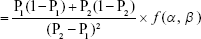Back to Journals » International Journal of Women's Health » Volume 9
Efficacy of intravenous tramadol and low-dose ketamine in the prevention of post-spinal anesthesia shivering following cesarean section: a double-blinded, randomized control trial
Authors Lema GF, Gebremedhn EG , Gebregzi AH , Desta YT, Kassa AA
Received 14 April 2017
Accepted for publication 24 August 2017
Published 26 September 2017 Volume 2017:9 Pages 681—688
DOI https://doi.org/10.2147/IJWH.S139655
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Girmay Fitiwi Lema,1 Endale Gebreegziabher Gebremedhn,1 Amare Hailekiros Gebregzi,1 Yilkal Tadesse Desta,1 Adugna Aregawi Kassa2
1Department of Anesthesia, School of Medicine, University of Gondar, Gondar, 2Department of Anesthesia, School of Medicine, Addis Ababa University, Black Lion Specialized Hospital, Addis Ababa, Ethiopia
Background: Shivering is a frequent and undesirable complication of spinal anesthesia. It is a physiologic response to increase the body core temperature in an attempt to raise metabolic heat production. However, shivering may trigger myocardial ischemia; increase intraocular and intracranial pressures, increase wound pain, delay wound healing and interfere with pulse rate, blood pressure and electrocardiogram monitoring. We aimed to compare the efficacy of intravenous (IV) ketamine with IV tramadol for the prevention of shivering in patients who underwent cesarean delivery under spinal anesthesia.
Patients and methods: A prospective, randomized, double-blind study was conducted. One hundred and twenty-three American Society of Anesthesiologist I and II patients, aged between 18 and 39 years, who underwent cesarean section were included in the study. Patients were randomly allocated to one of three groups: group S (n=41; control group) received saline, group K (n=41) received ketamine 0.2 mg/kg and group T (n=41) received tramadol 0.5 mg/kg. Incidence and grade of shivering and side effects between the treatment groups were recorded.
Results: The incidence of shivering was significantly reduced in the ketamine and tramadol groups (41.5% and 53.7%, respectively) compared to the saline group (70.7%; p=0.028). Grade 3 shivering occurred in 16 (39%) patients in the saline group, compared to 9 (22%) in the tramadol group and 8 (19.5%) in the ketamine group (p=0.011). Only two cases in the saline group developed grade 4 shivering (p<0.01). Neonatal outcome and perioperative complications were comparable among the three groups.
Conclusion: The prophylactic administration of low-dose IV ketamine or IV tramadol is effective for reducing the incidence and intensity of shivering. We recommend low-dose IV ketamine or tramadol prophylaxis for parturients undergoing cesarean section under spinal anesthesia.
Keywords: cesarean delivery, spinal anesthesia, shivering, prophylaxis, ketamine, tramadol, efficacy
Introduction
Shivering is defined as spontaneous, involuntary and repetitive muscular activity. It is a common problem during and after spinal anesthesia (SA) due to vasodilation, which could facilitate rapid heat loss and core to peripheral redistribution of body heat, resulting in hypothermia that lowers the threshold for shivering.1–4
Although shivering is not a life-threatening event, it may cause severe complications in patients with history of cardiorespiratory diseases, such as increased oxygen consumption that leads to hypoxemia, increased carbon dioxide production and lactic acidosis. Moreover, it increases intracerebral and intraocular pressure, can be uncomfortable for patients, aggravates wound pain and interferes with electrocardiography monitoring, blood pressure and pulse oximetry, which may pose a patient safety issue.5,6
Nonpharmacologic techniques such as forced air warmers, blankets, radiant heat and increasing the operating room ambient temperature prevent shivering through maintenance of the core body temperature by decreasing heat loss. However, these methods are expensive and cumbersome to use.7 Different pharmacologic agents including opioids, N-methyl D-aspartate receptor antagonists, magnesium sulphate, α2-agonists, cholinomimetics and biogenic amines (serotonin 5-HT3 receptor antagonist) have been used for prevention of post-spinal shivering.8
Tramadol, a centrally acting analgesic drug with μ-opioid agonist effects with minimum effect at kappa (κ) and delta (δ) receptors, has been shown to be effective in the prevention of post-spinal shivering. The mechanism of action is proposed to act through the modulatory effect on central monoaminergic pathways, inhibiting the neuronal uptake of noradrenaline and serotonin in the spinal cord and increasing hydroxyltryptamine secretion, which resets the body temperature regulation center.9–14 Ketamine, a competitive receptor antagonist of N-methyl D-aspartate has also been shown to inhibit postoperative shivering in many reports.15–20
However, there is limited information on the antishivering efficacy of prophylactic tramadol compared with low-dose intravenous (IV) ketamine. This prospective, randomized, double-blind, placebo-controlled study was designed to compare the effectiveness of low-dose IV ketamine (0.2 mg/kg) with that of 0.5 mg/kg IV tramadol for the prevention of post-spinal shivering in parturients who underwent elective cesarean section. In addition, side effects between the treatment groups were also evaluated.
