Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Efficacy of ICS versus Non-ICS Combination Therapy in COPD: A Meta-Analysis of Randomised Controlled Trials
Authors Ding Y, Sun L , Wang Y, Zhang J, Chen Y
Received 4 November 2021
Accepted for publication 24 March 2022
Published 5 May 2022 Volume 2022:17 Pages 1051—1067
DOI https://doi.org/10.2147/COPD.S347588
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Zhang
Yanling Ding *, Lina Sun *, Ying Wang, Jing Zhang, Yahong Chen
Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Zhang; Yahong Chen, Department of Respiratory and Critical Care Medicine, Peking University Third Hospital, Beijing, People’s Republic of China, Tel +86 13-8104-57631, Email [email protected]; [email protected]
Background: Several large randomized clinical trials (RCTs) have assessed the efficacy and safety of inhaled corticosteroid (ICS) combination regimens versus non-ICS therapy in patients with chronic obstructive pulmonary disease (COPD) at increased risk of exacerbation risk with mixed results.
Methods: We performed a systematic literature review and meta-analysis of RCTs comparing the effect of ICS-containing combination therapy and non-ICS regimen in patients with COPD.
Results: A total of 54 RCTs (N = 57,333) reported treatment effects on various outcomes and were eligible for inclusion. Overall, the number of patients experiencing moderate/severe exacerbations was significantly lower for ICS-containing combination therapy versus non-ICS therapy (RR: 0.86 [95% CI: 0.80– 0.93]). The annual rate of exacerbations was also significantly reduced by 22% (0.78 [0.72– 0.86]) with ICS-containing versus non-ICS therapy. The annual rate of exacerbations requiring hospitalisation was reduced by 31% versus non-ICS therapy (0.69 [0.54– 0.88]); similar reduction was observed for exacerbations requiring oral steroids (0.69 [0.66– 0.73]). Overall, the effect on trough FEV1 was comparable between ICS-containing and non-ICS therapies (follow-up: 6– 52 weeks); however, a significant improvement in lung function (trough FEV1) was observed for ICS/LABA versus LABA (MD: +0.04 L [0.03− 0.05]) and ICS/LABA/LAMA versus LAMA (MD: +0.09 L [0.05− 0.13]) regimens. In addition, a significant improvement in QoL was observed with ICS-containing versus non-ICS therapy (MD in SGRQ score: − 0.90 [− 1.50, − 0.31]).
Conclusion: This meta-analysis demonstrated that a wide range of patients with COPD could benefit from dual and triple ICS-containing therapy.
Keywords: meta-analysis, chronic obstructive pulmonary disease, inhaled corticosteroid, dual therapy, triple therapy, exacerbation
A Letter to the Editor has been published for this article.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by progressive deterioration of lung function and worsening of symptoms and health status, leading to persistent respiratory symptoms and airflow limitation.1 COPD is complicated by frequent and recurrent acute exacerbations, which result in high morbidity and mortality as well as enormous health-care expenditures; being the third leading cause of death worldwide.2,3 COPD exacerbations are estimated to result in ~110,000 deaths and >500,000 hospitalisations/year, with >US$18/year billion spent in direct costs.3 Additionally, acute exacerbations result in >50% mortality at 5 years following hospitalisation.4 Following an exacerbation, symptoms and lung function take several weeks to recover, which negatively affects patients’ quality of life (QoL). Furthermore, exacerbations accelerate the rate of irreversible worsening of pulmonary function.3,4
While short-term treatment goals include relief from symptoms and improvement of exercise tolerance and health status, the long-term treatment goal is to prevent disease progression, and reduce acute exacerbations and mortality.1 The role of inhaled corticosteroid (ICS) therapy to attenuate the underlying inflammatory process in COPD has been a topic of debate in the recent years. Studies with newer long-acting anti-muscarinic agents (LAMA) or their combination with long-acting β2 agonists (LABA) have shown comparable effects on the risk of exacerbations compared with ICS/LABA, raising questions on the utility of ICS-containing dual therapy in patients with stable disease.5–7 However, these studies were limited by short duration or were randomised withdrawal studies that stabilised subjects on an ICS prior to randomisation, which may have had an impact on study outcomes. In addition, the heterogeneity in the characteristics of patients with COPD should also be considered as patients with higher eosinophil count (≥300 cells/µL) or with a history of exacerbations may benefit more with ICS treatment.1 The current Global Initiative for Chronic Obstructive Lung Disease (GOLD) treatment algorithm recommends initial ICS-containing therapy in patients with group D disease; however, this is not based on high-quality evidence.1,7–9 Hence, there is ambiguity in the initial treatment approach for newly diagnosed patients with COPD.
