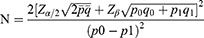Back to Journals » Journal of Pain Research » Volume 15
Efficacy of Electroacupuncture for the Treatment of Postherpetic Neuralgia: Study Protocol for a Multicenter Randomized Controlled Trial
Authors Sun R, Li S, Ren L, Xia Y, Wang Y , Bian Z, Fang J, Zhang Z
Received 19 January 2022
Accepted for publication 19 March 2022
Published 5 April 2022 Volume 2022:15 Pages 959—968
DOI https://doi.org/10.2147/JPR.S357435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Houman Danesh
Ruohan Sun,1,2,* Shimin Li,1,* Leilei Ren,2 Yunfan Xia,2 Yiyi Wang,2 Zhiyuan Bian,2 Jianqiao Fang,2 Zuyong Zhang1
1Hangzhou Third Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Acupuncture, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zuyong Zhang, Hangzhou Third Hospital Affiliated to Zhejiang Chinese Medical University, No. 38 West Lake Road, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected] Jianqiao Fang, The Third Affiliated Hospital of Zhejiang Chinese Medical University, No. 23 Qinchun Road, Hangzhou, Zhejiang, People’s Republic of China, Email [email protected]
Introduction: Postherpetic neuralgia (PHN) is a severe complication of herpes zoster (HZ), representing an important burden of disease in the elderly. Electroacupuncture (EA) has become growingly appreciated as a therapy of PHN with the situation that effectiveness of conventional therapy of PHN is less than ideal. Owing to its low price, no side effects, high safety and high patients acceptance, EA has been used in treating PHN more frequently. Therefore, the randomized controlled trial which is to evaluate the effectiveness and safety of EA in patients with PHN and whether EA could be an alternative therapy of medication is needed.
Patients and Methods: A total of 88 patients with PHN will be recruited from 2 hospitals and randomized assigned to EA group or Medication group in a 1:1 ratio, utilizing a central randomization system. The trial will involve a 4-week treatment period, and a 4-week follow-up period. All variables will be evaluated at week 0 (baseline), week 2 (treatment), week 4 (treatment), week 8 (follow-up) and week 16 (follow-up). Primary outcomes will be pain intensity. Secondary outcomes will contain quality of life, mood state and sleep quality. All adverse effects will be assessed during the trial.
Conclusion: This study will provide significant evidence that whether EA therapy is effective and safe for patients with PHN and whether EA could be an alternative therapy of medication.
Ethics and Dissemination: Ethics approval has been obtained from the Ethics Committee of the Hangzhou Third People’s Hospital (No. 2021KAO43). Informed consent will be signed before enrolment. Results of this trial will be presented to international journals for publication and be reported in relevant international conferences.
Trial Registration Number: This protocol has been registered in the Chinese Clinical Trial registry with the identification code ChiCTR2100054592.
Keywords: postherpetic neuralgia, electroacupuncture, pain, randomized controlled trial, protocol
Introduction
Postherpetic neuralgia(PHN) is a condition which results from nerve dysfunction following an episode of acute herpes zoster(AHZ) previously and is the most frequent and refractory sequela of AHZ. PHN can occur in 20% of patients at any age, whereas, people over 50 are more prone to developing PHN.1 Hence, most patients in a condition of PHN always take on more medications pertaining to other age-related issues that result in synergistic side effects when they add PHN pharmaceuticals, which leads to a less pain relief.2 Except increasing age, low immunity is a risk factor as well. Approximately 2% patients with AHZ may be evolved into a chronic pain syndrome and persist for 5 years or more,3 which may lead to a reduced quality of life and sleep of the afflicted. Patients with PHN generally has a longer duration, contributing to financial burdens on individuals and society, and describe different types of pain: a persistent deep aching, a burning pain, allodynia as well as itching which could be more irritating than the pain itself as many patients complain.2
At present, oral drug shows acceptable analgesia for PHN prevalently, for instance, Gabapentin and Pregabalin are the most commonly used first-line therapies for chronic PHN pain both approved by Food and Drug Administration(FDA) for the PHN management, furthermore, topical therapy, such as lidocaine 5% patch and the capsaicin 8% patch is also an effective method available for PHN.4,5 Whereas, this benefit usually comes with a cost- serious side effect and drug dependence to the human body, Besides the side effects, the effectiveness of pharmaceutical and surgical treatments for PHN is hardly satisfactory,2,6 thus it is still insufficient for chronic pain.7 Up to now, although the vaccine can prevent PHN cases and lower the incidence of PHN in part,8 we still lack a disease-modifying therapy.