Patients and methods
Approval was obtained from the University of Gondar School of Medicine Ethical Review Committee (Nr: SOM/60/09/08). Written informed consent was obtained from 123 American Society of Anesthesiologist (ASA) I and II women aged 18–39 years, who were planned for cesarean section under SA in Gondar University Hospital, Northwest Ethiopia from February to April 2016.
Parturients who had history of hypersensitivity to opioids, ketamine or bupivacaine, history of cardiovascular disease, hypertension, psychosis, antepartum hemorrhage, cord prolapse, fetal distress, initial temperature >38°C or <36°C, history of alcohol or substance abuse, those patients who needed blood transfusion during surgery or who received medications likely to alter thermoregulation, SA with combination of intrathecal fentanyl, SA with sedation, such as using propofol, pethidine or ketamine, or failed spinal converted to general anesthesia were excluded from the study.
Before SA, an 18-gauge IV cannula was inserted and 10 mL/kg normal saline was administered. Electrocardiogram, heart rate (HR), noninvasive blood pressure and pulse oximetry were attached for standard monitoring. Room temperature, tympanic temperature and hemodynamic variables (blood pressure, HR and oxygen saturation [SPO2]) were recorded before SA. Patients did not receive premedication. SA was instituted at either L3–L4 or L4–L5 in the sitting position by the anesthetist (blind to study) using 22–25 gauge Quincke spinal needles, and 2.5 mL of 5% (12.5 mg) isobaric bupivacaine was injected. SA block was assessed using pinprick and Bromage scale for the desired sensory and motor block, which was T6–T4 and Bromage scale 3, respectively. Oxygen at 4 L/minute was administered via a face mask, and patients were covered with drapes but not actively warmed. Just after SA, patients were randomized using the open envelope technique to one of the following three study groups: group K = ketamine (0.2 mg/kg IV), group T = tramadol (0.5 mg/kg IV) and group S = saline (5 mL saline), as shown in Figure 1. The study drugs were presented as coded syringes by the anesthetist who was not involved in patient management. Moreover, the study drugs and normal saline were prepared in 5 mL coded syringes and presented to the responsible anesthetists who were blinded to study group allocations, and were given as an IV bolus.
  | Figure 1 Consort flow chart. |
Tympanic temperature was monitored every 5 minutes until the end of the surgical procedure. Shivering scores were recorded at 5-minute intervals and every 10 minutes postoperatively for 1 hour. Shivering was graded using a scale similar to that validated by Tsai and Chu10 (Table 1). Pethidine (25 mg IV) was given as the rescue drug after the delivery of the fetus for parturients who developed grade 3 or 4 shivering within 15 minutes of SA despite the administration of prophylactic dose of one of the study drugs.
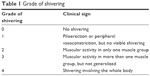  | Table 1 Grade of shivering |
Hemodynamic variables such as HR, mean arterial pressure and oxygen saturation SPO2 using pulse oximetry were recorded every 5 minutes intraoperatively and every 10 minutes postoperatively for 1 hour. Apgar score was assessed at 1 and 5 minutes.
