Back to Journals » Journal of Hepatocellular Carcinoma » Volume 5
Efficacy of loco-regional treatment for hepatocellular carcinoma prior to living donor liver transplantation: a report from a single center in Egypt
Authors Shaker MK , Montasser IF , Sakr M, Elgharib M, Dabbous HM, Ebada H , El Dorry A, Bahaa M, El Meteini M
Received 24 July 2017
Accepted for publication 14 December 2017
Published 27 February 2018 Volume 2018:5 Pages 29—36
DOI https://doi.org/10.2147/JHC.S147098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Mohammad Kamal Shaker,1 Iman F Montasser,1 Mohamed Sakr,1 Mohamed Elgharib,2 Hany M Dabbous,1 Hend Ebada,1 Ahmed El Dorry,2 Mohamed Bahaa,3 Mahmoud El Meteini3
1Department of Tropical Medicine, 2Department of Radiodiagnosis and Interventional Radiology, 3Department of Hepatobiliary Surgery and Liver Transplantation, Ain Shams Center for Organ Transplantation (ASCOT), Ain Shams University, Cairo, Egypt
Background and aim: The number of loco-regional therapies (LRTs) for hepatocellular carcinoma (HCC) has increased dramatically during the past decade, bridging or downstaging patients on the waiting list for liver transplantation. This study aimed to analyze the outcomes of LRTs prior to living donor liver transplantation in patients with HCC.
Methods: Sixty-two HCC patients received living donor liver transplantation at Ain Shams Center for Organ Transplantation over a 2-year period. Data from 29 HCC patients were analyzed. Twenty patients (68.97%) met the Milan Criteria and 4 patients (13.8%) exceeded the Milan Criteria, but met the University of California, San Francisco Criteria. Five patients (17.2%) exceeded the University of California, San Francisco Criteria. All patients underwent preoperative LRTs. The protocol of bridging/downstaging, methods, duration of follow-up, the number of patients who were successfully downstaged before liver transplantation (LT), and their outcomes after LT were recorded.
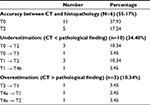
Results: There was a decrease in the mean overall size of focal lesions (from mean 5.46 to 4.11 cm) in the last abdominal computed tomography (CT) scan after LRT (p=0.0018). Discrepancies between the radiological findings and histopathology were as follows: in 16 patients (55.17%) the CT findings were consistent with the histopathological examination of the explanted liver. Underestimated tumor stage was documented in 10 patients (34.48%), and was overestimated by CT scan findings in 3 patients (10.34%). The 1-year survival rate was 93%. No patient had HCC recurrence after median follow-up of 21 months (range 1–46 months).
Conclusion: These results encouraged tumor bridging/downstaging as a potential treatment option among carefully selected patients with HCC beyond conventional criteria for LT. Further studies on a large number of patients are necessary.
Keywords: hepatocellular carcinoma, loco-regional therapy, LRT , liver transplantation, Milan criteria, beyond Milan, HCC recurrence, bridge/down staging
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers and a leading cause of cancer-related deaths worldwide.1 Mazzaferro et al reported that patients with a single tumor ≤5 cm in diameter, or no more than 3 tumors ≤3 cm, displayed a favorable long-term prognosis. These are known as the Milan Criteria.2 The Milan Criteria has been widely adopted as the main basis for listing HCC patients for liver transplantation (LT). However, many extended criteria beyond the Milan Criteria have been proposed recently.1
Loco-regional therapy (LRT) has been proposed as a strategy not only to retard HCC progression and prevent dropout from the transplant waitlist (bridging therapy), but also as a means of downstaging patients to within the Milan Criteria, and thus, to achieve eligibility for transplantation.3,4
Bridging therapy is used for patients with HCC who meet the Milan Criteria with an expected delay on the waiting list of >6 months,5 so as to prevent tumor progression.6,7
Downstaging,5,6 is used to convert tumors that initially do not meet the transplant criteria, usually intermediate multinodular asymptomatic tumors (stage B of the Barcelona Clinic Liver Cancer),8 into tumors that meet the Milan Criteria (the most frequent endpoint). Conversely, the University of California, San Francisco (UCSF) Criteria, also known as the up-to-seven criteria, aims to include patients on the waiting list once the tumor size has decreased. Tumors with more favorable histology are more likely to respond to treatment and exhibit a good outcome after LT.9 Thus, this study aims to analyze the outcome of LRTs prior to LT in patients with HCC.
