Back to Journals » Journal of Pain Research » Volume 10
Efficacy of flurbiprofen 8.75 mg delivered as a spray or lozenge in patients with sore throat due to upper respiratory tract infection: a randomized, non-inferiority trial in the Russian Federation
Authors Radkova E, Burova N , Bychkova V , DeVito R
Received 25 February 2017
Accepted for publication 17 May 2017
Published 6 July 2017 Volume 2017:10 Pages 1591—1600
DOI https://doi.org/10.2147/JPR.S135602
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Eugenia Radkova,1 Natalia Burova,2 Valeria Bychkova,3 Robert DeVito4
1OCT Clinical Trials, Saint Petersburg, Russia; 2Federal State Establishment Clinical Diagnostic Medical Center, Saint Petersburg, Russia; 3Reckitt Benckiser (Russia), Moscow, Russia; 4Reckitt Benckiser, Parsippany, NJ, USA
Objective: To assess the efficacy of flurbiprofen 8.75 mg delivered as a spray or lozenge in patients with sore throat due to upper respiratory tract infection (URTI).
Materials and methods: This multicenter, double-blind, double-dummy, non-inferiority study randomized 440 adults with recent-onset, moderate-to-severe sore throat due to URTI to a single dose of either flurbiprofen 8.75 mg spray (n=218) or flurbiprofen 8.75 mg lozenge (n=222). The presence or absence of beta-hemolytic streptococci (A or C) was confirmed by culture tests (throat swab). The primary efficacy end point was the difference from baseline to 2 hours post-dose in sore throat pain intensity scale (STPIS pain intensity difference [PID] 2h), a validated 100 mm visual analog scale (from 0=“no pain” to 100=“severe pain”), with a non-inferiority margin of −6 mm. Secondary end points included STPIS PID at 1 hour (STPIS PID 1h) and over 2 hours (STPIS sum of sore throat pain intensity differences [SPID]0–2h) and ratings of patient satisfaction and investigator assessment of drug efficacy at 2 hours. Safety (adverse events [AEs]) was also assessed.
Results: Reductions in sore throat pain intensity at 2 hours (STPIS PID 2h) were similar for spray (least square mean −40.51) and lozenge (−40.10) (difference: 0.41, 95% confidence interval [95% CI] −3.20, 4.01), with non-inferiority demonstrated. Subgroup analyses showed similar efficacy (STPIS PID 2h) for patients testing positive or negative for Strep A or C. There was no significant difference between spray and lozenge in STPIS PID 1h or STPIS SPID0–2h, and patient satisfaction and investigators’ assessment of efficacy at 2 hours were similar for both groups. There were no significant differences in AEs between the two groups, with 17 drug-related events across both groups, all being mild and none being serious.
Conclusion: Both formulations demonstrated comparable efficacy and safety profiles and provide patients with two different treatment formats to choose from for effective symptomatic relief of sore throat, depending on their preference.
Keywords: flurbiprofen, non-inferiority, spray, lozenge
Plain language summary
A sore throat is usually caused by a viral infection such as cold or flu, which results in pain and inflammation. This clinical study was done to test how effective the anti-inflammatory drug, flurbiprofen, relieves sore throat when it is provided as a throat spray or a lozenge. The study patients had moderate or severe sore throat caused by an infection. They were given a single dose of the drug provided as either a throat spray or lozenge, and then their throat pain was measured for the next 2 hours. The study found that both spray and lozenge were effective for reducing throat pain, with no difference seen between them. The study also showed that the spray and lozenge were both effective in the small number of patients with “Strep throat” (a bacterial infection that may need antibiotics). Side effects were similar for both spray and lozenge and none were serious. This study showed that flurbiprofen throat spray and flurbiprofen lozenge are both effective for sore throat, giving patients a choice of treatment, depending on their preference.