Side effects such as hypotension, nausea and vomiting, sedation and hallucinations were recorded. If the patient’s HR decreased below 50 bpm, IV atropine 0.5 mg was administered. In addition, hypotension was treated with 5 mg of IV ephedrine and crystalloids. If the patient developed grade 1 and above nausea and vomiting, metoclopramide 10 mg was administered IV. The degree of sedation was assessed according to a five-point scale, validated by Abdelrahman,3 where 1: fully awake and oriented patient, 2: drowsy, 3: eyes closed, arousable on command, 4: eyes closed, arousable to physical stimuli and 5: eyes closed and patient unarousable to physical stimuli.
Pan African clinical trial registry with ID of PACTR201609001759210. University of Gondar, College of Medicine and Health Science, supported the study.
Sample size calculation and statistical analysis
As there was no previous study conducted in our country on the efficacy of 0.2 mg/kg of ketamine in the prevention of shivering following cesarean section under SA, we calculated the sample size using the findings of a previous study, where the incidence of shivering following cesarean section under SA after taking 0.5 mg/kg of tramadol was 16% (P1 =0.16).20 We considered an assumption of 50% reduction in the incidence of shivering after taking the prophylaxis (P2= 0.5). Using an alpha value of 0.05% and 90% power, the sample size can be determined by the following formula:
|
Given
- P1 = 0.16, P2 = 0.5
- 1 − P1 = 0.84
- 1 − P2 = 0.5
- (P2 − P1)2 = 0.1156
- f (α, β) = 10.5
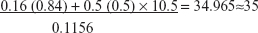 per group
per group
By considering an attrition rate of 15%, 41 participants were recruited. A total of 41×3=123 were enrolled.
Data were analyzed using SPSS version 20 statistical package software. The data were tested for normality using Shapiro–Wilk normality test, and homogeneity of variance was assessed using Levene’s test for equality of variances. Continuous variables with normal distribution were analyzed using one-way analysis of variance test, and temperature values over time were also analyzed using repeated-measures analysis of variance. Nonparametric data, shivering scores and sedation scores were compared using Kruskal–Wallis test and Mann–Whitney U test for two-group comparisons. Chi-square test was used to analyze the difference between ASA physical status, number of shivering patients, presence of nausea and vomiting, and hallucinations. Results are presented as medians (interquartile range), and exact numbers of proportions are expressed as percentages. A p-value <0.05 was considered statistically significant.
Results
A total of 123 parturients who underwent cesarean delivery under SA were enrolled in this study. Of these patients, 41 were allocated to the tramadol group (Group T), 41 to the ketamine group (Group K) and 41 to the saline group/placebo group (Group S).
Sociodemographic characteristics (age, weight and height), gestational age, parity, ASA physical status and duration of surgery were comparable among the groups (Table 2). There was no difference in recorded baseline hemodynamic data (mean arterial pressure), pulse rate and SPO2. Preoperative tympanic temperature and room temperature were also comparable among the groups (Table 3).
There was no significant difference among the groups with regard to Apgar scores, skin incision to delivery of neonates, total IV fluid given and estimated blood loss (Table 4).
In this study, with 41 patients in each group, shivering was seen in 22 patients (53.7%) in the tramadol group, 17 patients (41.5%) in the ketamine group and 29 patients (70.7%) in the normal saline group, and the overall incidence of shivering was 68/123 (55.3%; p=0.028). Post hoc test showed that there was significant difference among the groups (tramadol vs placebo =0.029, ketamine vs placebo =0.008). However, there was no significant difference between tramadol and ketamine groups (p=0.272).
Grade 2 shivering was recorded in 13 (31.7%) patients in the tramadol group and 9 (22%) patients in the ketamine group, whereas it was recorded in 11 (26.8%) patients in the normal saline group. The incidence of grade 3 shivering showed a statistically significant difference (p=0.011) in the normal saline group (16 [39%]) as compared to the tramadol group (9 [22%]) and the ketamine group (8 [19.5%]).