Patients and methods
Study design and sampling
This cohort study evaluated 29 adult HCC patients, who underwent LRT before living donor LT (LDLT) at Ain Shams Center for Organ Transplantation (ASCOT), over 2 years. All study participants provided written informed consent prior to study enrollment.
Ethical approval
This study was carried out after approval from the Research and Ethics Committee of Ain Shams University, Cairo, Egypt in accordance with local research governance requirements. All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and all subsequent revisions. The trial was registered with the federal clearinghouse for randomized trials: ClinicalTrials.gov (NCT02990351).
All included patients were subjected to:
Pre-treatment assessment
- Detailed history taking and thorough clinical examination: the initial hepatopathy of the HCC, any previous LRT (type, number of sessions, date of each maneuver) were recorded.
- Laboratory assessments, including: alpha fetoprotein (AFP), Child and model for end-stage liver disease (MELD) score.
- Radiological investigations: abdominal ultrasound and duplex, Tri-phasic spiral abdominal computed tomography (CT) scans or magnetic resonance imaging (MRI) before and after LRT.
- The waiting time from last intervention to transplantation and survival were recorded.
Patients within the Milan Criteria expecting to stay on the waiting list >3 months according to ASCOT protocol (due to limited availability of the donors, as we only have LDLT in our country) underwent LRT as bridging therapy to avoid tumor progression while on the waiting list.
Histopathological evaluation of the explants
Included the number of HCC nodules, size, and grade based on the Edmondson and Steiner Criteria,22,23 presence of viable malignant cells, microvascular or capsular invasion and lymph node examination.
Downstaging protocol
The eligibility criteria for downstaging were adapted to match with the UCSF Criteria for downstaging9 were as follows: 1) 1 lesion >5 cm and up to 8 cm; 2) 2 to 3 lesions with at least 1 lesion >3 cm and not exceeding 5 cm, with a total tumor diameter up to 8 cm; or 3) 4 to 5 lesions with none >3 cm, and a total tumor diameter up to 8 cm.9 A minimum follow-up period of 3 months after downstaging was required before LT, along with imaging studies meeting the defined criteria for successful downstaging.9 Patients with high AFP before downstaging were required to have the AFP reduced to <200 ng/dL according to our local protocol.
Successful downstaging was defined as a reduction in the size and number of viable tumors, using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) meeting the United Network for Organ Sharing (UNOS) T2 Criteria (Milan Criteria) or UCSF Criteria or complete tumor necrosis, indicating disappearance of the viable tumor in the CT scans of patients with HCC initially beyond the Milan Criteria.12
A complete response (CR) corresponded to the disappearance of all contrast-enhancement at the arterial phase for the mRECIST evaluation. In case of a mixed response, the sum of greatest dimensions prevailed in the evaluation of each target lesion measured separately. A partial response (PR) was defined as a 30% decrease in the sum of maximal dimensions, progressive disease was defined as a 20% increase in the sum of maximal dimensions, and all other variations were classified as stable disease (Sd), but documentation had to occur 6 weeks after the baseline determination. Every response had to be confirmed by a subsequent CT scan.
Patients who were not successfully downstaged to fall within the UNOS T2 Criteria or to meet the UCSF Criteria had their response to treatment evaluated by measuring their AFP levels and analyzing their radiological data. These patients were evaluated on a case-by-case basis by the transplantation committee.
Patients who had tumor progression on the waiting list and/or vascular invasion proved by imaging studies and/or extrahepatic or lymph node metastasis were delisted.
