Back to Journals » Journal of Blood Medicine » Volume 10
Efficacy of EHL N9-GP for on-demand treatment of bleeding episodes in hemophilia B: analysis of pivotal trial data
Authors Escobar MA , Walsh CE, Cooper DL , Young G
Received 19 April 2019
Accepted for publication 28 June 2019
Published 25 July 2019 Volume 2019:10 Pages 243—250
DOI https://doi.org/10.2147/JBM.S212690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Miguel A Escobar,1 Christopher E Walsh,2 David L Cooper,3 Guy Young4
1Department of Internal Medicine, University of Texas Health Science Center at Houston—McGovern Medical School, Houston, TX, USA; 2Icahn School of Medicine at Mount Sinai, New York, NY, USA; 3Clinical Development and Medical Affairs - Biopharm, Novo Nordisk Inc, Plainsboro, NJ, USA; 4Hemostasis and Thrombosis Center, Children’s Hospital Los Angeles, University of Southern California Keck School of Medicine, Los Angeles, CA, USA
The safety and efficacy of N9-GP (nonacog beta pegol; Novo Nordisk A/S, Bagsværd, Denmark), a recombinant glycoPEGylated factor IX(FIX) with extended half-life (EHL),1 was investigated in the multinational, Phase 3, paradigm 2 trial (NCT01333111), previously reported by Collins et al in Blood.2 The trial was conducted in accordance with the Declaration of Helsinki and written informed consent was provided by all participants. Prior to trial initiation, the protocol, the protocol amendments, the consent form, and the patient information sheet were reviewed and approved according to local regulations by appropriate health authorities and by independent ethics committees/institutional review boards (see Table S1). Patients 13–70 years of age with previously treated hemophilia B (≤2% baseline FIX) were allocated to either once-weekly prophylaxis or on-demand (OD) treatment. TheODtreatment was a US Food andDrugAdministration requirement prior to enrolling patients on prophylaxis in the USA.
The safety and efficacy of N9-GP (nonacog beta pegol; Novo Nordisk A/S, Bagsværd, Denmark), a recombinant glycoPEGylated factor IX (FIX) with extended half-life (EHL),1 was investigated in the multinational, Phase 3, paradigm 2 trial (NCT01333111), previously reported by Collins et al in Blood.2 The trial was conducted in accordance with the Declaration of Helsinki and written informed consent was provided by all participants. Prior to trial initiation, the protocol, the protocol amendments, the consent form, and the patient information sheet were reviewed and approved according to local regulations by appropriate health authorities and by independent ethics committees/institutional review boards (see Table S1). Patients 13–70 years of age with previously treated hemophilia B (≤2% baseline FIX) were allocated to either once-weekly prophylaxis or on-demand (OD) treatment. The OD treatment was a US Food and Drug Administration requirement prior to enrolling patients on prophylaxis in the USA.
While much of the focus on EHL FIX products has been around prophylaxis, some patients with severe hemophilia, and the majority with mild/moderate hemophilia, are treated OD. Recent epidemiologic studies have demonstrated that patients with FIX levels up to 15–20% may experience bleeding.3,4 Thus, renewed interest in the potential use of N9-GP as a single-dose OD treatment prompted a post hoc patient-level analysis from the paradigm 2 trial to describe the dosing and efficacy of N9-GP as an OD treatment.
Fifteen of 74 patients in paradigm 2 were enrolled to OD treatment, consisting of a single 40-IU/kg dose of N9-GP, with additional doses of 40 IU/kg as required. Thirteen patients (86.7%) had severe hemophilia (FIX <1 IU/dL) and 2 (13.3%) had moderate hemophilia (FIX 1–2 IU/dL). Twelve patients (80.0%) had target joint (TJ) bleeds at baseline. The study duration of OD treatment in paradigm 2 was 28 weeks.
Fourteen patients reported 143 bleeds, all mild/moderate in severity (Table 1). Most bleeds (83.9%) were treated with a single dose of N9-GP, and the remainder with 2 or more doses (Table 1). Considering patient-level data, seven patients had all 62 of their bleeds (100%) treated with 1 dose. Annualized bleeding rates in these patients were reduced by 37% from 26.1 prestudy to 16.5 on-study. Hemostatic response was rated excellent for 36 and good for 26 bleeds.
 |
Table 1 OD treatment of bleeds by the number of N9-GP doses |
The other seven patients had at least one bleed treated with 2 or more doses of N9-GP. While 28.4% of their bleeds were treated with 2 or more doses (hemostatic response: 18, good; 5, moderate), 71.6% were still treated with only 1 dose (hemostatic response: 7, excellent; 49, good; 1, moderate; 1, not reported).