Introduction
Pharyngitis, more commonly known as sore throat, is associated with pharyngeal inflammation and is usually caused by an upper respiratory tract infection (URTI) such as the common cold.1–4 Although painful, acute sore throat is self-limiting and most cases resolve within 3–7 days, even in the absence of treatment.3,5 However, the discomfort caused by sore throat can significantly impact quality of life and daily activities,6 and sore throat remains one of the most common reasons for primary care consultations.1,3,7–10
Only a small proportion (~10%) of sore throat cases are caused by bacterial infection, such as group A beta-hemolytic streptococcus (Strep A).1 Although less common, group C beta-hemolytic streptococcus (Strep C) can also cause sore throat.2 The vast majority of sore throats are caused by viral infection, but many patients seek – and are inappropriately prescribed – antibiotics.5,8,11–13 Antibiotics can be useful in certain circumstances, but their effectiveness is limited in most cases of acute sore throat and they do not always lead to faster symptom resolution even when bacterial infection is involved.8,14 Inappropriate use of antibiotics also contributes to the growing problem of antibacterial resistance.13,15 Furthermore, it has been suggested that patients who hope for antibiotic treatment may actually be seeking a treatment to relieve pain.12 Together, these factors show that a need remains for treatments that can provide rapid and effective pain relief for acute sore throat, even in those cases where antibiotics are warranted.3
Local therapies, such as lozenges and sprays, are useful for the symptomatic treatment of sore throats as they allow direct application to the inflamed and painful area, with a reduced risk of toxicity when compared with systemic treatments.16 Lozenges allow a high initial deposition of active ingredient in the mouth and throat, while sprays are effective in coating the posterior pharynx16 for rapid delivery to the affected area.17
Flurbiprofen is a non-steroidal anti-inflammatory drug (NSAID) with proven analgesic and anti-inflammatory effects,18,19 and lozenges containing 8.75 mg flurbiprofen have been shown to be safe and effective in relieving the symptoms of a sore throat (including pain).3,20–24 Flurbiprofen 8.75 mg lozenges are available over-the-counter in many countries, and they are the only formulation of flurbiprofen currently available in Russia. However, an innovative spray formulation containing flurbiprofen 8.75 mg has recently been developed to provide patients with another treatment option for the relief of sore throat symptoms.17 In the first safety and efficacy study of flurbiprofen 8.75 mg spray, treatment was well tolerated, and rapid, long-lasting relief from sore throat pain and other symptoms was observed.17
The primary objective of this randomized, non-inferiority study was to assess the efficacy of flurbiprofen 8.75 mg delivered as a spray or lozenge in patients with sore throat due to URTI. Treatment was assessed in the overall population and in subgroups of patients who tested positive or negative for beta-hemolytic streptococci (A or C).
Materials and methods
Study design
This multicenter, randomized (1:1), double-blind, double-dummy, active-controlled, parallel-group, single-dose, non-inferiority study was conducted at 16 investigational centers in the Russian Federation between November 28, 2014 and November 14, 2015. The study was conducted in accordance with the Declaration of Helsinki (EU Directive 2001/20/EC), International Conference on Harmonisation Good Clinical Practice guidelines, and all applicable Russian regulatory guidelines. It was also approved by the Ethics Council at the Ministry of Healthcare of the Russian Federation and by Independent Ethics Committees at each investigational site (Table S1). All patients provided written informed consent.
Study population
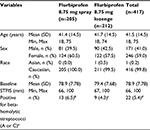
Adult patients (aged 18–75 years) who presented to one of the investigational sites were evaluated for sore throat due to URTI with recent onset (within ≤4 days). Patients were included if they rated their pain as moderate or severe on the throat pain scale (TPS), if they had at least one symptom of URTI on the URTI questionnaire, and if they had sore throat pain (score of ≥66 mm on the sore throat pain intensity scale [STPIS]), difficulty swallowing (score of ≥50 mm on the difficulty swallowing scale [DSS]), and the sensation of a swollen throat (score of ≥33 mm on the swollen throat scale [SwoTS]) at baseline. A score of ≥5 points on the Tonsillopharyngitis Assessment (TPA), as documented by a physician, was also required in order to confirm pharyngeal inflammation.