In patients with moderate-to-severe COPD, triple inhaled therapy with ICS/LAMA/LABA improves lung function, symptoms and patient-reported outcomes and reduces exacerbations and mortality compared to ICS/LABA, LABA/LAMA or LAMA monotherapy.10–14 These findings further highlight the importance of ICS-containing therapies in the management of COPD.
Several large randomised clinical trials (RCTs) have evaluated the effect on risk reduction of acute exacerbations with dual (ICS/LABA) and triple ICS-containing therapy (ICS/LAMA/LABA) versus non-ICS regimens in patients with COPD at increased exacerbation risk. FLAME (NCT01782326) study showed a significant reduction (by 17%) in exacerbations with LABA/LAMA compared with ICS/LABA in patients with a history of ≥1 exacerbation during the previous year. Given the range of heterogeneity across studies and the lack of a precise definition of acute exacerbation these studies showed contradictory results. Therefore, we performed a systemic literature review and meta-analysis of RCTs assessing the effects of ICS in dual and triple inhaled therapy versus non-ICS regimen on lung function, risk of exacerbation, patient symptoms and health status to obtain more comprehensive evidence on their efficacy.
Materials and Methods
Search Strategy and Selection Criteria
We conducted a systematic review of parallel-group RCTs comparing ICS versus non-ICS therapies in patients with COPD. A literature search was conducted using MEDLINE (PubMed), Cochrane CENTRAL and ClinicalTrials.gov databases from inception to January 2019.
Single-blind, double-blind and open-label RCTs comparing ICS-containing dual (ICS/LABA versus LABA; ICS/LABA versus LAMA; or ICS/LABA versus LABA/LAMA) or triple therapy (ICS/LABA/LAMA versus LAMA; ICS/LABA/LAMA versus LABA/LAMA) and reporting at least one of the outcomes of interest were included. There was no restriction in terms of study duration, type of device or study medications; however, studies with fewer than 50 participants were excluded.
Study treatments were restricted to all available combinations at the approved doses of these combinations and their comparators (Table 1).
 |  |  |  |
Table 1 Characteristics of Included Studies |
Data Extraction and Risk of Bias Assessment
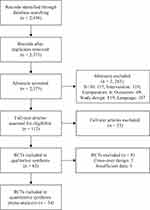
Two reviewers screened the search results for relevant titles or abstracts, followed by review of full-text articles. The reviewers worked independently during study selection and data extraction process. The results were compared to obtain consensus and avoid bias (Figure 1).
Risk of bias in eligible trials was assessed by the Cochrane collaboration’s tool (Version 2.0;). Two reviewers examined the risk of bias for the randomisation process, deviation from intended intervention, missing outcomes data, measurement of the outcome and selection of the reported results. Disagreements were resolved and the overall bias for each included trial was categorised as “low risk”, “some concerns” and “high risk”.
Endpoints
The outcomes of interest for the present meta-analysis included change from baseline in trough forced expiratory volume in one second (FEV1), number of patients experiencing moderate-to-severe exacerbations, annual rate of moderate-to-severe exacerbations, change in St George’s Respiratory Questionnaire (SGRQ) score and SGRQ response, use of rescue medication, frequency and severity of dyspnoea (change in modified Medical Research Council [mMRC] score) and other COPD symptoms (change in COPD Assessment Test [CAT] score).
Data Analysis
All statistical analyses were performed using the Stata software (version 15.0). Data were extracted using a standardised data form. Relative risks (RR), mean difference (MD) and 95% confidence intervals (95% CI) were calculated for each outcome. Pooled RR or MD was estimated using a fixed-effects model (Mantel-Haenszel method, inverse-variance method) if no significant heterogeneity was detected, or a random-effects model if a high level of heterogeneity was present. All the tests were two-sided and P ≤ 0.05 was considered statistically significant. For all the outcomes of interest, subgroup analyses were performed by therapeutic regimen.
Heterogeneity was assessed using I2 statistic (values <25, 25–50 and >50% indicated low, moderate and high heterogeneity, respectively) and chi-Square test (significance level at P < 0.1). Potential publication bias was evaluated using funnel plot and Harbord or Egger test. A sensitivity analysis was performed to evaluate the influence of each individual study.