Acupuncture, originated in ancient China, is a noninvasive treatment, and according to a variety of increasingly empirical and clinical evidences, has been shown to be an economical, effective, safe, and non-side-effect-causing therapy, used in various chronic pain,9–11 containing PHN.12–14 Based on our studies conducted previously,15–19 acupuncture, especially electroacupuncture (EA), has been found to be effective for alleviating neuropathic pain. The exact mechanism underlying EA analgesia is complex and remains unclear. Some researches reveal that EA analgesia associates with bioactive chemicals, such as opioids, serotonin, norepinephrine through peripheral, spinal, and supraspinal systems.11 In line with existing animal experiments, 2Hz EA shows more successful analgesia for chronic pain models of neuropathic pain,20–22 comparing with 100Hz. Furthermore, Combined with previous trials of our team that 2Hz EA was better than 2/100Hz EA in maintaining analgesic effects for Trigeminal neuralgia, EA at 2Hz are supposed to be a more effective frequency for chronic and neuropathic pain. Whereas, the efficacy of EA is not fully accepted worldwide up to now. Despite a number of researches suggesting its effectiveness and widespread use in clinical practice, the current evidence supporting is not strong.23,24 Thus, it is necessary to conduct a Randomized controlled trial(RCT) to obtain stronger and valid proof of EA and confirm the effectiveness and safety of EA compared with medication.
Methods
Study Design
This trial is a multicenter and randomized controlled design. Patients with a prior PHN diagnosis are diagnosed by the research doctors at the outpatient clinics and on the wards will be invited to participate in this trial. After enrollment, eligible participants will be divided into electroacupuncture(EA) group and medication group respectively in a 1:1 allocation ratio through a central randomized system. All participants will receive 4-week treatment and 12-week follow-up, and before entering, they will be asked to sign an informed consent. The trial design is summarized in Figure 1. This trial is aimed at evaluating the effectiveness and safety of electroacupuncture therapy compared with medication. The protocol is based on the standard for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA)25 and used the SPIRIT reporting guidelines.26
Participants Enrollment
Two centers contain the Hangzhou Third People’s Hospital and The Third Affiliated Hospital of Zhejiang Chinese Medical University. Participants will be recruited by means of local newspaper, programs, advertisement in communities and hospitals. Our researchers will screen patients who are interested in this trial through the following criteria, and after signing informed consent, eligible participants will be randomized into two groups with different treatments.
Inclusion Criteria
Participants will be included if they (1) meet the diagnostic criteria for PHN27; and (2) have a HZ history, and the duration persists for more than 90 days; and (3) have a Visual Analogue Scale(VAS) not lower than 4; and (4) are 18–80 years old; and (5) sign the written informed consent and can fully understand the protocol.
Exclusion Criteria
Participants will be excluded if they (1) do not meet the inclusion criteria; and (2) suffer from Herpes Zoster which occurs in the head and perineum or which is regarded as the special type of Herpes Zoster; and (3) are pregnant or nursing; and (4) have severe heart diseases, liver, kidney and other organ damages or have other serious skin diseases; and (5) have a cardiac pacemaker; and (6) have intolerance, uncooperative behavior to the study, and the inability to finish the self-evaluation questionnaires; and (7) have participated in other clinical trials in the last 3 months.
Discontinuation Criteria
Participants will discontinue this trial if they (1) have so serious adverse reactions during the study that it is impossible to continue; and (2) have disease progression or severe complications during the trial and need some urgent emergency measures; and (3) have poor compliance or ask to leave the trial halfway.
The physician will evaluate the severity of the situation and discontinue the trial.
Elimination Criteria
Participants will be eliminated if they (1) are not standards-compliant but they are wrongly recruited; and (2) fail to complete the prescribed treatment or data collection due to various reasons and affects the evaluation of efficacy and safety; and (3) leave this trial by themselves; and (4) receive other treatment rather than our intervention.