Patients were ineligible if they had any of the following: known allergy or hypersensitivity to the study drug or other NSAIDs; purulent plaques on the tonsils; mouth breathing due to nasal congestion; body temperature (oral) ≥38.0°C; severe coughing; use of any medicated confectionery, throat pastille, throat lozenge, throat spray, cough drop, or any product with demulcent properties such as boiled sweets within 1 hour before enrollment; use of cold medication (e.g., decongestants, expectorants) or immediate-release analgesics/anti-pyretics within 4 hours prior to enrollment; use of sustained-release analgesics or anti-pyretics within 12 hours prior to enrollment; or any disease that could compromise breathing (e.g., bronchopneumonia).
Study medications
Using a blocked randomization schedule, patients were randomized into one of two treatment groups: test drug (flurbiprofen 8.75 mg spray) or reference drug (flurbiprofen 8.75 mg lozenge). Patients were allocated a unique subject number, and the study drug was randomized to this sequence. As the test and reference drugs had different formulations, a double-dummy technique was used, whereby patients randomized to receive the test drug were given a single dose of flurbiprofen 8.75 mg spray and one placebo lozenge and patients randomized to receive the reference drug were given one flurbiprofen 8.75 mg lozenge and a single dose of placebo spray. Within each treatment group, the order of administration of spray and lozenge was also randomized (1:1) to control for dose-sequencing effects. All patients and investigators were blinded to the study medication.
Study medications were given to patients by study personnel. After finishing administration of the first randomized formulation (for lozenges, this was defined as the lozenge completely dissolving), there was a 10-minute interval before administration of the second randomized formulation. For administration of lozenges, study personnel instructed the patient to suck the lozenge (not to chew or bite the lozenge and not to swallow the lozenge until it was fully dissolved) and “swish” it around the mouth (i.e., not suck it in only one part of the mouth). A designated member of the study personnel administered the spray formulation: the patient was asked to open his or her mouth, and the designated person sprayed the patient’s throat with a single dose of flurbiprofen 8.75 mg spray (consisting of three sprays). Patients were not permitted to have anything by mouth for the next 2 hours. A washout period was permitted before the baseline screening assessments, if necessary, in order to allow patients to be considered for entry even if they had taken prohibited therapies (such as throat pastilles and boiled sweets). The duration of the washout period was determined by the type of prohibited therapy, and the patient must not have had a sore throat for >4 days after the washout period was completed. All study medications were provided by Reckitt Benckiser (Hull, UK).
Study assessments
At baseline, a medical history was obtained and patients underwent a physical examination (including the TPA and the Practitioner’s Assessment of Pharyngeal Inflammation [PAIN]). Throat swabs for bacterial culture were taken from each patient to confirm the presence or absence of beta-hemolytic streptococci (A or C); analysis was performed in a central laboratory (INVITRO Ltd, Moscow, Russia).
Throughout the study, patients reported sore throat pain intensity using the STPIS, a validated 100 mm visual analog scale (VAS) where 0=“no pain” and 100=“severe pain”. STPIS scores were reported at baseline and at 60 minutes and 120±5 minutes post-dose. Additional assessments conducted at 120±5 minutes post-dose were the Patient Satisfaction Scale and the Practitioner’s Clinical Assessment of Drug Efficacy (CLIN). For the Patient Satisfaction Scale, the patient indicated his or her level of satisfaction with study treatment using a 7-point categorical scale (extremely dissatisfied, very dissatisfied, dissatisfied, somewhat dissatisfied, satisfied, very satisfied, and extremely satisfied). Investigators used the CLIN to evaluate the study medication as a treatment for sore throat using a 5-point categorical scale (poor, fair, good, very good, and excellent). All treatment-emergent adverse events (AEs) were recorded throughout the study using Medical Dictionary for Regulatory Activities (MedDRA) terminology.