Results
Studies Included in the Analyses
Of the 2938 articles identified in the initial search, a total of 62 RCTs reporting data on 66 treatment comparisons were shortlisted based on full-text review. Three studies were excluded because of cross-over design and five were excluded because of insufficient data; hence, 54 RCTs with 58 treatment comparisons were included in the final analysis (Figure 1). A total of 23 studies compared ICS/LABA dual therapy with LABA, 14 with LABA/LAMA and seven with LAMA monotherapy. In addition, ICS/LABA/LAMA triple therapy was compared with LABA/LAMA in six studies and with LAMA in eight (Table 1).5,7,8,10,14–61Study duration ranged from 4 weeks to 3 years. Most of the studies showed a low risk of bias in the six domains of the Cochrane Risk of Bias instrument (Version 2.0), with five showing a high risk and five with some concerns.
Patient inclusion criteria of the studies included in the meta-analysis are summarised in Table 1. The included studies enrolled patients across a wide range of airflow limitation, symptoms and history of exacerbations (ABCD groups). A total of 21 studies included patients with no history of exacerbations, evaluating the potential benefit of ICS-containing therapy in patients with stable disease.
Effects of Treatment on Lung Function (Trough FEV1 Pre-Dose)
A total of 41 studies reported change in trough FEV1 (pre-dose) and were included in the pooled analysis of overall effect (follow-up ranged from 6 weeks to 1 year). There was no significant difference between ICS and non-ICS treatment regimens for the change from baseline in trough FEV1 (MD: +0.01 L [−0.01, 0.03];). The improvement in lung function was significant for the following: ICS/LABA versus LABA (MD: +0.04 [0.03, 0.05]) and ICS/LABA/LAMA versus LAMA (MD: +0.09 [0.05, 0.13]; Figure 2).
Effects of Treatment on COPD Exacerbations
A total of 26 and 24 studies reported effects on the number of patients experiencing COPD exacerbations and the annual exacerbation rate (AER), respectively. The number of patients experiencing exacerbations was significantly reduced by 14% (RR: 0.86 [0.80, 0.93]) with ICS compared with non-ICS treatment regimen. The AER was significantly reduced by 22% with ICS versus non-ICS treatment (RR: 0.78 [0.72, 0.86]). None of the non-ICS regimens were shown to significantly reduce exacerbations versus ICS-containing therapies (Figures 3 and 4). ICS-containing dual therapy (ICS/LABA) showed a significant reduction in the number of patients experiencing exacerbations compared with LABA or LAMA alone: 14% reduction versus LABA (RR: 0.86 [0.80, 0.92]) and 26% reduction versus LAMA (RR: 0.86 [0.36, 1.53]). The number of patients experiencing exacerbations was significantly reduced by 41% (RR: 0.86 [0.36, 0.98]) and by 29% (RR: 0.71 [0.49, 1.02]) with ICS-containing triple therapy compared with LABA/LAMA and LAMA, respectively. The AER was significantly reduced by 24% (RR: 0.76 [0.65, 0.89]) and by 33% (RR: 0.67 [0.46, 0.96]) with ICS-containing triple therapy compared with LABA/LAMA and LAMA, respectively. Skin thickening, candidiasis and pneumonia were the side effects associated with ICS therapy,62 while dry mouth, nausea, headache are the side-effects often associated with non-ICS therapy.63
A total of 7 and 10 studies reported effects on the AER requiring hospitalisations and oral steroids, respectively. The annual rate of severe exacerbations requiring hospitalisation was reduced by 31% with ICS versus non-ICS therapy (RR: 0.69 [0.54, 0.88]). There was a similar reduction in the AER requiring oral corticosteroids (RR: 0.69 [0.66, 0.73]; with ICS versus non-ICS treatment.
Effects of Treatment on Health Status (Change in SGRQ Score and SGRQ Response)
A total of 22 studies reported data on change in SGRQ score. ICS-containing treatment was associated with a significant reduction in SGRQ compared with non-ICS treatment (MD: −0.90 [−1.50, −0.31];). A greater change in SGRQ was reported with ICS/LABA/LAMA versus LAMA (weighted mean difference (WMD): −5.20 [−8.02, −2.38]).
Effects of Treatment on Rescue Medication Use, Dyspnoea and Other COPD Symptoms
The use of rescue medication (RMU) was reduced by −0.15 inhalation/day with ICS versus non-ICS treatment (WMD: −0.15 [−0.25, −0.06]);). The between-group difference for the change in mMRC favoured ICS-containing therapy (MD: −0.07 units; −0.18, 0.04); similar results were observed for CAT (MD: −0.07 units; −0.15, 0.02;).