Post-transplantation follow-up
All patients were followed up regularly post-LT with laboratory investigations and ultrasonography performed every 2 weeks for the first 3 months after hospital discharge, and then every month for the next 6 months.
Tri-phasic spiral abdomen CT scans were performed at 6 months post-transplantation and then as needed according to clinical evaluation. Suspected HCC recurrence was confirmed with Tri-phasic spiral abdomen CT scans and/or MRI. Chest CT bone scans with or without PET scans were performed for distant recurrences.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). Data are expressed as the mean±SD or median (range) and percentage. Student’s t-test, McNermer, Mann–Whitney U, and Chi-Square tests were used to compare differences between the subgroups. Overall survival (OS) was defined as the interval between LT and death or the last observation. Recurrence-free survival was calculated as those who were still alive at the last observation. P value <0.05 was considered statistically significant.
Results
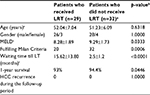
Sixty-two HCC patients received LDLT at our center over 2 years. Thirty-two patients (51.6%) had LT without LRT and 1 patient had LRT but died before transplantation and was excluded from this study. Twenty-nine HCC patients (46.7%) who had LRT then underwent LT, were included in this study. The mean age was (52.04±7.04) years and 93.1% of patients were male. The most common etiologies leading to transplantation were hepatitis C virus-related cirrhosis (79.3%), 2 patients (6.9%) had hepatitis B virus, and 4 patients (13.8%) had both hepatitis C and B. Most patients were in Child B and Child C (41.38% and 34.48%), respectively, and their mean MELD score was (8.28±1.89). Mean waiting time from first diagnosis of HCC until the time of LT was (15.62±13.80) months. Mean waiting time from last loco-regional treatment until LT was (8.48±6.83) months.
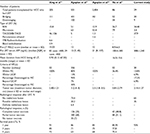
Site, size, and number of focal lesions (FLs) are detailed in Table 1. Initially, 20 patients (68.97%) were within the Milan Criteria, and 9 patients (31.03%) were beyond the Milan Criteria. Four patients (13.8%) met the UCSF Criteria and 5 patients (17.2%) exceeded the UCSF Criteria. Eleven patients (37.93%) had transarterial chemoembolization (TACE), 4 of these had 1 session (13.79%), 4 had 2 sessions (13.79%), 2 had 3 sessions (6.90%), and 1 had 5 sessions (3.45%). Ten patients (34.48%) had undergone radiofrequency ablation (RFA), 9 of these had 1 session of RFA (31.03%) and 1 had 2 sessions of RFA therapy (3.45%). Seven patients (24.14%) had several sessions of both TACE and RFA, 4 of them had 1 session of RFA plus 1 session of TACE (13.79%), and 3 had 1 session of RFA plus 2 sessions of TACE (10.34%). One patient (3.45%) had 1 session of microwave ablation.
With regard to the response to LRT (Table 2), well-ablated lesions (CR) were achieved in 52 FLs (65%) out of a total of 80 FLs in all patients. Residual enhancement (PR) was recorded in 28 FLs (35%).A decrease in overall size of FLs (from mean 5.46 to 4.11 cm) was found in the last abdominal CT scan (1 month before LT) after LRT that reached statistical significance (p=0.0018). Also, a statistically significant reduction in AFP serum levels was found after 1 loco-regional treatment, from 308±1240 ng/dL before intervention to 47.41±112.15 ng/dL after intervention but before LT (p<0.05).
According to tumor/node/metastasis (TNM) classification after loco-regional treatment, 15 patients (51.72%) had complete ablation (T0), 5 (17.24%) with T1, 5 (17.24%) with T2, 2 (6.89%) with T3, and 2 (6.89%) with T4a.
Reassessment of Milan Criteria with Tri-phasic spiral abdominal CT scan findings after LRT, and 1 month before LT, showed that 24 patients (86.21%) came within the Milan Criteria and 4 (13.79%) were beyond the Milan Criteria after intervention (Tables 3 and 4). Patients beyond the Milan Criteria (2 of them were exceeding UCSF Criteria) were discussed by a multi-disciplinary team before transplantation and their detailed characteristics were reviewed (data not shown).
