Back to Journals » Hepatic Medicine: Evidence and Research » Volume 12
Efficacy of Glucagon-Like Peptide-1 Analogs in Nonalcoholic Fatty Liver Disease: A Systematic Review
Authors Teshome G , Ambachew S , Fasil A , Abebe M
Received 11 June 2020
Accepted for publication 1 September 2020
Published 24 September 2020 Volume 2020:12 Pages 139—151
DOI https://doi.org/10.2147/HMER.S265631
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Getnet Teshome,1 Sintayehu Ambachew,2 Alebachew Fasil,2 Molla Abebe2
1University of Gondar Comprehensive Specialized Hospital, University of Gondar, Gondar, Amhara, Ethiopia; 2Department of Clinical Chemistry, University of Gondar, Gondar, Amhara, Ethiopia
Correspondence: Getnet Teshome
University of Gondar Comprehensive Specialized Hospital, University of Gondar, Gondar, Amhara, Ethiopia
Email [email protected]
Background: Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease. It is believed to be the hepatic manifestation of the metabolic syndrome. Many treatment approaches have been suggested so far, and several types of studies have been done to find treatment for NAFLD, the most promising of which are those with lifestyle interventions.
Objective: The aim of this systematic review was to evaluate the efficacy and safety of glucagon-like peptide-1 (GLP-1) analogs on the management of NAFLD.
Methods: The PubMed, MEDLINE, and Cochrane Central Library were searched to identify randomized controlled trials, single arm trials, and cohorts that compared GLP-1 analogs with a control treatment or baseline values with respect to efficacy and safety in patients living with NAFLD. The key outcomes were a change in serum transaminase, resolution of disease status measured by imaging or histological techniques, improvement in insulin resistance, and reduction in body weight.
Results: Initial searching retrieved 201 peer-reviewed articles and abstracts. Ten studies met all inclusion criteria. The review included a total of 590 participants with NAFLD. Following administration of GLP-1 analogs, a decrease in serum transaminases, improvement in liver histology and insulin resistance, and a reduction in body weight were observed. Compared with baseline, body weight, alanine aminotransferase, aspartate aminotransferase, and gamma glutamyltransferase were decreased by 5.5%, 59.5%, 52.8%, and 44.8%, respectively, due to GLP-1. Likewise, a reduction of proinflammatory cytokines and fibrosis markers and an enhancement of protective adipokines were observed in some of the studies.
Conclusion: The decrease in a key biochemical marker of liver injury following treatment with GLP-1 analogs, as well as improvements in imaging and histology, suggests that these agents may be effective alternatives for managing NAFLD.
Registration: CRD42018087262.
Keywords: GLP-1RA, GLP-1 analogs, GLP-1 and NAFLD
Introduction
NAFLD is one of the most common liver diseases in the developed world.1 The term NAFLD describes a wide spectrum of liver diseases ranging from isolated steatosis (accumulation of lipids in hepatocytes) to its more severe form of nonalcoholic steatohepatitis (NASH) with hepatic injury, inflammation, and often with fibrosis.2 NAFLD is defined as fat accumulation in the liver exceeding 5% by weight, but it is estimated practically as the proportion of fat-laden hepatocytes observed by microscopy,3 and it can be defined as liver disease not caused by excess use of alcohol (>20 g/day in women, >30 g/day in men), infection such as hepatitis virus, autoimmunity, use of hepatotoxic drugs, or other compounds.4
Insulin resistance (IR), obesity, and type 2 diabetes mellitus (T2DM) are associated with NAFLD. The main events in the pathophysiology of NAFLD are lipid accumulation, lipotoxicity, and inflammation.5 Increased dietary intake, de-novo lipogenesis, and influx of free fatty acids (FFA) are factors that lead to lipid accumulation in the liver. IR induces lipolysis in the adipose tissue which increases influx of FFAs in to the liver.6 IR in liver and muscle leads to diminished glucose uptake and hyperglycemia, and the resultant increase in secretion of insulin further favors triglyceride (TG) storage in the liver. Insulin resistant conditions have also proinflammatory effects observed in insulin sensitive tissues such as adipose tissue and liver. Once excess fat is deposited in the liver, this may enhance inflammation locally as fatty acids in high quantities have proinflammatory effects.7
One of the main limitations for assessment and treatment of NAFLD is the shortage of accurate noninvasive diagnostic methods. Currently, the gold standard diagnostic technique is liver biopsy, which is recommended by the 2009 European Association for the Study of Liver Disease and American Association for the Study of Liver Disease.8,9 However, liver biopsy is invasive, involves a time consuming and an expensive methodology, and is not exempted from some risks to health.8,9 Other frequently used diagnostic techniques are ultrasonography or nuclear magnetic resonance (MRI) based imaging. Even though these techniques are widely used, they are yet precise when steatosis is in less than 30% of the hepatocytes, whereas hepatic steatosis is diagnosed for values over 5%.10,11 Recently, noninvasive blood biomarkers have been widely explored. Despite these new biomarkers being promising, research is still needed to adopt them as a general diagnostic method. Currently, the most frequently used blood biomarkers, such as hepatic enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), or gamma-glutamyltransferase (GGT), do not offer the possibility of differentiating between the wide arrays of different features that usually accompany NAFLD progression, but they are accepted markers of liver damage.12