Sensitivity Analysis and Publications Bias
The sensitivity analysis demonstrated that the included studies had no excessive influence in the meta-analysis.
We used funnel plots to estimate publication bias, and these results did not show any evidence of obvious bias. However, funnel plot was not powerful enough for detecting publication bias for outcomes with insufficient number of studies: the number of patients experiencing exacerbations requiring oral steroids,4 change in CAT6 and mMRC.3 Nevertheless, no publication bias was founded by Egger test for the number of patients experiencing exacerbations requiring oral steroids (P=0.262) and the change in CAT (P=0.877).
Discussion
The current therapeutic landscape for patients with COPD mainly consists of bronchodilators and anti-inflammatory ICS therapy, and given the heterogeneity in the clinical characteristics and response to treatment, there is a persistent dilemma among physicians regarding the choice of ICS or non-ICS regimen. We conducted a meta-analysis using the totality of available data from RCTs to compare the effects of different ICS and non-ICS treatment regimens on clinical outcomes in a heterogeneous population concerning airflow limitation (assessed by FEV1), symptoms and exacerbations. The results provide insights into the treatment strategy for patients with COPD.
The clinical guidelines propose different therapeutic algorithms with a stepwise escalation of treatment based on persistence of symptoms and incidence of exacerbations.1,64,65 However, there is no high-quality evidence supporting a specific initial treatment in newly diagnosed patients with COPD. The results of our meta-analysis showed a significant benefit of ICS/LABA therapy on improving lung function (WMD: +0.04L) and QoL, and reducing exacerbations (AR reduced by 25%), as compared with LABA monotherapy. While these benefits are acknowledged by the guidelines, the use of ICS/LABA initial therapy is limited to patients with an annual history of ≥2 moderate-to-severe exacerbations. However, in clinical practice, there is a significant variability in the exacerbation rate over time, leading to uncertainty regarding use and possible underutilisation of ICS/LABA therapy in eligible patients. In addition, the guidelines do not distinctly define the patient population for ICS combination therapy based on exacerbations history. The GOLD 2020 guidelines recommend use of ICS in group D patients (those with a history of ≥2 exacerbations/year), which is not based on evidence, whereas it also recommends considering ICS-containing therapy in patients with at ≥1 moderate exacerbation/year.1 The results of our meta-analysis suggest a benefit of using ICS therapy regardless of exacerbations history. A recent ICS withdrawal guideline from the European Respiratory Society (ERS) recommended continuation of ICS therapy regardless of history of exacerbations in patients with eosinophils counts ≥300 cells/µL.66 In addition, chronic bronchitis could be another clinical marker for the use of ICS-containing regimen.67,68 Further research is needed to evaluate the use of low-dose ICS combination therapy in COPD based on different patient phenotypes, to mitigate the potential safety concerns.
Furthermore, the results of our meta-analysis showed a significant benefit of LABA/LAMA therapy on improving lung function compared with ICS/LABA, whereas the risk of exacerbations was comparable between the treatment regimens. Of note, a large majority of studies comparing LABA/LAMA with ICS/LABA therapy included patients with no history of exacerbations. A previous meta-analysis by Rodrigo et al showed that LABA/LAMA significantly reduced moderate/severe exacerbation rate compared with ICS/LABA (RR: 0.82 [0.75, 0.91]).69 These results were based on two studies7,41 and were mostly driven by the data from FLAME (NCT01782326) study which showed a significant reduction (by 17%) in exacerbations with LABA/LAMA compared with ICS/LABA in patients with a history of ≥1 exacerbation during the previous year.7 In contrast, the recently completed IMPACT (NCT02164513) study showed a 10% reduction in exacerbations with ICS/LABA versus LAMA/LABA in patients with a history of ≥1 moderate/severe exacerbation in the previous year.8 These mixed results could be explained by the different therapies used, as well as differences in the patient population (~80% of patients in the FLAME study had one exacerbation in the past year versus 54% of patients with a history of ≥2 exacerbations in the IMPACT study) and the study design — the FLAME study included a long run-in period of LAMA monotherapy which may have resulted in selection of patients with low disease burden and who could be managed with LAMA monotherapy.7,8 Moreover, >50% of patients in the FLAME study were using ICS therapy before enrolment, hence the study may have selected patients who were symptomatic or had exacerbations despite ICS therapy and were less likely to have benefited from ICS therapy.7 Overall, the present meta-analysis showed that in patients with frequent dyspnoea or exercise intolerance and low risk of exacerbations (as indicated by no history of exacerbations), use of dual bronchodilator (LABA/LAMA) therapy may provide optimal management of disease. This is in line with the recent recommendations from the American Thoracic Society (ATS) Clinical Practice Guideline for COPD.65