Comparison between the results of the last CT scan before transplantation and histopathological examination revealed a highly statistically significant difference with regard to the total size of FLs (5.46±2.81 and 6.16±2.96 cm, respectively; p=0.0184) and also, in interpretation of well-ablated lesions (p=0.0014). There were 28 FLs showing well ablations in spiral CT scans and complete necrosis in histopathological examination. Twenty-one lesions appeared well ablated in CT scans; however, in histopathological examination, viable tumor tissue was found. Four lesions did not fulfill the criteria of well ablation in CT scans; however, on histopathological examination showed complete necrosis. On the other hand, there was agreement between the results of CT scans and histopathological analysis in the presence of viable tumors in 27 lesions. With regard to the TNM classification, the results of the spiral Tri-phasic CT scan were in agreement with pathological results in 16 patients (55.17%). In ten patients, the CT findings (34.48%) were underestimating the histopathological results. However, in three patients (10.34%) CT findings were overestimating the histopathological findings (Table 5).
Comparison between patients who had LRT (n=29) and those who did not (n=32) as an internal comparative arm is summarized in Table 6. As expected, a statistically significant difference was found between both arms regarding the Milan Criteria and waiting time until LT.
Post-transplantation outcome and survival
Two patients died within the early post-operative period after LT, 1 died from graft infarction at day 12 and the other died from hepatic artery occlusion at day 5. The first patient was within the Milan Criteria and the other patient was beyond it. The 1-year survival rate was 93%. No patient had HCC recurrence after the median follow-up of 21 months (range 1–46 months).
Discussion
The purpose of this study was to evaluate the outcome of the pre-transplant LRTs (either for bridging or downstaging) for HCC patients prior to LDLT. HCC downstaging prior to transplantation has different potential goals.10 LRT is accepted as the standard of care for patients expected to stay on the LT waitlist for longer than 6 months.11,12
In this study, the effectiveness of LRT before LDLT was investigated based on the mRECIST Criteria from CT scan imaging, and then by tumor necrosis in the explant pathology.
The role of the different types of LRT was not analyzed due to heterogeneity in treatment modalities and the small number of patients. Several trials investigated the impact of pre-transplant bridging/downstaging on LRTs for patients with HCC. The results of the current study were compared with other studies (Table 7).
In our series, a complete radiological response according to mRECIST Criteria was achieved in 49 (61.2%) out of 80 hepatic FLs (HFLs) per all patients, with residual enhancement (PR) in the remaining 31 (38.75%) out of 80 HFLs/all patients. Also, there was a decrease in the overall size of FLs in the last abdominal CT scan after LRT that reached statistical significance, from the mean size of all HFLs at 5.46 cm before LRT to 4.11 cm (p=0.0018) after LRT.
Twenty-two patients did not develop new HFLs before LT. It should be noted that the mean waiting time from LRT until LT was 6 months (interquartile range [IQR]=4–8 months) for patients who were within the Milan Criteria and 8 months (IQR=6–10 months) for those who were beyond it. In addition, seven patients developed new HFLs in the last CT scan follow-up before LT (1 new HFL in 4 patients and 2 new HFLs in 3 patients) but they were still within the Milan Criteria.
Histopathological examination of explanted liver tissue revealed >85 focal lesions per all patients with a mean size of 2.16±1.24 cm compared with the total number of HFLs at 80 in the Tri-phasic CT scan with a mean size of 4.11 cm before LT. Histopathological grading showed a well-differentiated HCC in 4 FLs (4.49%) and a moderately differentiated HCC in 36 FLs (40.45%). Likewise, no poorly differentiated HCCs were found. Twenty-eight FLs showed complete necrosis (31.46%), and 21 FLs were discovered to be macrodegenerative and dysplastic nodules (23.6%).