The incidence and prevalence of NAFLD has been increasing steadily over the past two decades and is predicted to continue in line with the increasing incidence of the metabolic syndrome. Its continued rise can be associated with significant economic and resource utilization costs.13 In spite of the significance of this condition, there are no well proven pharmacotherapies. Currently lifestyle-mediated weight loss is the mainstay of managing NAFLD.14 It is, however, true that it is difficult to achieve and maintain weight loss with lifestyle intervention due to factors related to behavioral orientation, mobility, and social practices.15 Due to the limited availability of pharmacotherapies, there is much research interest in this area and currently glucagon like peptide-1 receptor (GLP-1R) analogs are under investigation.16 The aim of this systematic review was to assess the efficacy of GLP-1R analogs for the treatment of NAFLD.
GLP-1R analogs mimic the actions of glucagon like peptide-1 (GLP-1), the gastrointestinal hormone of the incretin class.17 GLP-1 is a naturally existing hormone secreted from enteroendocrine cells (EECs) of the gastrointestinal tract (GIT) in response to meal ingestion and induces insulin secretion and inhibits glucagon secretion at glucose levels above basal.18 It regulates glucose metabolism and energy homeostasis through regulation of islet hormone secretion, GIT motility, and food intake. Activation of the GLP-1R leads to delayed gastric emptying and inhibition of small bowel motility, which results in a reduced appetite and delayed food absorption leading to decreased food ingestion and weight loss.18 GLP-1 also regulates the immune system by suppressing inflammation.19
To date, treatment of NAFLD has been assessed by many clinical studies. Most clinical efforts have been directed at managing the components of metabolic syndrome, namely obesity, dyslipidemia, DM, and hypertension. Other interventions are directed against a specific pathway potentially involved in the pathogenesis of NAFLD, such as IR and inflammation. Recently it is accepted that the presence of a multifactorial approach in the management of NAFLD and the focus should be given not only on the disease itself but also on the management of metabolic comorbidities such as T2DM, IR, obesity, and dyslipidemia. Decrease in histologically measured hepatic steatosis is considered as a measure of treatment efficacy. But, due to the invasive nature of liver biopsy, many studies use biochemical improvement as the primary measure of efficacy. Lifestyle intervention remained at the center in management of NAFLD and in improving underline comorbidities. Evidences revealed that diet and exercise improves aminotransferases and steatosis as evaluated by imaging techniques and biochemical analysis in patients with NAFLD. Bariatric surgery is also indicated as an important treatment of patients with NAFLD.20–23 Administration of insulin sensitizing agents, antioxidants, polyunsaturated fatty acids (PUFA), and GLP-1R analogs seem to provide a promising effect in attenuating hepatic fat content (LFC).24
Even though the role of GLP-1 in NAFLD is insufficiently understood, experimental data suggested a link between GLP-1 and steatogenesis. It has been described that GLP-1 plays an important role in improving hepatic steatosis, particularly in T2DM patients with NAFLD. But, its half-life is very short (1–2 minutes) because it is digested by dipeptidyl peptidase-4 (DPP-4). Due to this digestion, only small amounts of active GLP-1 reach the circulation. Furthermore, up-regulation of DPP-4 and down-regulation of GLP-1R have been reported in liver biopsies of NAFLD patients.25,26 Therefore, it is better to find the synthetic version of GLP-1 (GLP-1 analog), and improvement of hepatic steatosis by these different GLP-1 analogs has been reported in rodents.27
Liraglutide, the GLP-1 analog, shares a 97% homology with amino acid sequence of the native human GLP-1. The half-life of GLP-1 is prolonged to 13 hours due to this small structural difference, making it suitable for once daily administration.28 Exenatide is a synthetic analog of exendin-4. It shares 53% homology with the amino acid sequence of human GLP-1 and cannot be digested easily by DPP-4.29 Dulaglutide and Semaglutide have 90% and 94% structural similarity with the native GLP-1.30,31 This review will contribute to increase the body of scientific knowledge in this area. In addition, still there is no approved pharmacotherapy for NAFLD so that this review may suggest treatment alternatives for NAFLD.