Our results also showed a significant reduction in the AER by 24% with ICS/LABA/LAMA triple therapy compared with LABA/LAMA dual therapy. These results are in line with the IMPACT, TRIBUTE (NCT02579850) and KRONOS (NCT02497001) studies and the more recent ETHOS (NCT02465567) study, which demonstrated superior efficacy of ICS/LABA/LAMA in preventing exacerbations compared with LABA/LAMA in patients with moderate-to-severe COPD.8,9,14,70 Similarly, another meta-analysis showed the superiority of ICS/LABA/LAMA triple therapy compared with LABA/LAMA in reducing the rate of moderate/severe exacerbation (RR: 0.78 [0.70, 0.88]).71 These results further reinforce the additive effect of ICS in combination with LABA/LAMA in the management of COPD. All the clinical guidelines recommend use of triple therapy in patients who are symptomatic despite dual LABA/LAMA therapy. In addition, it is yet to be determined whether certain subsets of patients with advanced disease deserve maximal treatment from the beginning.
While initiation of ICS treatment combination is strongly recommended in patients with high risk of exacerbations, its withdrawal is suggested in patients with low risk as indicated by no exacerbations in the previous year.65 The recent ERS guideline made a conditional recommendation for ICS withdrawal in patients with no history of exacerbations with eosinophil count <300 cells/µL; due to uncertainty whether the desirable outcomes of ICS withdrawal outweigh the undesirable consequences.66 However, the recently completed KRONOS study provides evidence against the withdrawal of ICS therapy in patients with low risk of exacerbations suggested by current guidelines. Most of the patients included in the KRONOS study had no exacerbations in the preceding 12 months (1411/1896, 74%). The study demonstrated that the addition of ICS to LABA/LAMA dual therapy significantly reduced the risk of exacerbations in these patients compared with LABA/LAMA therapy (RR: 0.48; 0.37–0.64; P<0.0001).14 ICS withdrawal may increase the airway inflammation severity and decrease lung function and health status, hence careful identification of patients who may benefit most from ICS treatment is required.
Furthermore, improving patient-reported outcomes is an important goal in the management of COPD. ICS-containing therapy significantly improved the QoL as assessed by SGRQ, as compared with non-ICS therapy. In addition, there was a significant reduction in the RMU with ICS compared with non-ICS therapy. The effects on symptom scores were comparable between ICS and non-ICS therapies; however, the number of the studies was small. Although the outcomes were statistically significant, they did not achieve a significant clinical improvement.
Based on the results of this meta-analysis, ICS therapy is an important part of the therapeutic armamentarium for COPD. A key question for physicians is when to prescribe ICS during the disease course. While the COPD guidelines provide a comprehensive approach to disease management, the suggested treatment algorithms based on grouping of patients by their symptoms and history of exacerbations are not evidence-based, adding to the uncertainty concerning appropriate choice of treatment. In this regard, MacDonald et al proposed a treatable trait approach where patients are individually assessed for a specified set of pulmonary, extra-pulmonary and behavioural traits, followed by systematic identification and treatment of the disease characteristics that contribute to poor outcomes.72 For example, when the treatment is guided by symptoms alone, some key disease traits such as inflammation are left untreated, which may lead to poor outcomes. Hence, a holistic treatment approach involving identification and treatment of all the modifiable disease characteristics is critical for achieving favourable patient outcomes.72