El-Gazzaz et al13 stated that the overall radiologic staging correlated with the explant pathology in 73 (57%) of 128 patients with HCC. Underestimated tumor stage was noted in 49 patients (38%), and an overestimated tumor stage in 6 patients (5%) when evaluating different levels of radiological response in correlation with the percentage of tumor necrosis in the explanted pathology.
The results presented here agree with Beal et al14 who stated that rates of viable tumors in the explant pathology were high compared with the radiological findings pre-LT. In their study, out of 43 HCC patients treated with at least 1 bridging therapy, 18 patients (42%) underwent TACE and 25 (58%) underwent ablation. Overall, 67% (including 20 patients [80%] who underwent ablation and 9 patients [60%] who underwent TACE) had CR based on imaging. However, viable tumors were identified in explant pathology in 32 patients (74%). The presence or absence of viable tumors was not associated with OS.
In the present study, 29 patients underwent LT, of whom 25 (86.20%) were within the Milan Criteria and 4 (13.79%) were beyond it. Histopathological results according to TNM classification were: 11 patients (37.93%) were T0 with complete necrosis, and two patients (6.89%) were T1, 12 patients (42.3%) were T2, one patient (3.45%) were T3, and three patients (10.3%) were T4b due to the presence of microvascular invasion. The 1-year survival rate post-transplantation was 93%. Two patients died in the early post-operative period (1 died on day 5, and the other on day 12) due to vascular complications in the transplanted liver. No patient had HCC recurrence post-transplantation. The recurrence-free survival rate was 100% during the median follow-up of 21 months (range 1–46 months). This finding agreed with Yao et al in a recent study that included 35 patients within the Milan Criteria who underwent LT. Post-transplantation pathological findings were 13 patients (37.1%) with complete necrosis, 17 (48.6%) met the T2 criteria, and 5 (14.3%) exceeded the T2 criteria. The 1-year post-transplantation survival rate was 96.2%. No patient had HCC recurrence after a median post-transplantation follow-up of 25 months.9
A similar study was conducted by Barakat et al15 on 13 patients who were within the Milan Criteria and underwent LT. Post-transplantation pathological findings were 12 patients (92.3%) met the T2 criteria and 1 (7.7%) exceeded the T2 criteria. The 1-year post-transplantation survival rate was 92%. Two patients (14.2%) had HCC recurrence after a median follow-up period of 35 months (range, 1.5–50 months) after LT. Lei and Yan16 studied 58 patients beyond the Milan Criteria and within the UCSF Criteria who underwent LT. The 1-year post-transplantation survival rate was 92%. However, our results are not in agreement with the results of Bargellini et al17, who reported on 33 patients exceeding the Milan Criteria who underwent LT after loco-regional treatment. Post-transplantation pathological findings were 8 patients (24.24%) had complete necrosis, 15 (45.5%) met the T2 criteria, and 10 (30.3%) exceeded the T2 criteria. The 1-year post-transplantation survival rate was 87.9%. Ten patients had HCC recurrence after a median post-transplantation follow-up of 36 months.
Reassessment of the Milan Criteria with Tri-phasic spiral abdominal CT scan findings after LRT and before LT showed that 25 patients (86.21%) came within the Milan Criteria. Of these, 19 (65.52%) were still within the Milan Criteria, 3 (10.34%) met the UCSF Criteria and came within the Milan Criteria, and 3 (10.34%) exceeded the UCSF Criteria and came within the Milan Criteria after LRT. Likewise, 4 patients (13.79%) were beyond the Milan Criteria after intervention. Two of these (6.9%) exceeded the UCSF Criteria and 2 (6.9%) met the UCSF Criteria.
In the present study, no significant differences were found between patients who were within or beyond the Milan Criteria with regard to the effect of LRT based on the last Tri-phasic CT scan before LT. Forty-eight FLs in patients within the Milan Criteria were well ablated (77.4%), and 14 lesions showed viable tumor tissue (22.6%). Patients beyond the Milan Criteria had 4 well-ablated lesions (34.5%) and 14 had viable tumor tissue (77.7%). Also, no significant differences were found between patients who were within or beyond the Milan Criteria with regard to histopathological examination results (p=0.13). Likewise, no significant difference was found with regard to microvascular or capsular invasion (p=1.00, 1.00), respectively. These results might be attributable to the low sample size in our series.