Patients completed the 120-minute assessment period under supervision and then left the clinic with routine care for sore throat due to URTI. Study personnel contacted patients by telephone within 1–3 days post-dose to collect data on concomitant medications and AEs experienced after the administration of study treatment.
Sample size
This study tested the hypothesis that flurbiprofen 8.75 mg spray is not worse than flurbiprofen 8.75 mg lozenges in the treatment of patients with sore throat due to URTI. Based on data from previous studies of flurbiprofen lozenges,20,21 the non-inferiority margin of −6 mm was selected. Assuming that flurbiprofen 8.75 mg spray would be at least as efficacious as flurbiprofen 8.75 mg lozenges in reducing sore throat pain intensity at 2 hours post-dose with the non-inferiority margin of −6 mm and assuming no actual difference between groups, a pooled standard deviation of 19.1 mm, a ratio of 1:1 in treatment group allocation, a 2.5% chance for type I error, and a 2.5% dropout rate over the first 2 hours of the study, 220 patients per treatment group were required to ensure 90% power.
Primary and secondary end points
The primary efficacy end point was the difference from baseline to 2 hours post-dose in sore throat pain intensity scale (STPIS pain intensity difference [PID] 2h). The main secondary end points were difference from baseline in sore throat pain intensity at 1 hour (STPIS PID 1h), the sum of sore throat pain intensity differences (SPID) over 2 hours (STPIS SPID0–2h), and change from baseline in sore throat pain intensity (STPIS) in patients with and without beta-hemolytic streptococci (A or C) infection. Scoring on both the Patient Satisfaction Scale and the CLIN at 2 hours post-dose was also compared between the treatment groups.
Statistical analyses
For the primary efficacy end point (STPIS PID 2h), STPIS PID 1h, and STPIS SPID0–2h, least square (LS) means and mean square error were compared between treatment groups using a mixed analysis of covariance (ANCOVA) model with baseline sore throat pain intensity as a covariate, treatment center and treatment sequence as fixed effects, and center as a random effect. Non-inferiority of flurbiprofen 8.75 mg spray was demonstrated if the two-sided 95% confidence interval (95% CI) for the difference between the LS means (lozenge − spray) was above the pre-defined non-inferiority margin of −6 mm. Patient satisfaction with study medication (Patient Satisfaction Scale) and the CLIN at 2 hours post-dose were analyzed as categorical parameters using the Mann–Whitney U test. Ordinal logistic regression models with baseline STPIS as a covariate, treatment group and treatment sequence as fixed effects, and center as a random effect were also performed. Safety analyses included all patients who took study medication, and the incidence of treatment-emergent AEs was compared between treatment groups using a Fisher’s exact test. All statistical tests were two-sided, with significance determined at the 5% significance level, and all between-treatment comparisons using continuous parameters were reported with 95% CI for the difference.
Efficacy was evaluated in both the full analysis set (FAS), which was based on the intent-to-treat (ITT) principle, and the per-protocol (PP) set; as this was a non-inferiority study, the PP set was used as the primary analysis set. The safety set, comprising all those who took study medication, was used to evaluate safety data and demographic, baseline, and treatment characteristics.
Results
Study population
In total, 441 patients were screened and 440 were enrolled (flurbiprofen 8.75 mg spray, n=218; flurbiprofen 8.75 mg lozenge, n=222) (Figure 1). One patient was withdrawn (flurbiprofen 8.75 mg spray group) due to a protocol violation (Figure 1) and was excluded from efficacy analysis in the FAS and PP set, since no post-baseline efficacy data were available. The FAS therefore comprised 439 patients (flurbiprofen 8.75 mg spray, n=217; flurbiprofen 8.75 mg lozenge, n=222), and the PP set included 417 patients (flurbiprofen 8.75 mg spray, n=205; flurbiprofen 8.75 mg lozenge, n=212). Both treatment groups in the PP set were well balanced in terms of demographic data and baseline characteristics (Table 1), as well as in the FAS (data not shown). Results of throat swab culture tests showed that similar proportions of patients in the flurbiprofen spray and lozenge groups tested positive for beta-hemolytic streptococci (A or C) (Table 1).