Severity of shivering was comparable between the tramadol and ketamine groups (p=0.354), but differed significantly in tramadol vs normal saline (p=0.031) and ketamine vs normal saline groups (p=0.004), as shown in Table 5.
Eighteen (39%) patients in the saline group, nine (22%) in the tramadol group and eight (19.5%) in the ketamine group received 25 mg of IV pethidine (p=0.027) for rescue. Mann–Whitney test showed that there was a statistically significant difference in tramadol vs saline (p=0.036) and ketamine vs saline (p=0.018) groups. However, there was no significant difference between ketamine and tramadol groups (p=0.787) in the reduction of shivering rates.
The time taken from administration of the study drugs to the onset of shivering was statistically different among the groups (p=0.001). Mann–Whitney test showed that patients in the saline group had earlier onset of shivering, as compared to patients in the tramadol (p<0.001) and ketamine (p=0.012) groups (Table 4).
In all groups, there was a significant decrease in tympanic temperature after SA with respect to baseline values. Temperature changes over time in each group were statistically significant (p<0.001); however, there were no significant differences among groups (Figure 2).
In the recovery room, tympanic temperature was recorded every 10 minutes for 1 hour. The mean tympanic temperature increased in all groups with respect to T0 values (tympanic temperature immediately after arrival in the recovery room), with p<0.001. However, there was no significant difference in the temperatures measured at 50 and 60 minutes. Post hoc testing showed that there was no significant difference among the groups (ketamine vs placebo, p=0.740; tramadol vs placebo, p=0.740; ketamine vs tramadol, p>0.05), as shown in Figure 3.
Perioperative complications such as hypotension, bradycardia, postoperative nausea and vomiting, and hallucination were not significantly different among the study groups. Hypotension was seen in five (12.2%) cases in the tramadol group, four (9.8%) cases in the ketamine group and four (9.8%) cases in the saline group. All hypotensive episodes were treated with IV crystalloid infusions and 5 mg of IV ephedrine. No patient in any group developed bradycardia. The sedation score was below 3 in all of the patients. Grade 2 sedation was seen in two cases (4.9%) in the tramadol group and seven cases (17.1%) in the ketamine group. Hallucinations were not noted in any of the study groups (Figure 4).
  | Figure 4 Incidence of perioperative complications. |
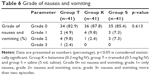
Nausea and vomiting were seen in seven (17.1%) patients in the tramadol group, five (12.2%) patients in the ketamine group and six cases (14.6%) in the saline group, and there was no significant difference among the groups regarding the severity of nausea and vomiting (p=0.613), as found in Table 6.
Discussion
This prospective, randomized, double-blind, placebo-controlled study revealed that 0.5 mg/kg IV tramadol was as effective as 0.2 mg/kg of IV ketamine in reducing the incidence and intensity of shivering during SA, compared to the control group.
In this study, the incidence of shivering in the control group was 70.7%. This incidence was high compared with previous studies,21,18 where the incidences were 53.3% and 40%, respectively. This discrepancy could be due to the use of higher ambient temperature (25°C), additional warming measures such as warming of the fluids and forced warm air that maintained body temperature of the patients in their study. Moreover, our incidence was high compared with a study carried out by Wason et al,22 where the incidence of shivering in the control group was 54%. This difference could be attributed to the median sensory block level used: T7 (in their study) vs T4 (in our study). High sensory block level in our study could increase the magnitude of vasodilation, which facilitates heat loss. Furthermore, in our study, the room temperature (20.4°C−27.5°C) was not adjusted and all fluids were not preheated to 37°C, which could increase the incidence of shivering in the control group.
In this study, the incidence of grade 3 and above shivering was 22% in the tramadol group, whereas it was 43.9% in the saline group. Our incidence was high compared with a study performed by Hidayah et al,20 where the incidence of postoperative shivering in tramadol-treated (0.5 mg/kg IV) parturients after SA was 16% vs 24% in the control group. This low incidence in the previous study could be due to the use of 25 mcg intrathecal fentanyl, which has antishivering effect.
