Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 9 » Issue 1
Efficacy of indacaterol on quality of life and pulmonary function in patients with COPD and inhaler device preferences
Authors Ohno T, Wada S, Hanada S, Sawaguchi H, Muraki M, Tohda Y
Received 30 October 2013
Accepted for publication 6 December 2013
Published 21 January 2014 Volume 2014:9(1) Pages 107—114
DOI https://doi.org/10.2147/COPD.S56777
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Takeshi Ohno,1 Shota Wada,1 Souichirou Hanada,1 Hirochiyo Sawaguchi,1 Masato Muraki,1 Yuji Tohda2
1Department of Respiratory Medicine and Allergology, Nara Hospital, Kinki University Faculty of Medicine, Ikoma, Japan; 2Department of Respiratory Medicine and Allergology, Kinki University School of Medicine, Osakasayama, Japan
Background: Indacaterol is a novel, once-daily, inhaled, long-acting β2-agonist for patients with chronic obstructive pulmonary disease (COPD). The study objective was to evaluate the efficacy of indacaterol on quality of life and pulmonary function in patients with COPD in a real-world setting, and also to evaluate its inhaler device (Breezhaler®), which is important for both adherence and management.
Methods: Twenty-eight outpatients with COPD were treated with indacaterol (150 µg once daily for 8 weeks), and the effects on pulmonary function were evaluated using a questionnaire survey with the modified Medical Research Council (mMRC) dyspnea scale and COPD assessment test (CAT) before and after treatment. Similar investigations were also performed separately among different baseline medications. Moreover, original questionnaire surveys for indacaterol and its device were performed.
Results: Overall, mMRC dyspnea scale and CAT scores significantly improved (1.96±1.04 to 1.57±1.07 and 17.39±8.23 to 12.82±8.42, respectively; P<0.05). Significant improvements in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were also observed on pulmonary function tests (2.91±0.66 L to 3.07±0.65 L and 1.46±0.60 L to 1.58±0.59 L, respectively; P<0.05). Replacement therapy from salmeterol to indacaterol significantly improved mMRC and FVC values, but did not significantly improve CAT scores or other pulmonary functions. Add-on therapy with indacaterol significantly improved mMRC score, CAT score, FVC, and FEV1, regardless of whether tiotropium was used as a baseline treatment. All subjects in a questionnaire survey found the inhaler device easy to use. There were no serious adverse events leading to treatment discontinuation.
Conclusion: Indacaterol is thought to be effective and well tolerated as a bronchodilator for the management of COPD. Treatment with indacaterol in addition to a long-acting muscarinic antagonist was also useful.
Keywords: indacaterol, COPD, quality of life, respiratory function, device
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide and results in an economic and social burden that is both substantial and increasing.1,2 It was reported that the overall lifetime risk of physician-diagnosed COPD at 80 years of age was 27.6% in a longitudinal cohort study.3 Improvements in quality of life (QoL), such as exercise tolerance and physical activity, and the improvement of pulmonary function in addition to the prevention of exacerbation are expected in the medication management of patients with COPD. The principal therapies for COPD management are inhaled medications. However, various devices for inhalation exist. Satisfaction with the inhaler device is also positively correlated with improved adherence and clinical outcomes.4 Especially for elderly patients, it is thought that the merits of these devices reflect treatment successes. Indacaterol (Onbrez®; Novartis Pharma, Tokyo, Japan) is a novel, inhaled, long-acting b2-agonist (LABA) providing a fast onset of action5 and 24 hours of bronchodilation6–8 on once-daily dosing. A dose of 150 μg of powder contained in a capsule is inhaled using its inhaler device (Breezhaler®; Novartis Pharma).
In the present study, an evaluation of the efficacy of indacaterol on QoL (the modified Medical Research Council [mMRC] dyspnea scale and COPD assessment test [CAT] scores) and pulmonary function was performed for patients with COPD in an open study in a real-world setting. Additionally, similar analyses were investigated in a salmeterol-to-indacaterol replacement group and in an indacaterol add-on group based on the presence or absence of the long-acting muscarinic antagonist (LAMA) tiotropium. Finally, a questionnaire survey was conducted for the inhaler device, which is important for both adherence and management.