A major strength of this meta-analysis was the large number of RCTs included, and to our best knowledge, this is the first analysis comparing several combinations of ICS and non-ICS therapies across a wide range of patients with COPD. However, there were some limitations. Firstly, the risk of mortality and adverse events (AEs) were not evaluated. There are few studies reporting the effects of ICS treatment on all-cause mortality; the IMPACT and ETHOS studies showed a significant reduction in mortality by 28% (HR: 0.72 [0.53, 0.99]) and by 46% (HR: 0.54 [0.34, 0.87]), respectively, with ICS/LABA/LAMA triple therapy compared with LABA/LAMA therapy in patients with moderate-to-severe COPD.68,71 The AE profile of ICS therapies appears to be product-specific, as indicated by varying risk of pneumonia with different ICS therapies. The risk of pneumonia was significantly lower with budesonide compared with fluticasone as demonstrated in RCTs24 and meta-analyses.73–76 In some studies, the risk of pneumonia with budesonide was comparable with that with non-ICS therapy,24–26 whereas even low doses of fluticasone therapy may increase the risk of pneumonia.1 Although safety was not evaluated, the effect of ICS-containing regimens on health status and QoL was assessed by changes in SGRQ score and SGRQ response. Secondly, only a few studies reported the number of patients experiencing exacerbations requiring hospitalisation and oral steroids, and the change in mMRC score. Finally, there was no restriction in terms of study duration, dosing regimen and type of device used among the included trials. We do acknowledge that a subgroup analysis by exacerbation history would add value to our study. However, such a subgroup analysis may not be balanced as most studies that were included in the exacerbation risk analysis included patients at high risk of exacerbations. In addition, we also acknowledge that data on tolerability or drop-outs would be helpful; however, our analysis is focused on efficacy outcomes and hence these data were not extracted from the studies. We also acknowledge that the comparison of LAMA vs ICS/LABA/LAMA is likely to be in favor of the combination therapy group; however, we conducted a systematic literature review and selected studies based on the predefined criteria of ICS vs non-ICS therapy comparison. We should have included studies where the outcomes of interest were not the primary endpoints. All these factors may affect the meta-analysis heterogeneity.
Conclusions
In conclusion, findings from this meta-analysis suggest that ICS-containing combination therapy is efficacious in reducing the RMU and improving QoL with a comparable effect on lung function and symptoms as compared to non-ICS therapy. ICS-containing regimen proved beneficial in reducing the annual rate of severe exacerbations requiring hospitalization. Long-term use of ICS regimen has shown fewer rehospitalization rates. This meta-analysis shows the important role played by ICS regimen in COPD patients. Findings from this meta-analysis could provide guidance to clinicians on the selection of suitable patients and choice of the optimal personalised ICS-containing regimen.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from Yahong Chen ([email protected]) on reasonable request.
Acknowledgments
Editorial assistance was provided by Syed Abdul Haseeb and Julia Ventura from MediTech Media (Singapore), which was funded by AstraZeneca China in accordance with Good Publication Practice (GPP3) guidelines.
Author Contributions
All authors had access to all relevant data. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Yanling Ding and Lina Sun are co-first authors.
Funding
This work was supported by AstraZeneca China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. GOLD-2020-REPORT-ver1.0wms.pdf [Internet]. [
2. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–118. doi:10.1183/09059180.00002610
3. May SM, Li JTC. Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy Asthma Proc. 2015;36(1):4–10. doi:10.2500/aap.2015.36.3812
4. Halpin DM, Miravitlles M, Metzdorf N, Celli B. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2017;12:2891–2908. doi:10.2147/COPD.S139470
5. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study - PubMed [Internet]. [
6. Magnussen H, Watz H, Kirsten A, et al. Stepwise withdrawal of inhaled corticosteroids in COPD patients receiving dual bronchodilation: WISDOM study design and rationale. Respir Med. 2014;108(4):593–599. doi:10.1016/j.rmed.2014.01.002
7. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
8. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
9. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial - PubMed [Internet]. [
10. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750. doi:10.1164/rccm.200904-0492OC
11. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD - PubMed [Internet]. [
12. Frith PA, Thompson PJ, Ratnavadivel R, et al. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study - A randomised controlled trial. Thorax. 2015;70(6):519–527. doi:10.1136/thoraxjnl-2014-206670
13. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi:10.1016/S0140-6736(16)31354-X
14. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