The incidences of grade 3 and above shivering in the ketamine group and the saline group were 19.5% and 43.9%, respectively. The incidence of shivering was low in ketamine-treated parturients in this study compared with a previous study where the incidence was reported to be 33% in ketamine-treated group.19 This discrepancy could be attributed to the type of anesthesia used for cesarean section, as SA was used in our study whereas general anesthesia was used in their study. On the other hand, our incidence was low compared with another study25 where 10% of patients in the ketamine group developed grade 3 and above shivering. Low incidence of shivering in the previous study could be due to controlled room temperature at 24°C±1°C, preheating all fluids to 37°C and use of lower median sensory block level (T6) compared with this study.
In this study, the mean tympanic temperature decreased after SA in all groups, compared to baseline values. The drop in temperature was not significantly different among the groups. The decrease in temperature was clinically significant, and hypothermia during SA can be explained by heat redistribution from the core to periphery, vasodilation with heat loss and inhibition of thermoregulation. This was in accordance with a previous study23 in which granisetron, dexmedetomidine, tramadol and control groups were compared and there were no differences among the groups with respect to tympanic temperature drop after SA. This could be attributed to the same mechanisms of actions of these drugs in the prevention of shivering via the central thermoregulatory center. However, another study25 showed a greater fall in core body temperatures in the placebo group, compared with ketamine, tramadol and clonidine groups.
Furthermore, in this study, postoperative temperature increased in the recovery room over time compared to temperatures recorded at admission in the recovery room immediately after operation, which could be clinically relevant, although statistically significant differences were not reached. The increase in tympanic temperature might be related to the use of blanket to cover the patient in the recovery room and the recovery of sensory and motor functions overtime as the effect of SA weaned.
Moreover, the incidences of perioperative complications such as hypotension, nausea and vomiting, sedation and hallucination were not significantly different among the groups in this study. However, Gangopadhyay et al24 observed large numbers of nausea and vomiting cases in parturients who were treated with tramadol (1 mg/kg). This could be attributed to large dose of tramadol used in the previous study compared with our study (1 vs 0.5 mg/kg).
In addition, sedation score was lower than grade 3 in all patients in this study. However, seven cases in the ketamine group developed grade 2 sedation, though not statistically significant compared to the tramadol group. This is inconsistent with a previous study25 where ketamine caused significant grade 3 and 4 sedation compared with saline, clonidine and tramadol groups. This could be due to the fact that large dose of ketamine was used in the previous study compared with this study (0.5 vs 0.2 mg/kg).
Limitation of the study
Room temperature and temperature of IV fluids were not tightly controlled.
Conclusion and recommendation
The prophylactic administration of low-dose IV ketamine (0.2 mg/kg) or 0.5 mg/kg IV tramadol is effective in reducing the incidence and intensity of shivering in patients having cesarean section under SA. We recommend low-dose IV ketamine or tramadol prophylaxis for parturients undergoing cesarean section under SA. Moreover, other shivering prevention methods should be established, as shivering is still high in the treatment groups.