Materials and methods
Subjects
Twenty-eight outpatients with COPD who had been commuting to the Department of Respiratory Medicine and Allergology at Nara Hospital, Kinki University Faculty of Medicine, Ikoma, Japan, were enrolled. COPD was defined according to the Global initiative for chronic Obstructive Lung Disease (GOLD) 2011 criteria.9 Subjects were over 40 years old, had >10 pack-years of smoking status, and had stable disease with no exacerbations during the 3 months prior to the study. Twenty-six patients (92.9%) were men and the mean age was 75.7±7.0 years. Maximum inspiratory flow using an In-Check Oral Inspiratory Flow Meter® (Matsuyoshi and Co, Ltd, Tokyo, Japan) was 78.2±20.7 L/min (using an adaptor for Diskus; A/A/D). The mean Brinkman Index was 1,175 (58.75 pack-years). Regarding the severity of airflow limitation,9 six patients were classified as mild (GOLD1), twelve were moderate (GOLD2), six were severe (GOLD3), and four were very severe (GOLD4). Two patients were undergoing long-term oxygen therapy and two had bronchial asthma complications. As baseline medications, 19 patients were receiving tiotropium, ten were receiving salmeterol, two were receiving theophylline, seven were receiving an inhaled glucocorticosteroid, and seven were receiving mucolytics (Table 1). All patients were ex-smokers and were not on active smoking cessation pharmacotherapy.
  | Table 1 Clinical characteristics of the subjects (n=28) |
Protocol (study design)
This study was performed in a real-world setting and was an open-label clinical trial with no randomization, no placebo group, and no blinding. All subjects visited the hospital in the morning and answered the mMRC and CAT questionnaires. A pulmonary function test using a CHESTAC-33 (CHEST MI, Tokyo, Japan) was performed 2–4 hours after the patients took their baseline medications. After receiving inhaler instructions from a pharmacist at the hospital, subjects started to inhale indacaterol (150 μg) once daily, starting the next morning. Eight weeks (±1 week) later, they visited the hospital at a similar time in the morning. Then, they answered the mMRC, CAT, and original questionnaires for indacaterol (Onbrez) and the Breezhaler device (it was confirmed that no subjects experienced problems with Breezhaler usage during the visit). Spirometry was also performed 2–4 hours after indacaterol inhalation and after the administration of other morning medications. In addition, adverse events were investigated during the 8 week (±1 week) follow-up visit.
All ten patients taking salmeterol as a baseline controlling medication were switched to indacaterol. Other baseline medications, except for salmeterol, were continued.
Second, similar subanalyses were performed on the basis of different baseline medications: the groups treated with and without an LAMA (tiotropium) and the groups treated with and without an LABA (salmeterol).
The study protocol was approved by the Institutional Review Board at Nara Hospital, Kinki University Faculty of Medicine, and informed consent was obtained from all subjects.
Statistical analysis
Data are summarized as the mean ± standard deviation. Statistical differences before and after treatment with indacaterol were assessed using paired t-tests. Statistical analyses were performed using JMP® version 10.0.2 statistical software (SAS Institute Japan, Tokyo, Japan), and a difference of P<0.05 was considered statistically significant.
Results
As one subject among the 28 patients could not perform the pulmonary function test after treatment, resulting from a mistake, their pulmonary function data were excluded from the analyses. In the questionnaire survey, some blank responses were also excluded from the analyses. All patients continued treatment with indacaterol during the study period, had no exacerbations, and did not require the use of a rescue inhaler.
Pre- and post-treatment mMRC dyspnea scale scores were significantly improved by treatment with indacaterol (1.96±1.04 to 1.57±1.07; P=0.0013) (Figure 1A). CAT scores also significantly improved from 17.39±8.23 to 12.82±8.42 (P=0.0003) after treatment with indacaterol (Figure 1B). Scores for each item on the CAT questionnaire are shown in Figure 2. Scores for questions one to seven of the total of eight questions were significantly improved (P<0.05). In pulmonary function tests, treatment with indacaterol significantly improved forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and flow at 25% forced vital capacity (V25) (P<0.05 for each) (Figure 3).
Subanalyses performed based on treatment with or without an LAMA (tiotropium) as a baseline medication are shown in Table 2. In patients treated with tiotropium, mMRC scale, CAT score, FVC, and V25 significantly improved (2.33±1.12 to 1.67±1.12, 13.78±4.99 to 10.22±4.87, 1.45±0.65 L to 1.64±0.56 L, and 0.17±0.10 L to 0.26±0.10 L; P<0.05 for each). In patients treated with tiotropium, mMRC scale score, CAT score, FVC, and FEV1 significantly improved (1.79±0.98 to 1.53±1.07, 19.11±8.99 to 14.05±9.53, 2.92±0.68 L to 3.08±0.66 L, and 1.47±0.60 L to 1.56±0.61 L; P<0.05 for each).