15. Cazzola M, Di Lorenzo G, Di Perna F, Calderaro F, Testi R, Centanni S. Additive effects of salmeterol and fluticasone or theophylline in COPD. Chest. 2000;118(6):1576–1581. doi:10.1378/chest.118.6.1576
16. Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. doi:10.1016/S0140-6736(03)12459-2
17. Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–919. doi:10.1183/09031936.03.00027003
18. O’Donnell DE, Sciurba F, Celli B, et al. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647–656. doi:10.1378/chest.130.3.647
19. Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(2):144–149. doi:10.1164/rccm.200602-244OC
20. Calverley PMA, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
21. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease. Springerlink. [
22. Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50μg) or salmeterol (50μg) on COPD exacerbations. Respir Med. 2008;102(8):1099–1108. doi:10.1016/j.rmed.2008.04.019
23. Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6(5):320–329. doi:10.1080/15412550903140881
24. Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease. Drugs. 2009;69(5):549–565. doi:10.2165/00003495-200969050-00004
25. Calverley PMA, Kuna P, Monsó E, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respir Med. 2010;104(12):1858–1868. doi:10.1016/j.rmed.2010.09.008
26. Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106(2):257–268. doi:10.1016/j.rmed.2011.07.020
27. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed-dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52-week placebo-controlled trials - PubMed [Internet]. [
28. Kerwin EM, Scott-Wilson C, Sanford L, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 μg; 100/25 μg) on lung function in COPD. Respir Med. 2013;107(4):560–569. doi:10.1016/j.rmed.2012.12.014
29. Fukuchi Y, Samoro R, Fassakhov R, et al. Budesonide/formoterol via Turbuhaler® versus formoterol via Turbuhaler® in patients with moderate to severe chronic obstructive pulmonary disease: Phase III multinational study results. Respirology. 2013;18(5):866–873. doi:10.1111/resp.12090
30. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi:10.1016/S2213-2600(13)70040-7
31. Martinez FJ, Boscia J, Feldman G, et al. Fluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: a randomised trial. Respir Med. 2013;107(4):550–559. doi:10.1016/j.rmed.2012.12.016
32. Wedzicha JA, Singh D, Vestbo J, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir Med. 2014;108(8):1153–1162. doi:10.1016/j.rmed.2014.05.013
33. Rossi A, van der Molen T, Del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556. doi:10.1183/09031936.00126814
34. Bhatt SP, Dransfield MT, Cockcroft JR, et al. A randomized trial of once-daily fluticasone furoate/vilanterol or vilanterol versus placebo to determine effects on arterial stiffness in COPD. Int J Chron Obstruct Pulmon Dis. 2017;19(12):351–365. doi:10.2147/COPD.S117373
35. Siler TM, Nagai A, Scott-Wilson CA, Midwinter DA, Crim C. A randomised, phase III trial of once-daily fluticasone furoate/vilanterol 100/25 μg versus once-daily vilanterol 25 μg to evaluate the contribution on lung function of fluticasone furoate in the combination in patients with COPD. Respir Med. 2017;123:8–17. doi:10.1016/j.rmed.2016.12.001
36. Ferguson GT, Tashkin DP, Skärby T, et al. Effect of budesonide/formoterol pressurized metered-dose inhaler on exacerbations versus formoterol in chronic obstructive pulmonary disease: the 6-month, randomized RISE (Revealing the Impact of Symbicort in reducing Exacerbations in COPD) study. Respir Med. 2017;132:31–41. doi:10.1016/j.rmed.2017.09.002
37. Ferguson GT, Papi A, Anzueto A. Budesonide/formoterol MDI with co-suspension delivery technology in COPD: the TELOS study. Eur Respir Soc. 2018;52. Available from: https://erj.ersjournals.com/content/52/3/1801334.
38. Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest. 2008;134(2):255–262. doi:10.1378/chest.07-2138
39. Magnussen H, Paggiaro P, Schmidt H, Kesten S, Metzdorf N, Maltais F. Effect of combination treatment on lung volumes and exercise endurance time in COPD. Respir Med. 2012;106(10):1413–1420. doi:10.1016/j.rmed.2012.05.011
40. Donohue JF, Worsley S, Zhu C-Q, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109(7):870–881. doi:10.1016/j.rmed.2015.04.018
41. Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026. doi:10.2147/COPD.S84436
42. Singh D, Worsley S, Zhu C-Q, Hardaker L, Church A. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med. 2015;19(15):91. doi:10.1186/s12890-015-0092-1
43. Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of Aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48(4):1030–1039. doi:10.1183/13993003.00216-2016
44. Beeh K-M, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat(®) is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler(®) (ENERGITO(®) study). Int J Chron Obstruct Pulmon Dis. 2016;11:193–205. doi:10.2147/COPD.S95055