Randomization and Blinding
This trial is randomized and allocated by a Dynamic Randomization Method of Central Stochastic System. After enrollment, randomization will be performed by an independent researcher who will not be involved in treatment and statistical analyses. The randomization scheme, which allows no one except the top system administrator to view, will be generated by an independent person not involved in this trial. Therefore, the variability of variables such as age and gender will be reduced to a minimum. All assessors, data recorder and statistical analysts will be blinded to assignment and corresponding intervention. Nonetheless, acupuncture manipulators and participants will not be blinded considering the peculiarity of acupuncture. All staff of this trial will perform independently in case of the information exchange about this trial.
Intervention
Participants will receive either EA or medication. Duration of treatment in both groups is 4 weeks with a follow-up period of 12 weeks.
Medication Group
Participants allocated to medication group shall receive oral 150-mg Pregabalin capsules (Produced by Pfizer Pharmaceutical Co., Ltd) twice daily (total daily dose,300mg) for 4 weeks.
EA Group
Participants in EA groups receiving EA treatment of deep needling on planned acupoints use disposable stainless steel needles with a specification of 0.25 mm×45mm and 0.18 mm×25mm (Suzhou Medical Products Factory Co., Ltd, China). The acupoints prescriptions for all are as follow: Ashi acupoints, Shenting (DU24), Baihui (DU20), Sishencong (EX-HN1),and Jiaji acupoints (EX-B2), Hegu (LI4), Waiguan (TE5), Yanglingquan (GB34), Qiuxu (GB40) on the affected side. Besides, specific needles are connected to EA apparatus and the frequency of electrical stimulation is 2 Hz. The locations of acupoints chosen in this trial are displayed in Table 1.
 |
Table 1 Location and Indication of Acupoints for Treating PHN |
Every participant will be asked to be settled in a lateral position so that the pain region can be exposed fully. After routine disinfection, needles with a specification of 0.25 mm×40mm will be inserted to a depth of 25 to 40 mm vertically and unilaterally on EX-B2, LI4,TE5,GB34 and GB40 on the affected side. EX-B2 on the ipsilateral side will be selected according to the corresponding ganglia involved by herpes zoster. Ashi acupoints related to the lesion area will be selected (every 2-to-3-cm interval of the painful region of PHN is used as an acupoint) and needles with a specification of 0.25 mm×40mm will spur the direction of the center of the lesion at 30° on Ashi acupoints. Needles with a specification of 0.18 mm×25mm will also be used to spur the direction of the center of the lesion at 15° on DU24, DU20 and EX-HN. Then, needles at EX-B2 and Ashi acupoints are connected to EA apparatus respectively. The treatment will last for 30 minutes. The frequency of electrical stimulation will be set as 2 Hz and the stimulation intensity should be increased gradually and to the scope that participants can be tolerant.
In addition, if the neuropathic pain cannot be endured during the trial, Acetaminophen orally will be allowed as the rescue analgesia but instructed not to exceed 3 grams per day and all therapies ought to be carefully recorded in detail on CRF in time.
All treatment will be operated by doctors who have at least 3-year clinical experience in acupuncture. Participants will cooperate with the treatment 3 times a week for 4 weeks continuously, moreover, any other treatment which may affect the trial will be prohibited. The adverse events will be reported, handled and recorded in a timely manner.
Outcome Measures
Participants suffering from PHN are always troubled with chronic pain, insomnia, and emotional handicap. Therefore, we chose related outcomes to evaluate their mental and physical condition in the trial.
Participants in both two groups will be evaluated at week 0 (baseline), week 2 (treatment), week 4 (treatment) week, week 8 (follow-up), and week 16 (follow-up), assessors to evaluate the changes of pain intensity, insomnia severity, emotional status and life quality. Moreover, assessors will monitor reasons for participants discontinuing trial during the treatment period as well as adverse reactions at each treatment session to evaluate the security of the therapies.
The schedule of this trial is presented in Table 2.
 |
Table 2 Schedule of Enrolment, Treatments, and Assessments |
Primary Outcome Measure
Primary outcome measures is Visual Analogue Scale (VAS) which ranges from 0 (no pain) to 10 (The worst unbearable pain) and is chosen to assess merely pain severity. Due to its simplicity and objectivity, VAS is widely used in clinical study.