Author contributions
All authors have been involved sufficiently in the intellectual content, conception and design, analysis and interpretation of the data, as well as the writing of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Crowley LJ, Buggy DJ. Shivering and neuraxial anesthesia. Reg Anesth Pain Med. 2008;33(3):241–252. | ||
Yimer HT, Hailekiros AG, Tadesse YD. Magnitude and associated factors of postanaesthesia shivering among patients who operated under general and regional anesthesia, Northwest Ethiopia: a cross sectional study. J Anesth Clin Res. 2015;2015. | ||
Abdelrahman RS. Prevention of shivering during regional anesthesia: comparison of midazolam, midazolam plus ketamine, tramadol, and tramadol plus ketamine. Life Sci J. 2012;9(2):132–139. | ||
Kim YA, Kweon TD, Kim M, Lee HI, Lee YJ, Lee KY. Comparison of meperidine and nefopam for prevention of shivering during spinal anesthesia. Korean J Anesthesiol. 2013;64(3):229–233. | ||
Talakoub R, Meshkat SN. Tramadol versus meperidine in the treatment of shivering during spinal anesthesia in cesarean section. J Res Med Sci. 2006;11(3):151–155. | ||
De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. J ASA. 2002;96(2):467–484. | ||
Dhimar AA, Patel MG, Swadia V. Tramadol for control of shivering (comparison with pethidine). Indian J Anaesth. 2007;51(1):28–31. | ||
Han JW, Kang HS, Choi SK, Park SJ, Park HJ, Lim TH. Comparison of the effects of intrathecal fentanyl and meperidine on shivering after cesarean delivery under spinal anesthesia. Korean J Anesthesiol. 2007;52(6):657–662. | ||
Mathews S, Al Mulla A, Varghese P, Radim K, Mumtaz S. Postanaesthetic shivering-a new look at tramadol. Anaesthesia. 2002;57(4):394–398. | ||
Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93(5):1288–1292. | ||
T M, Kaparti L. A randomised trial comparing efficacy, onset and duration of action of pethidine and tramadol in abolition of shivering in the intra operative period. J Clin Diagn Res. 2014;8(11):GC07–GC09. | ||
Reddy VS, Chiruvella S. Clonidine versus tramadol for post spinal shivering during caesarean section: A randomized double blind clinical study. J Obstet Anaesth Crit Care. 2011;1(1):26–29. | ||
Javaherforoosh F, Akhondzadeh R, Aein KB, Olapour A, Samimi M. Effects of tramadol on shivering post spinal anesthesia in elective cesarean section. Pak J Med Sci. 2009;25(1):12–17. | ||
Atashkhoyi S, Negargar S. Effect of tramadol for prevention of shivering after spinal anesthesia for cesarean section. Res J Biol Sci. 2008;3(12):1365–1369. | ||
Al Maruf A, Islam MS, Hoq N. Effect of tramadol and pethidine on shivering during cesarean section under spinal anaesthesia. J Armed Forces Med Coll Bangladesh. 2015;10(2):27–32. | ||
Honarmand A, Safavi M. Comparison of prophylactic use of midazolam, ketamine, and ketamine plus midazolam for prevention of shivering during regional anaesthesia: a randomized double-blind placebo controlled trial. Br J Anaesth. 2008;101(4):557–562. | ||
Elmawgood AA, Rashwan S, Rashwan D. Efficacy of prophylactic use of hydrocortisone and low dose ketamine for prevention of shivering during spinal anesthesia. Eg J Anaesth. 2012;28(3):217–221. | ||
Kose EA, Honca M, Dal D, Akinci SB, Aypar U. Prophylactic ketamine to prevent shivering in parturients undergoing cesarean delivery during spinal anesthesia. J Clin Anesth. 2013;25(4):275–280. | ||
Eydi M, Golzari SE, Aghamohammadi D, Kolahdouzan K, Safari S, Ostadi Z. Postoperative management of shivering: a comparison of pethidine vs. ketamine. Anesth Pain Med. 2014;4(2):e15499. | ||
Hidayah MN, Liu CY, Joanna OS. Ketamine and tramadol for the prevention of shivering during spinal anaesthesia. Clin Ter. 2014;165(4):193–198. | ||
Ejiro B, Edomwonyi N, Imarengiaye C. Ondansetron versus tramadol in the prevention of postanaesthesia shivering following caesarean section under spinal anaesthesia. African J Anaesth Intensive Care. 2014;14(1):6–11. | ||
Wason R, Jain N, Gupta P, Gogia AR. Randomized double-blind comparison of prophylactic ketamine, clonidine and tramadol for the control of shivering under neuraxial anaesthesia. Indian J Anaesth. 2012;56(4):370–375. | ||
Mohamed AZE. Different drugs for prevention of post subarachnoid block shivering. Randomized, controlled, double blind study. Eg J Anaesth. 2016;32:195–200. | ||
Gangopadhyay S, Gupta K, Acharjee S, Nayak SK, Dawn S, Piplai G. Ketamine, tramadol and pethidine in prophylaxis of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. 2010;26(1):59–63. | ||
Shakya S, Chaturvedi A, Sah BP. Prophylactic low dose ketamine and ondansetron for prevention of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. 2010;26(4):465–469. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.