Conclusion
In conclusion, these results encourage tumor downstaging as a potentially viable treatment option among carefully selected patients with HCC beyond conventional criteria for LT. Downstaging provides a good test of time against aggressive tumors that are likely to progress despite treatment, whereas tumors with more favorable histology are more likely to respond to treatment and do well after orthotopic liver transplantation. Further studies on a larger number of patients and with longer follow-up are emphasized.
Acknowledgment
The authors gratefully acknowledge all members of Ain Shams Center for Organ Transplantation (ASCOT) for their valuable contributions.
Disclosure
The authors report no conflicts of interest in this work.
References
Xu DW, Wan P, Xia Q. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria: a review. World J Gastroenterol. 2016;22(12):3325–3334. | ||
Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. | ||
Yao FY, Hirose R, LaBerge JM, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11(12):1505–1514. | ||
Bharat A, Brown DB, Crippin JS, et al. Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survival. J Am Coll Surg. 2006;203(4):411–420. | ||
Fujiki M, Aucejo F, Choi M, Kim R. Neo-adjuvant therapy for hepatocellular carcinoma before liver transplantation: where do we stand? World J Gastroenterol. 2014;20(18):5308–5319. | ||
Cescon M, Cucchetti A, Ravaioli M, Pinna AD. Hepatocellular carcinoma locoregional therapies for patients in the waiting list. Impact on transplantability and recurrence rate. J Hepatol. 2013;58(3):609–618. | ||
Roberts JP, Venook A, Kerlan R, Yao F. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl. 2010;16(8):925–929. | ||
European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. | ||
Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48(3):819–827. | ||
Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15(4):993–1000. | ||
Prasad MA, Kulik LM. The role of bridge therapy prior to orthotopic liver transplantation. J Natl Compr Canc Netw. 2014;12(8):1183–1191. | ||
Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2011;13(1):e11–e22. | ||
El-Gazzaz G, Sourianarayanane A, Menon KV, et al. Radiologic-histological correlation of hepatocellular carcinoma treated via pre liver-transplant locoregional therapies. Hepatobiliary Pancreat Dis Int. 2013;12(1):34–41. | ||
Beal EW, Dittmar KM, Hanje AJ, et al. Pretransplant locoregional therapy for hepatocellular carcinoma: evaluation of explant pathology and overall survival. Front Oncol. 2016;6:143. | ||
Barakat O, Wood RP, Ozaki CF, et al. Morphological features of advanced hepatocellular carcinoma as a predictor of downstaging and liver transplantation: an intention-to-treat analysis. Liver Transpl. 2010;16(3):289–299. | ||
Lei J, Yan L. Comparison between living donor liver transplantation recipients who met the Milan and UCSF criteria after successful downstaging therapies. J Gastrointest Surg. 2012;16(11):2120–2125. | ||
Bargellini I, Vignali C, Cioni R, et al. Hepatocellular carcinoma: CT for tumor response after transarterial chemoembolization in patients exceeding Milan criteria-selection parameter for liver transplantation. Radiology. 2010;255(1):289–300. | ||
Xing M, Sakaria S, Dhanasekaran R, et al. Bridging locoregional therapy prolongs survival in patients listed for liver transplant with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2017;40(3):410–420. | ||
Agopian VG, Morshedi MM, McWilliams J, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg. 2015;262(3):536–545. | ||
Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: analysis of 3601 patients from the US multicenter HCC transplant consortium. Ann Surg. 2017;266(3):525–535. | ||
Na GH, Kim EY, Hong TH, You YK, Kim DG. Effects of loco regional treatments before living donor liver transplantation on overall survival and recurrence-free survival in South Korean patients with hepatocellular carcinoma. HPB (Oxford). 2016;18(1):98–106. | ||
Paradis V. Histopathology of hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:21–32. | ||
Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.