Subanalyses based on groups who had not been treated with salmeterol, those who received indacaterol add-on therapy (the add-on group), and those who had been treated with salmeterol and switched to indacaterol (replacement group) are shown in Table 3. In the add-on group, mMRC scale score, CAT score, FEV1, and V25 significantly improved (1.94±1.00 to 1.50±0.86, 17.28±6.44 to 12.17±6.60, 1.63±0.59 L to 1.75±0.55 L, and 0.20±0.09 L to 0.22±0.08 L; P<0.05 for each). In the replacement group, mMRC and FVC significantly improved (2.00±1.15 to 1.70±1.42 and 2.66±0.52 L to 2.84±0.61 L; P<0.05 for each).
Original questionnaire surveys for indacaterol and the Breezhaler device were conducted, and responses were obtained from 26 subjects. A total of 61.5% of the total responded “I want to continue Onbrez”, and all subjects responded “Yes” for device ease of handling and ease of use. Furthermore, 24 subjects (92.3%) responded that they felt like they could inhale (Table 4).
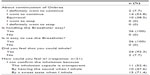  | Table 4 Questionnaire survey for indacaterol (Onbrez®) and the Breezhaler® device |
Responses for reasons the patients felt they could inhale were obtained from 21 of those 24 subjects. Reasons included (multiple answers were allowed) 71.4% responding “By the sweet taste when I inhale”, 52.4% responding “I can confirm the inhalation because the inhalation capsule is transparent”, and 47.6% responding “By hearing the sound when I inhale” (Table 4).
The most frequent adverse event observed was coughing immediately after inhalation, in six patients. In addition, three reports of hoarseness and two reports of dry mouth were observed in four subjects. The two patients who complained of dry mouth were both treated with tiotropium as a baseline medication. However, these symptoms were mild and did not lead to discontinuation of indacaterol treatment. All patients continued treatment with indacaterol after the present study (Table 5).
  | Table 5 Adverse events |
Discussion
Once, it was considered that anticholinergics (muscarinic antagonists) demonstrated a greater bronchodilation effect than β2-agonists in patients with COPD.9,10 In fact, of the long-acting bronchodilators, tiotropium showed a higher efficacy than salmeterol.11 However, indacaterol, a new LABA, was shown to be at least as effective as tiotropium and with a faster onset of action.12 Indacaterol has shown a powerful bronchodilation effect with a mechanism different from that of the LAMAs, and its efficacy has been shown. Pharmacologic therapy in COPD is used to reduce symptoms, reduce the frequency and severity of exacerbations, and improve health status and exercise tolerance.13 There are several validated questionnaires to assess symptoms in patients with COPD. GOLD recommends the use of the mMRC questionnaire or the CAT. The well-known mMRC questionnaire assesses only disability resulting from breathlessness; however, the CAT covers a broader range of the impact of COPD on the patient’s daily life and well-being.13 In the present study, these two questionnaires were used for the assessment of the patients’ QoL, and the efficacy of bronchodilation was assessed by spirometry. Because inhaler adherence is very important in the management of COPD,14 questionnaire surveys for indacaterol (Onbrez) and its inhaler device (Breezhaler) were performed.
It has been reported that patients receiving indacaterol had clinically significant improvements in symptoms of dyspnea compared with placebo.15 Among the 23 eligible patients, 14 were receiving tiotropium 18 μg/day (by a Handihaler®), and nine were receiving no therapy. Treatment with indacaterol improved both pulmonary function and QoL.16 Add-on therapy with indacaterol also improved expiratory reserve volume and exercise capacity, although a significant difference was not noted in the improvement of FEV1.17 Overall, indacaterol therapy improved the QoL of patients with COPD in the present study. In addition, FVC, FEV1, and V25 were improved in pulmonary function tests. Significantly more patients receiving indacaterol reported less need for a rescue inhaler (short-acting b2-agonist) than LABA recipients.18,19 In the present study, no patients used a short-acting b2-agonist in a rescue capacity. Indacaterol might be a useful option, even for the treatment of acute exacerbations of COPD.20
Indacaterol was at least as effective as tiotropium in improving clinical outcomes.21,22 However, a comparison of indacaterol and tiotropium under blinded conditions confirmed a statistically significant improved effect of indacaterol over tiotropium for dyspnea (transition dyspnea index) and health status (the St George’s Respiratory Questionnaire).23 Furthermore, compared with tiotropium monotherapy, indacaterol plus tiotropium provided greater bronchodilation and lung deflation (reflected by an increased resting inspiratory capacity).24 Regardless of the use or nonuse of tiotropium, add-on therapy with indacaterol significantly improved mMRC scale score, CAT score, and FEV1 in the present study. These results support COPD guideline recommendations to combine bronchodilators with different mechanisms of action.
Using trough FEV1 as a measure of therapeutic effect, indacaterol was superior to other β2-agonists, tiotropium, and placebo at weeks 12, 26, and 52. Indacaterol had a greater effect on the transition dyspnea index than salmeterol.25 Although this study did not consider the trough value, the switch from salmeterol did not significantly improve pulmonary function, except for FVC. However, mMRC score was significantly improved, even in the present small sample size. Therefore, it is thought that indacaterol treatment contributed to improvements of QoL more than salmeterol.