Secondary Outcome Measure
Secondary outcome measures contain Short-Form of McGill Pain Questionnaire (SF-MPQ), Brief Pain Inventory (BPI-SF), the Medical Outcomes Study-Sleep Scale (MOS-SS), Hamilton Depression Scale (HAMD), Hamilton Anxiety Scale (HAMA), and Short Form-36 Health Survey (SF-36). SF-MPQ focuses on characteristics, intensity, applying syndromes of pain as well as their change during the treatment, besides, it does not spend too much time, so it could be sensitive to clinical change and reflect the effectiveness of the therapy. BPI-SF includes descriptive words such as the cause of pain, the nature of pain, the impact on life and the location of pain, and is used to describe the degree of pain and evaluate the pain in many aspects. MOS-SS consists of 12 items related to the sleep quality within a 4-week recall period. Participants with PHN are usually beset by imaginable chronic pain, thus emotion status will affect their pain intensity conversely, thus HAMA and HAMD will be adopted to assess mental condition of participants, containing anxiety and depression respectively. SF-36 is a questionnaire that has been clarified to have reliability and validity and is used widely to evaluate people’s quality of life.
Informed Consent and Ethic
Before inclusion, all participants will be fully aware of this trial in detail including benefits and risks, and asked to sign the informed consent. Moreover, they will be informed of that it’s free to participate in this trial and they can withdraw after participating at their own will without any reason at any time. It’s a patient’s prerogative to decide whether to participant in this trial or not. All information related to participants will be kept confidential.
This trial has obtained ethics approvals from Hangzhou Third People’s Hospital (No. 2021KAO43).
Study Registration
This trial was registered in the Chinese Clinical Trial Registry (ChiCTR2100054592).
Quality Control
For the sake of minimizing treatment bias, two centers will designate one professional acupuncture doctor respectively who has at least 4-year experience of acupuncture to receive intensive training based on the treatment procedure of this trial. Simultaneously, data assessors will be trained together, and practice to collect outcome assessments and fill in case report forms normatively. Data related to this trial will be recorded in case report forms and there will be a specific person in charge of entering those raw data in every center. Considering the bias minimization, except acupuncture doctors and participants, clinical research coordinators (CRCs)(including outcome assessors, data recorders, data entry clerks and statistical analysts) will be blinded to assignment. All staff in two centers will meet every 3 months to talk over emerging issues and corresponding solutions during our trial.
To enhance the adherence of participants and avoid attrition bias induced by the dropout and loss to follow up, all of them will obtain 100 RMB per person transport allowance.
Safety Evaluation
Researchers will record adverse events caused by EA and Pregabalin during treatment in detail (time, degree, duration and solution), containing severe pain, bleeding, skin pigmentation, fainting, sweating, dizziness (acupuncture related), and gastrointestinal discomfort, drug allergies (medication related). At the end of the trial, researchers will calculate the frequency of adverse events arisen. If encountering extremely severe adverse events, researchers should keep ethics committee advised of this immediately, who will determine whether the participant should be withdrawn from the trial.
Sample Size
Sample size is calculated by PASS software (version 15.0.5, NCSS, LLC). The primary outcome is VAS, and based on the literature, the effective rate of Pregabalin is 60%,28 meanwhile, according to our early trials, the effective rate of 2 Hz EA is 92.3%, with the following assumptions: α of 0.05 (two-tailed), power of 90%, and a 1:1 ratio, at least 35 participants in each group will be obtained. Accounting for a possible dropout rate of 20%, each group should include no fewer than 44 participants and there will be at least 88 participants included in this trial. The following formula was used:
Statistical Analysis
The data will be performed by SPSS 19.0. Data will be expressed as mean ± standard deviation (xˉ ± s) and percentage. Continuous data in normal distribution will be examined by analysis of variance and t-test with Dunnett’s adjustment for multiple comparisons, and categorical data will be examined by Chi-square test. Difference is considered statistically significant at P < 0.05. Besides, all the data will be analyzed using both ITT and PP datasets to reduce deviation.