Method
Methods of the analysis and eligibility criteria were specified prior to the literature search and documented in a protocol registered with Prospero (CRD42018087262). This systematic review was conducted in accordance with the preferred reporting item for systematic review and meta-analysis (PRISMA) guidelines32 and recommendations described in the Cochrane Handbook for Reviews on Interventions33 including independent execution of search strategy and bias assessment.
Literature Search
The PubMed, MEDLINE, and Cochrane databases were searched from conception through February 14, 2018. In order to incorporate all available articles during data extraction, additional search engines like Google Scholar were also searched. The search terms were “NAFLD and GLP-1”, “NAFLD treatment and GLP-1”, and “glucagon like peptide-1 receptor agonists and NAFLD”.
Inclusion Criteria
The search items were limited to clinical trials, cohorts, and randomized control trials (RCT) of any age, gender, and ethnicity, human studies, and English language. The studies with a diagnosis of NAFLD, NASH, or fibrosis made on validated biochemical, radiological, or histological evidence were included. Trials using normalization of liver enzymes, improvement of NAFLD, improvement of lobular inflammation, hepatocellular ballooning, and fibrosis as outcome measurement were included.
Exclusion Criteria
Review papers, retrospective, case report, case series, cross-sectional and case control studies were excluded.
Data Extraction
Data extraction was taken into two phases. The first phase involved screening of eligible articles through titles and/or abstracts. Titles and/or abstracts of studies retrieved using the search strategy and those from additional sources were screened independently by two review authors to identify studies that potentially meet the inclusion criteria outlined above. In the second phase, the full text of these potentially eligible studies were retrieved and independently assessed by two review team members. Any disagreement between them over the eligibility of particular studies were resolved through discussion with a third reviewer. Extracted information included study setting/methodology, study population, details of the intervention and control, participant demographics and baseline characteristics, recruitment and study completion rate, outcomes and time of measurement, suggested mechanism of intervention action, and information for assessment of the risk of bias. Missing data was requested from the study authors.
Strategy for Data Synthesis
Narrative synthesis of the findings was provided by stating the dose of intervention, the follow-up period, and the key findings of each study. Additionally, tables was utilized to summarize the study characteristics, the population under study, and critical outcomes. The reviewers anticipate that there was limited scope for meta-analysis because of the range of different outcomes measured across the small number of existing trials. In addition, we recognized the possibility of a measurement of a single outcome differently in different studies, particularly for histologic improvement. Therefore, the scope of this review was a systematic review. Level of liver enzymes like ALT and AST and liver histological features were the primary outcomes of this review. IR, metabolic biochemistry, body weight change, and inflammation were considered as additional outcome measures. The result is presented either with change from baseline and/or the proportion of patients being improved from baseline status of the disease.
Quality Assessment
Quality assessment was done independently by two review authors, and discrepancies were resolved through discussion with a third reviewer. The quality of each included study was assessed by using the Cochrane Library’s risk of bias table for RCTs34 and the Quality Assessment Tool for Before–After (Pre–Post) Studies With No Control Group for interventional studies without control.35 Information was obtained from each included trial, specifically: 1) the objective of the intervention; 2) the method of recruitment of participants; 3) the inclusion and exclusion criteria; 4) if informed consent was obtained; 5) whether or not ethical approval had been granted; 6) whether or not there were any funding sources; 7) the statistical methods used; 8) methods of randomization (whether or not methods of allocation, blinding of participants and personnel, blinding of the outcome assessment where appropriate); 9) incomplete outcome data reporting, any selective reporting, and other sources of bias as described in the Cochrane handbook.33 In addition, further information recorded from each trial as identified by the authors such as the methods of assessing outcome measures, the reliability and validity of the outcome measures, the methods of follow-up for non-respondents, the timing of the outcome measurement, and any adverse events were assessed (Tables 1 and 2).
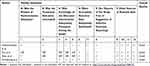 |
Table 1 Quality of Evidences for Randomized Control Trials |
 |
Table 2 Quality of Evidences for Uncontrolled Studies |
Results
Through the database searches, we identified and selected eligible studies included in this review Figure 1. From the database, overall 201 records were identified out of which 82 were removed due to duplication. Following removal of duplicates, 119 citations remained. These citations were screened for eligibility by reading the titles and abstracts (Table 3). A total of 18 papers were selected for further assessment after title and abstract screening. Eight papers were excluded after reading the full print of each paper for the following reasons; one was retrospective, two evaluated a primary outcome not related to NAFLD, one was a case series, one was a conference paper, and the other was an experimental study (Table 3).