Adherence is crucial for optimizing clinical outcomes in COPD, with nonadherence resulting in significant health and economic burdens.26 Adherence in patients with COPD is affected by multiple factors associated with the patient, their clinician, and society.27 Patient-related factors include health beliefs and self-efficacy. Treatment-related factors include each treatment requiring a different technique for administration, in addition to the need for multiple inhalers, and are especially burdensome for older COPD patients. In the present study, indacaterol and its device seemed to be regarded favorably by patients with COPD. These results may contribute to better clinical outcomes through satisfactory adherence.
The most frequently observed adverse event was coughing immediately after inhalation; hoarseness and dry mouth were also observed. However, these adverse events were mild and did not result in the discontinuation of indacaterol treatment. Indacaterol demonstrated excellent tolerability.
A limitation of the present study is the small sample size. There were no analyses of the patients’ adherence as distinguished from inhaler device preference; therefore, future investigations regarding inhaler adherence are needed. However, despite the small sample size and regardless of the quality of adherence, pulmonary functions and QoL were improved. In addition, there are no analyses comparing inhaler devices in the present study with other inhaler devices, and further studies are needed to better clarify patient preferences.
Conclusion
In summary, indacaterol is both safe and effective in the management of COPD. Furthermore, add-on therapy with an LAMA is also effective in improving clinical outcomes. The inhaler device is easy to use and was well regarded by the patients, which can contribute to medication adherence.
Disclosure
The authors report no conflicts of interest in this work.
References
Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27:397–412. | |
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. | |
Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991–996. | |
Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490. | |
Balint B, Watz H, Amos C, Owen R, Higgins M, Kramer B; INSURE Study Investigators. Onset of action of indacaterol in patients with COPD: comparison with salbutamol and salmeterol-fluticasone. Int J Chron Obstruct Pulmon Dis. 2010;5:311–318. | |
Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65:473–479. | |
Bleecker ER, Siler T, Owen R, Kramer B. Bronchodilator efficacy and safety of indacaterol 150 μg once daily in patients with COPD: an analysis of pooled data. Int J Chron Obstruct Pulmon Dis. 2011;6:431–438. | |
Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37:273–279. | |
Gross NJ, Skorodin MS. Role of the parasympathetic system in airway obstruction due to emphysema. N Engl J Med. 1984;311:421–425. | |
Schocken DD, Roth GS. Reduced beta-adrenergic receptor concentrations in ageing man. Nature. 1977;267:856–858. | |
Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–1103. | |
Vogelmeier C, Ramos-Barbon D, Jack D, et al. Indacaterol provides 24-hour bronchodilation in COPD: a placebo-controlled blinded comparison with tiotropium. Respir Res. 2010;11:135. | |
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of chronic obstructive lung disease [updated 2013]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed December 9, 2013. | |
Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64:939–943. | |
Han J, Dai L, Zhong N. Indacaterol on dyspnea in chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized placebo-controlled trials. BMC Pulm Med. 2013;13:26. | |
Hataji O, Naito M, Ito K, Watanabe F, Gabazza EC, Taguchi O. Indacaterol improves daily physical activity in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:1–5. | |
Mroz RM, Minarowski L, Chyczewska E. Indacaterol add-on therapy improves lung function, exercise capacity and life quality of COPD patients. Adv Exp Med Biol. 2013;756:23–28. | |
Korn S, Kerwin E, Atis S, Amos C, Owen R, Lassen C; INSIST study group. Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med. 2011;105:719–726. | |
Feldman GJ. Improving the quality of life in patients with chronic obstructive pulmonary disease: focus on indacaterol. Int J Chron Obstruct Pulmon Dis. 2013;8:89–96. | |
Segreti A, Fiori E, Calzetta L, et al. The effect of indacaterol during an acute exacerbation of COPD. Pulm Pharmacol Ther. 2013;26(6):630–634. | |
Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155–162. | |
Mahler DA, Buhl R, Lawrence D, McBryan D. Efficacy and safety of indacaterol and tiotropium in COPD patients according to dyspnoea severity. Pulm Pharmacol Ther. 2013;26:348–355. | |
Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J. 2011;38:797–803. | |
Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax. 2012;67:781–788. | |
Jiang FM, Liang ZA, Zheng QL, Wang RC, Luo J, Li CT. Safety and efficacy of 12-week or longer indacaterol treatment in moderate-to-severe COPD patients: a systematic review. Lung. 2013;191:135–146. | |
Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:401–406. | |
Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:371–384. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