Discussion
PHN representing a major health problem is the most intractable complication of HZ, and is a complex neuropathy with regarded of peripheral nerve system. Pharmaceutical therapy, including systemic therapy generally with anticonvulsants (gabapentin or pregabalin), tricyclic antidepressants and topical therapy (lidocaine or capsaicin) has long been regarded as the primary option for PHN,29,30 mostly affecting the elderly who has a lower immunity.31 Electroacupuncture, as a non-pharmaceutical therapy, is recognized as a strikingly effective analgesic means against various types of pain,32–34 which bases on traditional acupuncture combined with electrical stimulation. The mechanism of its analgesia involving peripheral and central nerve system has been poorly identified. As is reported, researches34–37reveal that EA exerts inhibitory effect on pain via the interaction and integration between acupuncture signals and pain signals at different levels of the nervous system, with plenty of bioactive substances participating in, such as serotonin,38,39 γ-aminobutyric acid (GABA),40 opioid peptide,41 TRPV1 and P2X3,42,43 and so on.44 Although various clinical experience and previous studies demonstrate that14,45–47 acupuncture will alleviate the intensity of pain in PHN patients and concomitant clinical symptoms of PHN, like insomnia, depression, anxiety, and low quality of life, most exiting trials exhibited poor methodological quality and evidence.12,48,49
Thus, this RCT with rigorous methodological quality is urgently needed to further investigate the effectiveness and safety of EA for PHN and explore whether 2 Hz EA can serve as an alternative therapy of Pregabalin or not. First, we choose SF-MPQ, BPI-SF, MOS-SS, HAMD, HAMA, and SF-36 as outcome measures for PHN participants whose chronic pain of high intensity could disturb their daily life,30 encompassing 4 aspects involving pain, sleep quality, emotion and life quality, which will enable a better overview of participants’ general status comprehensively and systematically. Second, with numerous studies having proved the curative effectiveness of acupuncture for PHN,12,13,47,50,51 our trial will utilize a fixed acupuncture regimen consisted of Jiaji acupoints (EX-B2), local Ashi acupuncture, distal acupuncture on the affected side (LI4, TE5, GB34, GB40) and scalp acupoints (DU24, DU20, EX-HN1). Jiaji acupunctures are located on both sides of the spinal column, 0.5 cun lateral to the lower border of each spinous process, stimulations on which could inhibit the expression of proinflammatory cytokines and Nogo-NgR signal pathway to facilitate nerve growth,52 and awake the cutaneous-visceral reflexes to regulate related nerve and organs.53 It has been reported that54 scalp acupuncture has a targeted stimulation for cerebral cortex function areas that ordinary acupuncture does not have, and could facilitate the balance of physiological functions of the cerebral cortex and regulate the autonomic nervous system, thus scalp acupuncture has more advantages in the treatment of neurological diseases. According to one literature,55 scalp acupuncture plus conventional acupuncture could reduce depressive symptoms significantly which were regarded as the most effective approach for treating Post-stroke depression (PSD) in the 12 types of acupuncture treatment. So scalp acupuncture will be utilized to promote participants’ psychological condition in this trial. Third, as is reported,56 EA analgesia at different frequency was mediated through different endogenous opioid pathways in rats. Moreover, 2 Hz had a better effect on spared nerve injury (SNI) model,57 and in a rat trial,58 researchers observed that stimulation at 2 Hz or 100 Hz both had a reduction of post-incision hyperalgesia, whereas 2 Hz EA last longer, whose finding is similar with our advance trial’s that 2 Hz EA has a better long-lasting analgesic effect. Therefore, we choose 2 Hz as the final frequency.
Conclusion
In conclusion, it’s a study protocol of a multicenter, assessor-blinded, randomized, controlled trial, whose purpose is to explore the effectiveness and safety of EA, compared to medication and whether EA could be an alternative modality of medication for patients with PHN. The research results will prove whether EA could mitigate pain intensity, improve sleep quality and emotion condition. This finding will be a meaningful evidence in clinical practice for treating PHN, and will provide a therapy with fewer side effects for patients with PHN.