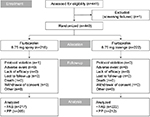  | Figure 1 Patient disposition. Abbreviations: FAS, full analysis set; PP, per-protocol. |
Primary end point
In the PP set, reductions in sore throat pain intensity from baseline to 2 hours post-dose (STPIS PID 2h) were similar for patients in the flurbiprofen 8.75 mg spray group and those in the flurbiprofen 8.75 mg lozenge group (p=0.82 for the difference between LS means) (Table 2; Figure 2). Similar results were observed for the FAS (Table 2). For both the PP set and the FAS, non-inferiority of the spray formulation compared with the lozenge formulation was demonstrated, as the lower limit of the two-sided 95% CI for the difference between LS means was greater than the predefined non-inferiority margin of −6 mm.
Secondary end points: STPIS PID 1h, STPIS SPID0–2h, and STPIS according to Strep status
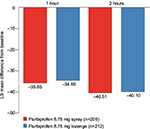
In the PP set, there were similar LS mean (95% CI) reductions in STPIS PID 1h in the flurbiprofen 8.75 mg spray group (−35.85 [−41.79, −29.90] mm) and flurbiprofen 8.75 mg lozenge group (−34.65 [−40.55, −28.74] mm) (Figure 2). Similar results were observed in the FAS (flurbiprofen 8.75 mg spray, −35.95 [−41.85, −30.05] mm; flurbiprofen 8.75 mg lozenge, −34.83 [−40.70, −28.96] mm). The differences between the treatment groups were not significant in either the PP set (p=0.4597) or the FAS (p=0.4784).
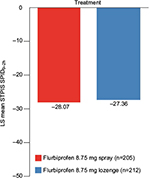
In the PP set, the STPIS SPID0–2h was −28.07 mm (95% CI, −32.34, −23.80) and −27.36 mm (95% CI, −31.60, −23.12) in the flurbiprofen 8.75 mg spray and flurbiprofen 8.75 mg lozenge groups, respectively (Figure 3). Corresponding results for the FAS were −28.14 mm (95% CI, −32.36, −23.91) in the flurbiprofen 8.75 mg spray group and −27.52 mm (95% CI, −31.72, −23.31) in the flurbiprofen 8.75 mg lozenge group. The LS mean difference in STPIS SPID0–2h was not significant between the two treatment groups (PP set, p=0.5469; FAS, p=0.5901).
Additionally, mean (±standard deviation [SD]) change from baseline in STPIS at 2 hours (STPIS PID 2h) post-dose was similar between patients who were positive for beta-hemolytic streptococci (A or C) in the PP set (flurbiprofen 8.75 mg spray, −36.5 [20.89] mm; flurbiprofen 8.75 mg lozenge, −44.2 [24.83] mm) and those who were negative for beta-hemolytic streptococci (A or C) (flurbiprofen 8.75 mg spray, −41.1 [21.84] mm; flurbiprofen 8.75 mg lozenge, −40.1 [22.28] mm). Similar results were obtained in the FAS.
Secondary end points: treatment ratings
There were no significant differences between treatment groups in patient satisfaction with study medication at 2 hours post-dose (PP set, p=0.1609; FAS, p=0.1802). Approximately 89% and 84% of patients in the flurbiprofen spray and lozenge groups, respectively, were either “satisfied”, “very satisfied”, or “extremely satisfied” with study medication (in both PP set and FAS).
Similarly, there were no significant between-group differences in CLIN ratings at 2 hours post-dose (PP set, p=0.2053; FAS, p=0.2390). “Good”, “very good”, or “excellent” assessment of drug efficacy was reported for ~86% and 79% of patients in the flurbiprofen spray and lozenge groups, respectively, in both the PP set and the FAS.