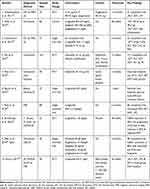 |
Table 3 Trials Evaluating the Role of GLP-1 Analoges on Nonalcoholic Fatty Liver Disease |
 |
Figure 1 Study screening flow. |
Trial Characteristics
Overall, 590 individuals participated in a total of 10 trials with durations ranging from 12 weeks to 2 years. Of the ten trials that met the inclusion criteria, four assessed the effect of liraglutide, out of which two were RCT, two RCTs assessed exenatide, one compared exenatide, liraglutide, sitagliptin, and pioglitazone, one evaluated the effect of both exenatide and liraglutide, one evaluated exenatide and one was comparing exenatide, liraglutide, gliclazide, pioglitazone, and sitagliptin on NAFLD. The protocols of the included trials were very diverse. The trials assessing the effect of liraglutide ranged from 0.6 mg to 3 mg, whereas trials with exenatide ranged from 10 µg to 20 µg daily. T2DM patients with NAFLD were taken as a study participant in most of the trials except two trials that were conducted among non-diabetic obese NAFLD patients36 and among both diabetic and non-diabetic NAFLD patients.37 This review included one cohort, five single arm trials, and four RCTs.
Our review considered change in the level of liver enzymes and histological features of liver as inclusion criteria for primary outcome measures. However, associated or secondary outcome measures varied markedly between studies, with some examining only liver outcomes, while others also reported data relating to metabolic syndrome like weight, body mass index, IR, glucose and lipid profile levels, and inflammatory markers linked with NAFLD. The heterogeneity was also seen in the methods of diagnosis and measuring change in NAFLD, the most common being ultrasound,38–41 two trials utilized biopsy in addition to ultrasound42 and liver enzymes,37 the remaining four used MRS43,44 and MRI36,45 besides liver enzymes.
Quality Assessment Evidence
Overall, the studies were of good quality. Common limitations of the studies were inadequate randomization, inadequate allocation concealment, lack of personnel blinding, and inadequate description of baseline (Tables 1 and 2).
Liraglutide Group
Ohki et al38 reported a significant reduction of serum ALT and AST and improvement of inflammation after administration of 0.9 mg/day liraglutide for 48 weeks, but no significant change was seen in body weight. Armstrong et al37 compared the placebo to liraglutide (0.9–1.8 mg once daily) treatment for over 48 weeks and observed a significant reduction of ALT and GGT levels from baseline in patients with NAFLD. Armstrong et al also reported a weight reduction of more than 5% and an improvement of NASH in 39% of participants. Similarly, Petit et al45 showed a significant reduction of LFC, ALT, and GGT in patients with NAFLD after 6months of treatment with liraglutide 1.2 mg/day. This effect was mainly driven by body weight reduction. Diaz et al42 reported a marked improvement of fibrosis, inflammation, and aminotransferases after administration of 0.9 mg/day liraglutide for 96 weeks. Another trial, done by Khoo et al,36 showed the similarity of results of treatment of liraglutide as compared to hypocaloric diet (400 kcal/day) and aerobic exercise in reducing ALT, AST, LFC, IR, and weight. Trials with liraglutide achieved a significant reduction in body weight in NAFLD patients with or without T2DM compared with sitagliptin and pioglitazone38 and placebo (Table 3).37
Exenatide
Fan et al39, and Shao et al41 observed a significant reduction of ALT, AST, and GGT levels after exenatide treatment compared with metformin and insulin therapy, respectively. A weight loss of approximately 6%39 and 10%41 from baseline was observed in studies with exenatide among patients with NAFLD. Improvement of steatosis by 56.7% was also reported after intervention of exenatide on NAFLD patients.41 According to Sathyanarayana et al,43 combined exenatide and pioglitazone therapy provided a significant decrease in LFC and an increase in plasma adiponectin level without significant change in body weight. Hepatic injury biomarkers, ALT and AST, were significantly decreased by both treatments (exenatide+pioglitazone and pioglitazone alone); however, the reduction in ALT was significantly greater following combined pioglitazone and exenatide therapy (Table 3).43
Liraglutide vs Exendin-4
Exenatide treatment significantly reduced ALT and AST compared with metformin39 and insulin,41 but liraglutide did not reduce AST levels compared with placebo.37 Both exenatide and liraglutide improved liver histology. The improvement of NASH was reported in 39%37 and 33%40 of patients who received liraglutide of 1.8 mg/day for 48 weeks and 1.2 mg/day for 6 months respectively. Six months of treatment with GLP-1 R analogs (either exenatide or liraglutide) was associated with a significant relative reduction of intrahepatic lipid by 42% and median weight loss of 5 kg, a relative reduction of 4.3% (Tables 3 and 4).44
 |
Table 4 Summary of the Overall Impact of GLP-1 Analogs NAFLD |
Comparison of RCT and Single Arm Trials
The mean level of liver enzymes was significantly reduced in RCT compared to single arm trials. Weight loss, on the other hand, was not significantly different between RCT and single arm trials. At baseline there was no statistically significant difference. (Table 5).