Ethics Statement
This trial was approved by the Hangzhou Third People’s Hospital (No. 2021KAO43) and the Third Affiliated Hospital of Zhejiang Chinese Medical University. And this trial will be conducted in accordance with the ethical guidelines of the Declaration of Helsinki. Before enrollment, all individuals in this trial will be asked to write a informed consent. Written informed consent will be obtained from the participants for the publication of this trial and any accompanying images.
Acknowledgments
The authors would like to express their gratitude to the participants who will be involved in this trial. This manuscript is based on the protocol version 3.0 (date 1 January 2022).
Author Contributions
All authors have made a significant contribution to the manuscript, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. And they will also accept the responsibility for the study protocol.
Funding
This trial was financially supported by the Traditional Chinese Medicine Science and Technology Program of Zhejiang (NO:2022ZZ029).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saguil A, Kane S, Mercado M, Lauters R. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2017;96(10):656–663.
2. Hadley GR, Gayle JA, Ripoll J, et al. Post-herpetic neuralgia: a review. Curr Pain Headache Rep. 2016;20(3):17. doi:10.1007/s11916-016-0548-x
3. Watson P. Postherpetic neuralgia. Am Fam Physician. 2011;84(6):690–692.
4. Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31(4):235–243. doi:10.3344/kjp.2018.31.4.235
5. Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of postherpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125–132. doi:10.2147/JPR.S57242
6. Gan EY, Tian EA, Tey HL. Management of herpes zoster and post-herpetic neuralgia. Am J Clin Dermatol. 2013;14(2):77–85. doi:10.1007/s40257-013-0011-2
7. Gross GE, Eisert L, Doerr HW, et al. S2k guidelines for the diagnosis and treatment of herpes zoster and postherpetic neuralgia. J Dtsch Dermatol Ges. 2020;18(1):55–78.
8. Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine. 2018;36(33):5037–5045. doi:10.1016/j.vaccine.2018.07.005
9. Kelly RB, Willis J. Acupuncture for pain. Am Fam Physician. 2019;100(2):89–96.
10. Vickers AJ, Linde K. Acupuncture for chronic pain. JAMA. 2014;311(9):955–956. doi:10.1001/jama.2013.285478
11. Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120(2):482–503. doi:10.1097/ALN.0000000000000101
12. Pei W, Zeng J, Lu L, Lin G, Ruan J. Is acupuncture an effective postherpetic neuralgia treatment? A systematic review and meta-analysis. J Pain Res. 2019;12:2155–2165. doi:10.2147/JPR.S199950
13. Ruengwongroj P, Muengtaweepongsa S, Patumanond J, Phinyo P. Effectiveness of press needle treatment and electroacupuncture in patients with postherpetic neuralgia: a matched propensity score analysis. Complement Ther Clin Pract. 2020;40:101202. doi:10.1016/j.ctcp.2020.101202
14. Wang L, Qiu L, Zheng X, et al. Effectiveness of electroacupuncture at Jiaji acupoints (EX-B2), plus moxibustion and intermediate on postherpetic neuralgia: a randomized controlled trial. J Tradit Chin Med. 2020;40(1):121–127.
15. Du J, Fang J, Xiang X, et al. Effects of low- and high-frequency electroacupuncture on protein expression and distribution of TRPV1 and P2X3 in rats with peripheral nerve injury. Acupunct Med. 2020;39(5):478–490.