Safety
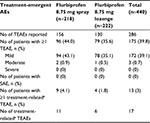
There were no significant differences in the incidence of treatment-emergent AEs between the two treatment groups (Table 3). The percentage of patients who reported at least one AE during the study was similar between the flurbiprofen 8.75 mg spray group (44%) and the flurbiprofen 8.75 mg lozenge group (35.6%; p=0.0796); most AEs were mild in severity, and no serious AEs (SAEs) were reported (Table 3).
The most common AEs in both treatment groups were throat irritation, asthenia, somnolence, and headache (Table 4). A small proportion of patients (4.1% and 1.8% in the flurbiprofen 8.75 mg spray and lozenge groups, respectively) reported AEs that were considered to be certainly, possibly, or probably related to study treatment. There were 11 treatment-related AEs in the flurbiprofen 8.75 mg spray group: throat irritation (n=5), dyspepsia (n=3), malaise, cough, and hiccups (n=1 each). The six treatment-related AEs in the flurbiprofen 8.75 mg lozenge group were glossodynia (n=2), tachycardia, dyspepsia, hypoesthesia, and somnolence (n=1 each). All treatment-related AEs in both treatment groups were mild in severity. The AE data reported here that are related to the study treatment are consistent with the known safety and tolerability profile of flurbiprofen.
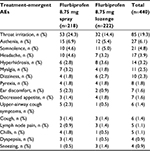  | Table 4 Most common treatment-emergent AEs (safety set) Abbreviation: AEs, adverse events. |
Discussion
This randomized, double-blind, single-dose, non-inferiority study demonstrated that flurbiprofen 8.75 mg spray is non-inferior to flurbiprofen 8.75 mg lozenges in the treatment of sore throat due to URTI. For the primary efficacy end point (STPIS PID 2h), non-inferiority of the spray formulation versus the lozenge was demonstrated in both the PP set and the FAS, as the lower limit of the two-sided 95% CI for the difference between LS means was greater than the predefined non-inferiority margin of −6 mm. Both formulations also showed comparable efficacy profiles in key secondary end points (STPIS PID 1h and STPIS SPID0–2h), and both were effective in patients with and without beta-hemolytic streptococci (A or C). At 2 hours post-dose, the majority of patients were at least “satisfied” with study medication and most practitioners rated treatment efficacy highly, with no significant differences between the spray and lozenge formulations. There were no serious safety concerns, and the two formulations showed favorable and comparable safety profiles.
This study demonstrates that flurbiprofen 8.75 mg delivered as a lozenge or spray provides relief for the pain associated with sore throat due to URTI, giving patients two different treatment formats to choose from according to their own preferences. Patients may, for example, prefer the demulcent effects of a lozenge or the convenience of a spray. Advantages of a spray include delivery of a full dose immediately at the site of pain and inflammation, whereas lozenges take time to dissolve in the mouth (~3–5 minutes) in order to deliver a full dose.25 A patient’s choice of treatment format may also depend on the time of day they require relief.