 |
Table 5 Comparison Between Randomized Controlled Trials and Single Arm Trials |
Adverse Events
All studies recorded adverse events. Overall, GLP-1 receptor agonists minimally increased the dropout rate during the trial periods and no serious adverse events such as hypoglycemia and acute pancreatitis were observed. The major side-effects involved gastrointestinal discomfort, including nausea, vomiting, bloating, decreased appetite, transient abdominal pain, constipation, flatulence, and diarrhea. The frequency of adverse events increased with higher doses. Armstrong et al37 noted the withdrawal of three participants, one (4%) due to needle phobia and the other two (8%) withdrew due to sustained tuberculosis and migraine, which were deemed unrelated to treatment with liraglutide. The trials observed gastrointestinal disorders in 0–81%, eye disorder in 0–4%, cardiac disorders in 0–12%, fatigue in 0–15%, influenza-like symptoms in 0–12%, peripheral edema in 0–8%, anorexia in 0–31%, musculoskeletal and connective tissue disorders in 0–31%, back pain in 0–12%, chills in 0–15%, dizziness in 0–23%, headaches or migraines in in 0–35%, psychiatric disorders in 0–23%, depression in 0–8%, and renal and urinary disorders in 0–8% of study participants, most of which were unrelated with GLP-1 treatment.
Discussion
Treatment of GLP-1 analogs are used to treat obesity. As displayed in Table 4, treatment of exenatide and liraglutide was associated with a significant reduction in body weight, with a mean change of 5.3 kg (6.7%) and 3.7 kg (4.6%) respectively. Overall treatment with GLP-1 analogs was found to bring a 5.5% weight reduction. Exenatide treatment achieved a greater weight loss than metformin upon administration of 10 µg exenatide twice daily and 2 g/day of metformin for 12 weeks.39 Similar findings were reported in a network meta-analysis on T2DM patients in that exenatide and liraglutide showed more advantages on weight control than traditional hypoglycemic drugs.46 A weight loss of more than 5% is recommended as one of the treatments of NAFLD by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology.9
Treatment with GLP-1 analogs showed a significant role in reducing IR which can be measured either through HOMA-IR36,37,39,42 or adiponectin level (Table 3).43–45 All trials observed a significant impact of GLP-1 analogs on metabolic biochemistry such as glucose, glycated hemoglobin, serum triglyceride, total cholesterol, low, and high density lipoproteins. Evidence from animal models showed activation of adenomonophosphate-activated protein kinase (AMPK) by either elevated endogenous GLP-1 level or treatment with liraglutide.27 AMPK is a critical signal molecule involved in the regulation of hepatic insulin sensitivity. An increased expression of GLP-1R increased the expression of insulin receptor substrate 1 (IRS-1) leading to activation of protein kinase C (PKC). This decreases the production of glucose and fatty acid in the liver.47 AMPK also has an inhibitory effect on lipogenic genes such as SREBP which might lead to diminished lipid production.48 Treatment with exendin-4 stimulated the phosphorylation of other key elements which are involved in the insulin signaling pathway, including phosphoinositide-dependent kinase-1 (PDK-1) and protein kinase B (also called AKT), in hepatocytes. Furthermore, GLP-1R knockdown in these cells abolished the effects of exendin-4 on PDK-1 and PKC.18 Exendin-4 treatment was also indicated in increasing protein kinase A (PKA) in the hepatocytes isolated from NAFLD rats.25 Overall, this indicates that the GLP-1 analogs can improve insulin sensitivity in NAFLD patients.