16. Fang J, Du J, Xiang X, et al. SNI and CFA induce similar changes in TRPV1 and P2X3 expressions in the acute phase but not in the chronic phase of pain. Exp Brain Res. 2021;239(3):983–995. doi:10.1007/s00221-020-05988-4
17. Liang Y, Du JY, Qiu YJ, Fang JF, Liu J, Fang JQ. Electroacupuncture attenuates spinal nerve ligation-induced microglial activation mediated by p38 mitogen-activated protein kinase. Chin J Integr Med. 2016;22(9):704–713. doi:10.1007/s11655-015-2045-1
18. Yu J, Du J, Fang J, et al. The interaction between P2X3 and TRPV1 in the dorsal root ganglia of adult rats with different pathological pains. Mol Pain. 2021;17:17448069211011315. doi:10.1177/17448069211011315
19. Hu H, Shen Y, Li X, et al. Efficacy of electroacupuncture therapy in patients with postherpetic neuralgia: study protocol for a multicentre, randomized, controlled, assessor-blinded trial. Front Med. 2021;8:624797. doi:10.3389/fmed.2021.624797
20. Han JS. Further proof of the specificity of acupuncture analgesia frequency. Zhen Ci Yan Jiu. 2001;26(03):224–227. doi:10.3969/j.issn.1000-0607.2001.03.045
21. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011 Mar;152(3 Suppl):S41–S48. doi:10.1016/j.pain.2010.10.012. PMID: 21078546
22. Han JS. [Research on Acupuncture Anesthesia-analgesia]. Zhen Ci Yan Jiu. 2016 Oct 25;41(5):377–87. Chinese. PMID: 29071939
23. Ju ZY, Wang K, Cui HS, et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;12(12):Cd012057. doi:10.1002/14651858.CD012057.pub2
24. Macone A, Otis JAD. Neuropathic Pain. Semin Neurol. 2018;38(6):644–653. doi:10.1055/s-0038-1673679
25. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi:10.1016/j.ijsu.2011.10.001
26. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi:10.1142/S0192415X20500135
27. Nalamachu S, Morley-Forster P. Diagnosing and managing postherpetic neuralgia. Drugs Aging. 2012;29(11):863–869. doi:10.1007/s40266-012-0014-3
28. Liqin Zhang, Feng Ding, Ying Jiao. Clinical efficacy of Guishao Granules, bloodletting cupping combined with pregabalin in patients with postherpetic neuralgia. Chinese Patent Medicine. 2021;43(2):560–562. doi:10.3969/j.issn.1001-1528.2021.02.056
29. Johnson RW, Rice AS, Solomon CG. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371(16):1526–1533. doi:10.1056/NEJMcp1403062
30. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274–280. doi:10.4065/84.3.274
31. Forbes HJ, Thomas SL, Smeeth L, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157(1):30–54. doi:10.1097/j.pain.0000000000000307
32. Liang Q, Zhang K, Wang S, et al. Acupuncture for cancer pain - an adjuvant therapy for cancer pain relief. Am J Chin Med. 2020;48(8):1769–1786. doi:10.1142/S0192415X20500883
33. Lin JG, Chen WL. Review: acupuncture analgesia in clinical trials. Am J Chin Med. 2009;37(1):1–18. doi:10.1142/S0192415X09006679
34. Qiao L, Guo M, Qian J, Xu B, Gu C, Yang Y. Research Advances on Acupuncture Analgesia. Am J Chin Med. 2020;48(2):245–258.
35. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41–s48. doi:10.1016/j.pain.2010.10.012
36. Wang SM, Kain ZN, White P. Acupuncture analgesia: i. The scientific basis. Anesth Analg. 2008;106(2):602–610. doi:10.1213/01.ane.0000277493.42335.7b
37. Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–375. doi:10.1016/j.pneurobio.2008.05.004
38. Zhang Y, Li A, Xin J, et al. Involvement of spinal serotonin receptors in electroacupuncture anti-hyperalgesia in an inflammatory pain rat model. Neurochem Res. 2011;36(10):1785–1792. doi:10.1007/s11064-011-0495-1
39. Zhang Y, Zhang RX, Zhang M, et al. Electroacupuncture inhibition of hyperalgesia in an inflammatory pain rat model: involvement of distinct spinal serotonin and norepinephrine receptor subtypes. Br J Anaesth. 2012;109(2):245–252. doi:10.1093/bja/aes136
40. Wang JY, Bai WZ, Gao YH, Zhang JL, Duanmu CL, Liu JL. GABAergic inhibition of spinal cord dorsal horns contributes to analgesic effect of electroacupuncture in incisional neck pain rats. J Pain Res. 2020;13:1629–1645. doi:10.2147/JPR.S242330