Throat swab culture results from our study showed that only a small proportion of patients had beta-hemolytic streptococci (A or C), indicating that antibiotics would have been inappropriate for the majority of participants. Many patients who hope or ask for antibiotics may, in fact, be seeking treatment to alleviate pain;12 however, findings from a systematic review showed that antibiotics are among the least effective treatments for symptomatic relief of sore throat.14 In contrast, symptomatic treatments such as NSAIDs and analgesics were found to be up to 93% more effective than placebo.14 Together with the growing problem of antibiotic resistance, these findings suggest that non-antibiotic treatments should be considered instead of antibiotics for relieving sore throat symptoms.26 Importantly, in our study, flurbiprofen 8.75 mg delivered as a spray or lozenge provided comparable pain relief in patients both with and without beta-hemolytic streptococci (A or C) infection. Both formulations may therefore represent an alternative and preferable first-line treatment for patients with acute sore throat without any of the “red flags” that might indicate a more serious illness.10 Even when the symptoms and course of sore throat suggest that antibiotics are warranted, flurbiprofen 8.75 mg spray or lozenge could be combined with antibiotic therapy to effectively relieve pain and other symptoms.24
Our findings support those of previous studies demonstrating that flurbiprofen 8.75 mg provides effective symptomatic relief of sore throats due to URTI when delivered as either a lozenge3,20–24 or a spray.17 Previous studies have compared flurbiprofen 8.75 mg lozenge or spray with placebo. To our knowledge, our study is the first that directly compares the relief provided by flurbiprofen 8.75 mg, using two different formats in the same study population. Although this study only monitored efficacy for up to 2 hours post-dose to determine non-inferiority, previous studies have shown that both formulations provide relief for up to 6 hours post-dose,3,17 suggesting that patients may be able to resume normal activities quickly without the need for regular re-dosing.17
While patients in this study were recruited from different sources (general practices, community pharmacies, advertizing) and may therefore have presented with a diverse range of symptoms, this may be more representative of “real-world” practice.17 Additionally, although the two products could not be matched due to their different formulations, this was countered by using a double-dummy design: each patient randomized to receive the spray formulation received one dose of flurbiprofen 8.75 mg spray and one placebo lozenge, and patients randomized to receive the lozenge received one flurbiprofen 8.75 mg lozenge and one dose of placebo spray. Results showed that the factor of treatment sequence was not significant, suggesting that the randomized order of drug intake within each treatment group did not affect the results significantly.
Conclusion
Flurbiprofen 8.75 mg delivered as a spray or lozenge provides effective relief from the pain associated with sore throats due to URTI. Non-inferiority of the spray versus the lozenge formulation was established, and both formulations demonstrated comparable efficacy and safety profiles. The spray and lozenge formulations offer patients two different treatment formats to choose from for effective symptomatic relief of sore throat, depending on their own preferences.
Acknowledgments
Medical writing assistance was provided by Hannah Chatfield at Elements Communications Ltd, Westerham, UK, and was funded by Reckitt Benckiser Healthcare Ltd, Slough, UK.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Valeria Bychkova and Robert DeVito are employees of Reckitt Benckiser. This study was funded by Reckitt Benckiser. Dr Eugenia Radkova received funding from Reckitt Benckiser to develop the content of all study-related documents (protocol, ICF, IB, CSR). Professor Natalia Burova’s organization (Federal State Establishment Clinical Diagnostic Medical Center) received funding from Reckitt Benckiser for the study. The authors report no other conflicts of interest in this work.
References
Worrall GJ. Acute sore throat. Can Fam Physician. 2007;53(11):1961–1962. | ||
ESCMID Sore Throat Guideline Group, Pelucchi C, Grigoryan L, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18(suppl 1):1–28. | ||
Schachtel BP, Shephard A, Shea T, et al. Flurbiprofen 8.75 mg lozenges for treating sore throat symptoms: a randomized, double-blind, placebo-controlled study. Pain Manag. 2016;6(6):519–529. | ||