Serum liver enzymes are moderately elevated in patients with NAFLD and it was demonstrated that GLP-1 analogs can reduce their levels, thereby improving hepatocyte damage in patients with NAFLD. Both liraglutide and exenatide brought a significant reduction of the level of liver enzymes such as ALT, AST, and GGT. Ohki et al38 reported a significant reduction of ALT, AST, and GGT levels after administration of 0.9 mg liraglutide for 48 weeks. A similar result was observed by Fan et al39 in exenatide 5–10 µg treated NAFLD for 12 weeks. Armstrong et al37 observed a significant reduction of ALT, GGT, ALP, and total bilirubin in NAFLD patients upon administration of 1.8 mg of liraglutide daily for 48 weeks as compared to placebo. On the other hand, they observed an increased albumin level after treatment. Sathyanarayana et al43 have shown a significant effect of combined regimen of 10 µg exenatide twice daily and 45 mg/day pioglitazone for 1year on ALT and AST levels as compared to patients treated with pioglitazone alone. Overall, treatment of NAFLD patients with these GLP-1 analogs brought a reduction of the levels of ALT, AST, and GGT by 59.5%, 52.8%, and 44.8%, respectively, as compared to baseline (Table 4). Therefore, both liraglutide and exenatide have promising effects in improving liver injury. Evidence from in vitro and animal studies showed that GLP-1 has direct effects on hepatocytes through the activation of a GLP-1R regulating glucose metabolism in liver cells and protecting against hepatocellular injuries.18,49 GLP-1 analogs have also been shown to lower liver enzymes in patients with diabetes and obesity.50 Thus, this finding suggests that GLP-1 analogs may have beneficial effects on liver injury in NAFLD and lead to lessening of disease.
Histological improvement was also observed after treatment of GLP-1 analogs, exenatide, and liraglutide. Eguchi et al42 observed an improvement of steatosis in seven of ten subjects after 96 weeks of intervention with 0.9 mg/day liraglutide. In this trial, six of ten subjects demonstrated an improvement of liver fibrosis and six of ten subjects showed reduction of histological inflammation as determined by NAS grade and stage indicated by Brunt’s classification. Petit et al45 indicated a significant reduction of LFC (relative reduction 31% from baseline) after 6 months of treatment with 1.2 mg/day liraglutide. Shao et al41 compared 10 µg exenatide and insulin, and a treatment of 10 µg exenatide twice daily for 12 weeks was found to be superior in reducing LFC compared to insulin. Cuthbertson et al44 have also observed a significant reduction of LFC after a combined treatment of 1.2 mg/day of liraglutide and 10 µg of exenatide twice daily for 6 months. Following exenatide (10 µg twice daily) and pioglitazone (45 mg/day) combined therapy for 12 months, Sathyanarayana et al43 found a significantly decreased amount of LFC as compared to pioglitazone alone. In a randomized placebo controlled clinical Phase II trial which conducted on diabetic (n=8) and nondiabetic (n=15) histologically proven NAFLD patients, an improvement of NASH, steatosis, hepatocellular ballooning, hepatocellular inflammation, liver fibrosis, and decreased NAS was seen in 39%, 83%, 61%, 48%, 26%, and 74% of 23 subjects who received 1.8 mg/day of liraglutide for 48 weeks.37 In the same way Shao et al41 indicated a significant improvement of liver inflammation and fibrosis score in a cohort of 80 NAFLD patients treated with 0.9 mg/day of liraglutide for 12 weeks (Table 3).
Administration of GLP-1 analogs may contribute to improve histological features of liver by facilitating oxidation of fatty acids. Evidence from animal studies has shown the effectiveness of administration of exendin-4 in enhancing the expression of mRNA level of acyl coenzyme-A oxidase 1 (ACOX1) and carnitine palmitoyl transferase 1A (CPT1A), which are the rate limiting enzymes involved in β-oxidation in the liver.25
GLP-1 analogs have an impact on hepatocytes, by activating genes like PPAR-α that are involved in fatty acid β-oxidation. This is in effect through a cAMP-dependent activation of PKA. By incubating with exenatide, Svegliati‐Baroni et al25 observed an increased level of PKA in hepatocytes of high-fat diet treated rats, from which the signal diverged to activate AMPK and the ERK/PI3K pathway needed to transduce the message to PPAR-α. Investigations on PPAR-α knockdown mice indicated that PPAR-α regulates fatty acid oxidation and its uptake as well as lipoprotein assembly and transport.51 Besides, PPAR-α is involved in the degradation of fat and removal of triglycerides.25 Exendin-4 treatment was reported to play a role in enhancing microsomal triglyceride transfer protein (MTTP) level, which is an important regulator of hepatic lipid excretion through VLDL52 and synthesis, and release of VLDL is thought to be a key factor in the progression of NAFLD in humans.53 Moreover, in human studies, the GLP-1 analog, exenatide, inhibited postprandial absorption of chylomicrons that are a main source for hepatic triglyceride accumulation.54 Further inhibition of hepatic de novo lipogenesis can be one of the mechanisms by which GLP-1 analogs reverse hepatic steatosis. Xiaokun et al55 observed increased mRNA expression of PPAR-α and AOX along with a decreased level of SREBP-1c and its target ACC after treatment of ob/ob mice with exendin-4. In this study, the expression of streayol CoA desaturase 1 (SCD1) was significantly decreased after exendin-4 treatment. SREBP-1c and SCD1 are known lipogenic genes.