41. Han JS. Acupuncture and endorphins. Neurosci Lett. 2004;361(1–3):258–261. doi:10.1016/j.neulet.2003.12.019
42. Fang JQ, Du JY, Fang JF, et al. Parameter-specific analgesic effects of electroacupuncture mediated by degree of regulation TRPV1 and P2X3 in inflammatory pain in rats. Life Sci. 2018;200:69–80. doi:10.1016/j.lfs.2018.03.028
43. Tang Y, Yin HY, Liu J, Rubini P, Illes P. P2X receptors and acupuncture analgesia. Brain Res Bull. 2019;151:144–152. doi:10.1016/j.brainresbull.2018.10.015
44. Chen T, Zhang WW, Chu YX, Wang YQ. Acupuncture for pain management: molecular mechanisms of action. Am J Chin Med. 2020;48(4):793–811. doi:10.1142/S0192415X20500408
45. Zhou Q, Wei S, Zhu H, et al. Acupuncture and moxibustion combined with cupping for the treatment of post-herpetic neuralgia: a meta-analysis. Medicine. 2021;100(31):e26785. doi:10.1097/MD.0000000000026785
46. Wu CH, Lv ZT, Zhao Y, et al. Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia. Mol Pain. 2013;9:18. doi:10.1186/1744-8069-9-18
47. Ma K, Zhou QH, Xu YM, et al. Peripheral nerve adjustment for postherpetic neuralgia: a randomized, controlled clinical study. Pain Med. 2013;14(12):1944–1953. doi:10.1111/pme.12254
48. Li X, Wang R, Shi X, et al. Reporting characteristics and risk of bias in randomised controlled trials of acupuncture analgesia published in PubMed-listed journals. Acupunct Med. 2017;35(4):259–267. doi:10.1136/acupmed-2016-011149
49. Liu K, Zeng J, Pei W, et al. Assessing the reporting quality in randomized controlled trials of acupuncture for postherpetic neuralgia using the CONSORT statement and STRICTA guidelines. J Pain Res. 2019;12:2359–2370. doi:10.2147/JPR.S210471
50. Ursini T, Tontodonati M, Manzoli L, et al. Acupuncture for the treatment of severe acute pain in herpes zoster: results of a nested, open-label, randomized trial in the VZV pain study. BMC Complement Altern Med. 2011;11(1):46. doi:10.1186/1472-6882-11-46
51. Zou J, Dong X, Wang K, Shi J, Sun N. Electroacupuncture inhibits autophagy of neuron cells in postherpetic neuralgia by increasing the expression of miR-223-3p. Biomed Res Int. 2021;2021:6637693. doi:10.1155/2021/6637693
52. Xu Q, Yang JW, Cao Y, et al. Acupuncture improves locomotor function by enhancing GABA receptor expression in transient focal cerebral ischemia rats. Neurosci Lett. 2015;588:88–94. doi:10.1016/j.neulet.2014.12.057
53. Cabioglu MT, Arslan G. Neurophysiologic basis of Back-Shu and Huatuo-Jiaji points. Am J Chin Med. 2008;36(3):473–479. doi:10.1142/S0192415X08005916
54. Liu FG, Tan AH, Peng CQ, Tan YX, Yao MC. Efficacy and safety of scalp acupuncture for insomnia: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2021;2021:6621993. doi:10.1155/2021/6621993
55. Hang X, Li J, Zhang Y, et al. Efficacy of frequently-used acupuncture methods for specific parts and conventional pharmaceutical interventions in treating post-stroke depression patients: a network meta-analysis. Complement Ther Clin Pract. 2021;45:101471. doi:10.1016/j.ctcp.2021.101471
56. Qi D, Wu S, Zhang Y, Li W. Electroacupuncture analgesia with different frequencies is mediated via different opioid pathways in acute visceral hyperalgesia rats. Life Sci. 2016;160:64–71. doi:10.1016/j.lfs.2016.06.025
57. Du J, Fang J, Xiang X, et al. Effects of low- and high-frequency electroacupuncture on protein expression and distribution of TRPV1 and P2X3 in rats with peripheral nerve injury. Acupunct Med. 2021;39(5):478–490. doi:10.1177/0964528420968845
58. Fais RS, Reis GM, Silveira JW, Dias QM, Rossaneis AC, Prado WA. Amitriptyline prolongs the antihyperalgesic effect of 2- or 100-Hz electro-acupuncture in a rat model of post-incision pain. Eur J Pain. 2012;16(5):666–675. doi:10.1002/j.1532-2149.2011.00034.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.