Aspley S, Shephard A, Schachtel E, Sanner K, Savino L, Schachtel B. Efficacy of flurbiprofen 8.75 mg lozenge in patients with a swollen and inflamed sore throat. Curr Med Res Opin. 2016;32(9):1529–1538. | ||
Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;(11):CD000023. | ||
Addey D, Shephard A. Incidence, causes, severity and treatment of throat discomfort: a four-region online questionnaire survey. BMC Ear Nose Throat Disord. 2012;12:9. | ||
Bisno AL, Peter GS, Kaplan EL. Diagnosis of strep throat in adults: are clinical criteria really good enough? Clin Infect Dis. 2002;35(2):126–129. | ||
van der Velden A, Bell J, Sessa A, Duerden M, Altiner A. Sore throat: effective communication delivers improved diagnosis, enhanced self-care and more rational use of antibiotics. Int J Clin Pract Suppl. 2013;(180):10–16. | ||
Kenealy T [webpage on the Internet]. Sore throat. Systematic review 1509. BMJ Clin Evid. 2014. Available from: http://clinicalevidence.bmj.com/x/systematic-review/1509/overview.html. Accessed August 31, 2016. | ||
Centor RM, Samlowski R. Avoiding sore throat morbidity and mortality: when is it not “just a sore throat?” Am Fam Physician. 2011;83(1):26–28. | ||
Shephard A, Zybeshari S. Virucidal action of sore throat lozenges against respiratory viruses parainfluenza type 3 and cytomegalovirus. Antiviral Res. 2015;123:158–162. | ||
van Driel ML, De Sutter A, Deveugele M, et al. Are sore throat patients who hope for antibiotics actually asking for pain relief? Ann Fam Med. 2006;4(6):494–499. | ||
Russo M, Bloch M, de Looze F, Morris C, Shephard A. Flurbiprofen microgranules for relief of sore throat: a randomised, double-blind trial. Br J Gen Pract. 2013;63(607):e149–e155. | ||
Thomas M, Del Mar C, Glasziou P. How effective are treatments other than antibiotics for acute sore throat? Br J Gen Pract. 2000;50(459):817–820. | ||
Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459):579–587. | ||
Farrer F. Sprays and lozenges for sore throats. S Afr Pharm J. 2011;78(4):26–31. | ||
de Looze F, Russo M, Bloch M, et al. Efficacy of flurbiprofen 8.75 mg spray in patients with sore throat due to an upper respiratory tract infection: a randomised controlled trial. Eur J Gen Pract. 2016;22(2):111–118. | ||
Buchanan WW, Kassam YB. European experience with flurbiprofen. A new analgesic/anti-inflammatory agent. Am J Med. 1986;80(3A):145–152. | ||
Sefia E, Mann A, Lambkin R, et al. Flurbiprofen lozenges rapidly reduce levels of the inflammatory mediator prostaglandin E in human respiratory cells in vitro. Abstract presented at: Annual Scientific Meeting of the British Pain Society; April 24–27; 2007; Glasgow, UK. | ||
Schachtel B, Aspley S, Shephard A, et al. Onset of action of a lozenge containing flurbiprofen 8.75mg: a randomized, double-blind, placebo-controlled trial with a new method for measuring onset of analgesic activity. Pain. 2014;155(2):422–428. | ||
Schachtel B, Aspley S, Shephard A, Shea T, Smith G, Schachtel E. Utility of the sore throat pain model in a multiple-dose assessment of the acute analgesic flurbiprofen: a randomized controlled study. Trials. 2014;15:263. | ||
Watson N, Nimmo WS, Christian J, Charlesworth A, Speight J, Miller K. Relief of sore throat with the anti-inflammatory throat lozenge flurbiprofen 8.75 mg: a randomised, double-blind, placebo-controlled study of efficacy and safety. Int J Clin Pract. 2000;54(8):490–496. | ||
Benrimoj SI, Langford JH, Christian J, Charlesworth A, Steans A. Efficacy and tolerability of the anti-inflammatory throat lozenge flurbiprofen 8.75mg in the treatment of sore throat: a randomised, double-blind, placebo-controlled study. Clin Drug Investig. 2001;21(3):183–193. | ||
Blagden M, Christian J, Miller K, Charlesworth A. Multidose flurbiprofen 8.75 mg lozenges in the treatment of sore throat: a randomised, double-blind, placebo-controlled study in UK general practice centres. Int J Clin Pract. 2002;56(2):95–100. | ||
Schachtel BP, Homan HD, Gibb IA, Christian J. Demonstration of dose response of flurbiprofen lozenges with the sore throat pain model. Clin Pharmacol Ther. 2002;71(5):375–380. | ||
Shephard A, Smith G, Aspley S, Schachtel BP. Randomised, double-blind, placebo-controlled studies on flurbiprofen 8.75 mg lozenges in patients with/without group A or C streptococcal throat infection, with an assessment of clinicians’ prediction of ‘strep throat’. Int J Clin Pract. 2015;69(1):59–71. |
Supplementary material
  | Table S1 Institutional ethics committees at each investigational site |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.