45. Frith PA, Ashmawi S, Krishnamurthy S, et al. Efficacy and safety of the direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in non-frequently exacerbating COPD patients: the FLASH randomized controlled trial. Respirology. 2018;23(12):1152–1159. doi:10.1111/resp.13374
46. Greulich T, Kostikas K, Gaga M, et al. Indacaterol/glycopyrronium reduces the risk of clinically important deterioration after direct switch from baseline therapies in patients with moderate COPD: a post hoc analysis of the CRYSTAL study. COPD. 2018;16(13):1229–1237. doi:10.2147/COPD.S159732
47. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD - PubMed [Internet]. [
48. Bateman ED, van Dyk M, Sagriotis A. Comparable spirometric efficacy of tiotropium compared with salmeterol plus fluticasone in patients with COPD: a pilot study. Pulm Pharmacol Ther. 2008;21(1):20–25. doi:10.1016/j.pupt.2006.10.001
49. Wedzicha JA, Calverley PMA, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi:10.1164/rccm.200707-973OC
50. Perng D-W, Tao C-W, Su K-C, Tsai -C-C, Liu L-Y, Lee Y-C. Anti-inflammatory effects of salmeterol/fluticasone, tiotropium/fluticasone or tiotropium in COPD. Eur Respir J. 2009;33(4):778–784. doi:10.1183/09031936.00115308
51. Effects of tiotropium and salmeterol/fluticasone propionate on airway wall thickness in chronic obstructive pulmonary disease - PubMed [Internet]. [
52. Covelli H, Pek B, Schenkenberger I, Scott-Wilson C, Emmett A, Crim C. Efficacy and safety of fluticasone furoate/vilanterol or tiotropium in subjects with COPD at cardiovascular risk. Int J Chron Obstruct Pulmon Dis. 2015;18(11):1–12. doi:10.2147/COPD.S91407
53. Chen H, Sun J, Huang Q, et al. Inhaled corticosteroids and the pneumonia risk in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Front Pharmacol. 2021;12:1592.
54. Paggiaro PL, Vagaggini B, Di Franco A, Zingoni M, Fano M, Biraghi M. Efficacy of nebulized flunisolide combined with salbutamol and ipratropium bromide in stable patients with moderate-to-severe chronic obstructive pulmonary disease. Respiration. 2006;73(5):603–609. doi:10.1159/000089816
55. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154
56. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–339. doi:10.1164/rccm.201803-0405OC
57. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555. doi:10.7326/0003-4819-146-8-200704170-00152
58. Jung KS, Park HY, Park SY, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir Med. 2012;106(3):382–389. doi:10.1016/j.rmed.2011.09.004
59. Hanania NA, Crater GD, Morris AN, Emmett AH, O’Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106(1):91–101. doi:10.1016/j.rmed.2011.09.002
60. Lee S-D, Xie C-M, Yunus F, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomized, multicentre study in East Asia. Respirology. 2016;21(1):119–127. doi:10.1111/resp.12646
61. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5
62. Janson C. Treatment with inhaled corticosteroids in chronic obstructive pulmonary disease. J Thorac Dis. 2020;12(4):1561–1569. doi:10.21037/jtd.2020.02.51
63. Montuschi P. Pharmacological treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2006;1(4):409–423. doi:10.2147/copd.2006.1.4.409
64. Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017;53(6):324–335. doi:10.1016/j.arbres.2017.03.018
65. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e56–e69.
66. Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55(6):2000351. doi:10.1183/13993003.00351-2020
67. Hurst JR. The rhythm of chronic obstructive pulmonary disease exacerbations. J Clin Epidemiol. 2010;63(12):1285–1286. doi:10.1016/j.jclinepi.2010.04.021
68. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
69. Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907.
70. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
71. Zheng Y, Zhu J, Liu Y, et al. Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis. BMJ. 2018:363. Available from: https://www.bmj.com/content/363/bmj.k4388.
72. McDonald VM, Fingleton J, Agusti A, et al. Treatable traits: a new paradigm for 21st century management of chronic airway diseases: treatable traits down under international workshop report. Eur Respir J. 2019;53(5):1802058. doi:10.1183/13993003.02058-2018
73. Halpin DMG, Gray J, Edwards SJ, Morais J, Singh D. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 2011;65(7):764–774. doi:10.1111/j.1742-1241.2011.02685.x
74. Hollis S, Jorup C, Lythgoe D, Martensson G, Regnell P, Eckerwall G. Risk of pneumonia with budesonide-containing treatments in COPD: an individual patient-level pooled analysis of interventional studies. Int J Chron Obstruct Pulmon Dis. 2017;12:1071–1084. doi:10.2147/COPD.S128358
75. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;10(3):CD010115.
76. Yang M, Du Y, Chen H, Jiang D, Xu Z. Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2019;77:105950. doi:10.1016/j.intimp.2019.105950
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.