Ones excess lipid is accumulated in the liver, it may trigger inflammation, and treatment of GLP-1 analogs was found to be effective in the regulation of inflammation. Following administration of 1.8 mg/day liraglutide for 48 weeks, Armstrong et al37 reported a reduction of the levels of proinflammatory markers. Treatment of liraglutide reduces proinflammatory cytokines such as TNF-α, monocyte chemotactic protein-1 (MCP-1), leptin, nuclear factor κB, and resistin.37 On the other hand, a significant increased protective cytokine, adiponectin, was observed following treatment of liraglutide37 and combined therapy of exenatide and pioglitazone.43,43 An increased level of adiponectin due to GLP-1 analogs may be of particular advantage in ameliorating NASH, which is characterized by widespread hepatic inflammation.
Conclusion
The aim of this systematic review was to assess the efficacy of GLP-1 analogs for the treatment of NAFLD. Based on included trials, interventions with GLP-1 analogs were effective in improving hepatic endpoints in NAFLD, such as reducing serum transaminases, LFC, histologically measured hepatic steatosis, and fibrosis. Studies involving liraglutide and exenatide were included and it was noted that treatment with exenatide was seem to be more effective than treatment with liraglutide regarding improvement in liver enzymes and weight reduction. The effect of both interventions on components of metabolic syndrome and liver histological outcomes was considered as comparable. From these findings, we conclude that GLP-1R analogs can improve liver injury, LFC, and distribution and lipid metabolism. With these promising findings, large scale randomized controlled trials are needed to evaluate the efficacy and safety of GLP-1 analogs in the treatment of patients with NAFLD.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52.
2. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419.
3. Cairns S, Peters T. Biochemical analysis of hepatic lipid in alcoholic and diabetic and control subjects. Clin Sci. 1983;65(6):645–652.
4. Liver EAftSot, Diabetes EAftSo. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016;9(2):65–90.
5. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846.
6. Marra F, Gastaldelli A, Baroni GS, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med. 2008;14(2):72–81.
7. Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13):1331–1346.
8. Bedossa P, Patel K. Biopsy and noninvasive methods to assess progression of nonalcoholic fatty liver disease. Gastroenterology. 2016;150(8):1811–22. e4.
9. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American gastroenterological association, American association for the study of liver diseases, and American college of gastroenterology. Gastroenterology. 2012;142(7):1592–1609.
10. Burt AD, Lackner C, Tiniakos DG, editors. Diagnosis and Assessment of NAFLD: Definitions and Histopathological Classification. Seminars in Liver Disease. Thieme Medical Publishers; 2015.
11. Benedict M, Zhang X. Non-alcoholic fatty liver disease: an expanded review. World J Hepatol. 2017;9(16):715.
12. Fitzpatrick E, Dhawan A. Noninvasive biomarkers in non-alcoholic fatty liver disease: current status and a glimpse of the future. World J Gastroenterol. 2014;20(31):10851.
13. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–30. e1.
14. Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372–384.
15. Teuscher D, Bukman AJ, van Baak MA, Feskens EJ, Renes RJ, Meershoek A. Challenges of a healthy lifestyle for socially disadvantaged people of Dutch, Moroccan and Turkish origin in the Netherlands: a focus group study. Crit Public Health. 2015;25(5):615–626.
16. Samson SL, Bajaj M. Potential of incretin-based therapies for non-alcoholic fatty liver disease. J Diabetes Complications. 2013;27(4):401–406.
17. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705.
18. Gupta NA, Mells J, Dunham RM, et al. Glucagon‐like peptide‐1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51(5):1584–1592.
19. Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24(1):15–30.
20. Mehta SR. Advances in the treatment of nonalcoholic fatty liver disease. Ther Adv Endocrinol Metab. 2010;1(3):101–115.
21. Demir M, Lang S, Steffen HM. Nonalcoholic fatty liver disease–current status and future directions. J Dig Dis. 2015;16(10):541–557.
22. Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Lonardo A. The role of nuclear receptors in the pathophysiology, natural course, and drug treatment of NAFLD in humans. Adv Ther. 2016;33(3):291–319.
23. Billeter AT, Senft J, Gotthardt D, et al. Combined non-alcoholic fatty liver disease and type 2 diabetes mellitus: sleeve gastrectomy or gastric bypass?—a controlled matched pair study of 34 patients. Obes Surg. 2016;26(8):1867–1874.
24. Chalasani N, Younossi Z, Lavine J, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Am J Gastroenterol. 2012;107:811–826.
25. Svegliati‐Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon‐like peptide‐1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high‐fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31(9):1285–1297.
