alternate-day cinacalcet represents a cost saving of up to 520 US dollar per patient for the duration of the 4 months of the study period.
Limitations
There were some limitations to our study. First, it was a retrospective, nonrandomized study that led to a selection bias. Patients in the low-dose group were younger, had higher serum phosphate levels, and had better nutritional conditions compared to the controls. These clinical characteristics could be associated with the response rate for the cinacalcet use. Moreover, there was a single-center study with small number of subjects and relatively short period of follow-up. Thus, these results need to be revalidated in well-designed multicenter, randomized clinical trial with a longer follow-up period. Second, there was no standard protocol for the dosage adjustment of cinacalcet and other related medications such as vitamin D and phosphate binders, particularly in the control group. However, the second limitation did not affect the study quality as most of the patients in our study were managed based on the current recommendations from KDIGO, and the dose of vitamin D and phosphate binders did not change throughout the study period. Third, there was no data to determine if the patients were taking medications that affected cinacalcet blood levels or pharmacokinetic tests such as trough levels or area under the curve to confirm the efficacy of the low-dose, alternate-day cinacalcet.22 Finally, tertiary HPT could not be excluded in some patients with long-standing renal replacement therapy, especially in the control group. Thus, it could be associated with poor response to cinacalcet.
Conclusion
In summary, among hemodialysis patients, initial treatment with cinacalcet 25 mg on alternate days may be used for the treatment of secondary HPT. The role of low-dose cinacalcet in secondary HPT should be confirmed in a large-scale, adequately powered, randomized controlled trial.
Acknowledgment
The authors acknowledge group captain Thaweepong Pajareya, Deputy Director of Bhumibol Adulyadej Hospital, and group captain Sayom Bunnag, Chief of the Department of Radiology, Bhumibol Adulyadej Hospital, for their support in data collection during the study period.
Disclosure
The authors report no conflicts of interest in this work.
References
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. | ||
Melamed ML, Eustace JA, Plantinga L, et al. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70(2):351–357. | ||
Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–780. | ||
Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52(3):519–530. | ||
Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–1955. | ||
Ketteler M, Wüthrich RP, Floege J. Management of hyperphosphataemia in chronic kidney disease-challenges and solutions. Clin Kidney J. 2013;6(2):128–136. | ||
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;(113):S1–S130. | ||
Fotheringham J, Balasubramanian SP, Harrison B, Wilkie M. Post-parathyroidectomy parathyroid hormone levels: the impact on patient survival – a single-centre study in a stage 5 chronic kidney disease population. Nephron Clin Pract. 2011;119(2):c113–c120. | ||
Gagné ER, Ureña P, Leite-Silva S, et al. Short- and long-term efficacy of total parathyroidectomy with immediate autografting compared with subtotal parathyroidectomy in hemodialysis patients. J Am Soc Nephrol. 1992;3(4):1008–1017. | ||
Nemeth EF, Heaton WH, Miller M, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004;308(2):627–635. | ||
Poon G. Cinacalcet hydrochloride (Sensipar). Proc (Bayl Univ Med Cent). 2005;18(2):181–184. | ||
Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–1525. | ||
Moe SM, Chertow GM, Coburn JW, et al. Achieving NKF-K/DOQITM bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int. 2005;67(2):760–771. | ||
Fukagawa M, Yumita S, Akizawa T, et al. Cinacalcet (KRN1493) effectively decreases the serum intact PTH level with favorable control of the serum phosphorus and calcium levels in Japanese dialysis patients. Nephrol Dial Transplant. 2008;23(1):328–335. | ||
Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol. 2008;3(6):1718–1725. | ||
Block GA, Zeig S, Sugihara J, et al. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2008;23(7):2311–2318. | ||
Urena P, Jacobson SH, Zitt E, et al. Cinacalcet and achievement of the NKF/K-DOQI recommended target values for bone and mineral metabolism in real-world clinical practice – the ECHO observational study. Nephrol Dial Transplant. 2009;24(9):2852–2859. | ||
Zhang Q, Li M, You L, et al. Effects and safety of calcimimetics in end stage renal disease patients with secondary hyperparathyroidism: a meta-analysis. PLoS One. 2012;7(10):e48070. | ||
Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. | ||
Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26(4):1327–1339. | ||
Moe SM, Abdalla S, Chertow GM, et al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE trial. J Am Soc Nephrol. 2015;26(6):1466–1475. | ||
Padhi D, Harris R. Clinical pharmacokinetic and pharmacodynamic profile of cinacalcet hydrochloride. Clin Pharmacokinet. 2009;48(5):303–311. | ||
National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–S201. | ||
Wilson PW, Kauppila LI, O’Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103(11):1529–1534. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