26. Miyazaki M, Kato M, Tanaka K, et al. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5(3):729–733.
27. Zhang L, Yang M, Ren H, et al. GLP‐1 analogue prevents NAFLD in ApoE KO mice with diet and Acrp30 knockdown by inhibiting c‐JNK. Liver Int. 2013;33(5):794–804.
28. Agersø H, Jensen L, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45(2):195–202.
29. Gentilella R, Bianchi C, Rossi A, Rotella C. Exenatide: a review from pharmacology to clinical practice. Diabetes Obes Metab. 2009;11(6):544–556.
30. Jensen L, Helleberg H, Roffel A, et al. Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species. Eur J Pharm Sci. 2017;104:31–41.
31. Eli Lilly and Company. Trulicity (Dulaglutide) Prescribing Information Indianapolis. Indiana; 2014.
32. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):10092–10097.
33. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0.. The Cochrane Collaboration; 2015.
34. Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the cochrane back review group. Spine. 2009;34(18):1929–1941.
35. National LaBI. Quality assessment tool for before-after (pre-post) studies with no control group [cited 2017 December 14]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
36. Khoo J, Hsiang J, Taneja R, Law NM, Ang TL. Comparative effects of liraglutide 3 mg versus structured lifestyle modification on body weight, liver fat and liver function in obese patients with non‐alcoholic fatty liver disease: a pilot randomized trial. Diabetes Obes Metab. 2017.
37. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled Phase 2 study. Lancet. 2016;387(10019):679–690.
38. Ohki T, Isogawa A, Iwamoto M, et al. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci World J. 2012.
39. Fan H, Pan Q, Xu Y, Yang X. Exenatide improves type 2 diabetes concomitant with non-alcoholic fatty liver disease. Arq Bras Endocrinol Metabol. 2013;57(9):702–708.
40. Díaza EG, Guagnozzib D, Gutiérrezc V, et al. Effect of incretin therapies compared to pioglitazone and gliclazide in non-alcoholic fatty liver disease in diabetic patients not controlled on metformin alone: an observational, pilot study. Endocrinol Nutr. 2016;63(5):194–201.
41. Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non‐alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30(6):521–529.
42. Eguchi Y, Kitajima Y, Hyogo H, et al. Pilot study of liraglutide effects in non‐alcoholic steatohepatitis and non‐alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN‐J). Hepatol Res. 2015;45(3):269–278.
43. Sathyanarayana P, Jogi M, Muthupillai R, Krishnamurthy R, Samson SL, Bajaj M. Effects of combined exenatide and pioglitazone therapy on hepatic fat content in type 2 diabetes. Obesity. 2011;19(12):2310–2315.
44. Cuthbertson DJ, Irwin A, Gardner CJ, et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One. 2012;7(12):501–517.
45. Petit J-M, Cercueil J-P, Loffroy R, et al. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the lira-NAFLD study. J Clin Endocrinol Metab. 2016;102(2):407–415.
46. Sun F, Chai S, Li L, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J Diabetes Res.2015.
47. Lee Y-S, Shin S, Shigihara T, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56(6):1671–1679.
48. Hwahng SH, Ki SH, Bae EJ, Kim HE, Kim SG. Role of adenosine monophosphate‐activated protein kinase–p70 ribosomal S6 kinase‐1 pathway in repression of liver X receptor‐alpha–dependent lipogenic gene induction and hepatic steatosis by a novel class of dithiolethiones. Hepatology. 2009;49(6):1913–1925.
49. Seino Y, Yabe D. Glucose‐dependent insulinotropic polypeptide and glucagon‐like peptide‐1: incretin actions beyond the pancreas. J Diabetes Investig. 2013;4(2):108–130.
50. Fadini GP, Frison V, Rigato M, et al. Trend 2010–2018 in the clinical use of GLP-1 receptor agonists for the treatment of type 2 diabetes in routine clinical practice: an observational study from Northeast Italy. Acta Diabetol. 2020;57(3):367–375.
51. Aoyama T, Peters JM, Iritani N, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J Biol Chem. 1998;273(10):5678–5684.
52. Yamamoto T, Nakade Y, Yamauchi T, et al. Glucagon-like peptide-1 analogue prevents nonalcoholic steatohepatitis in non-obese mice. World J Gastroenterol. 2016;22(8):2512.
53. Fujita K, Nozaki Y, Wada K, et al. Dysfunctional very‐low‐density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50(3):772–780.
54. Xiao C, Bandsma RH, Dash S, Szeto L, Lewis GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol. 2012;32(6):1513–1519.
55. Ding X, Saxena NK, Lin S, Gupta N, Anania FA. Exendin‐4, a glucagon‐like protein‐1 (GLP‐1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43(1):173–181.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