Two of the 7 patients experienced 11 recurrent TJ bleeds, 4 (36.4%) of which required treatment with 2 or more doses of N9-GP. One patient, 18 years of age, reported three elbow TJ bleeds in 2 months, which were treated with 1, 5, and 2 doses of N9-GP, respectively. The second patient, 27 years of age, was treated prophylactically with plasma-derived FIX (pdFIX) 100 IU/kg every 3 days prior to entering the OD arm of paradigm 2. He reported two bleeds in 2 weeks in his right ankle TJ; one TJ bleed was treated with 2 doses (1 for early rebleeding) and the second bleed was treated with 6 doses, after which the patient was withdrawn.
The other 5 of the 7 patients experienced 70 bleeds; 72.9% were treated with 1 dose (hemostatic response: 2, excellent; 47, good; 1, moderate; 1, not reported) and 27.1% with 2 or more doses (hemostatic response: 17, good; 2, moderate). Four of these five patients had previously received 2 or 3 high FIX doses (60–81 IU/kg) for treating a bleed before entering the study and reported 63 bleeds while on study, which accounted for 44% of all bleeds in the OD treatment arm (Figure 1). The average N9-GP dose in these four patients ranged from 41.7 to 71.1 IU/kg per bleed and FIX utilization was reduced by 56–80% compared with the patient’s historical FIX utilization (Figure 1). The fifth patient was treated prestudy with 1 prescribed infusion of 34 IU/kg pdFIX per bleed with unknown effectiveness and had seven bleeds while on study (6 treated with 1 dose of ~40 IU/kg per protocol and 1 with 2 doses; mean 46.8 IU/kg per bleed).
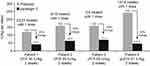 |
Figure 1 Treatment of patients with a history of requiring multiple high-dose FIX. Abbreviations: FIX, factor IX; pdFIX, plasma-derived factor IX; rFIX, recombinant factor IX. |
This post hoc analysis from paradigm 2 supports an important potential role for N9-GP in the OD treatment of mild/moderate bleeds. Modeling based upon the Phase 1 pharmacokinetic study to achieve recommended FIX levels suggested by the World Federation of Hemophilia1,5 indicated that N9-GP could potentially reduce the number of doses and total FIX utilization compared with recombinant FIX (rFIX) or pdFIX: 1 vs 2 doses for mild/moderate bleeds (40 vs 95–110 IU/kg), 1 vs 6 doses for severe bleeds (80 vs 310–350 IU/kg), and 5 vs 28 doses for intracranial hemorrhage (240 vs 1450–1490 IU/kg).6
The recent Bridging Hemophilia Experiences Results and Opportunities into Solutions (B-HERO-S) study in patients with mild-moderate-severe hemophilia in the USA reported that increasing education about self‐infusion may be of benefit to individuals, particularly those with mild/moderate hemophilia: treatment is typically given at a hemophilia clinic or hospital and/or patients need assistance from family members or health care professionals; fewer than 10% of patients reported that all their infusions were at home.7 Delays in recognizing bleeds or receiving help with infusions may also impact outcomes over time. Specifically, B-HERO-S showed that pain, functional impairment, and anxiety/depression were present at higher-than-expected levels in patients with mild/moderate hemophilia B, including affected women, suggesting unmet needs in the management of this population and perhaps undertreatment of bleeding episodes.8 Coupled with improved education to increase the recognition of bleeds, the ability to treat most bleeds with 1 dose with sustained FIX activity, within the World Federation of Hemophilia guidelines5 recommendations over many days, offers a potential pathway to improved outcomes.
In conclusion, a single 40 IU/kg dose of N9-GP was effective as an OD treatment for most bleeds in patients with hemophilia B investigated in paradigm 2. For patients who required additional N9-GP doses, the majority had either recurrent TJ bleeds or a history of multiple high-dose treatment. A prolonged duration of treatment after bleeding and potential change to routine prophylaxis is typical for patients with recurrent TJ bleeds. For patients who may not have required additional dosing of rFIX/pdFIX based upon their phenotype or individual pharmacokinetics, the paradigm 2 analysis supports the predictive modeling that a change to N9-GP would likely be associated with fewer infusions and FIX utilization than rFIX/pdFIX.
Data sharing statement
Novo Nordisk’s policy on data sharing may be found at https://www.novonordisk-trials.com/how-access-clinical-trial-datasets.
Acknowledgments
The paradigm 2 trial was sponsored by Novo Nordisk A/S (Bagsværd, Denmark) and is registered with ClinicalTrials.gov (NCT01333111). The sponsor was responsible for trial operations, including data analysis. The authors acknowledge the medical writing assistance of Vathsala Jayanth (Parexel), which was funded by Novo Nordisk A/S.
Author contributions
MAE, CEW, and GY were principal investigators and enrolled and cared for patients during the trial. All authors designed the trial protocol, directed the data analysis, and wrote the manuscript. All authors had access to the primary clinical trial data. All authors were involved in interpretation of the trial results and preparation of the manuscript outline, provided input during the review stages, and approved the final manuscript. The authors assume full responsibility for the accuracy and completeness of the reported data, and agree to be accountable for all aspects of the work.
Disclosure
MAE reports research funding from Pfizer and consulting fees from CSL Behring, Genentech, Roche, Hemabiologics, Novo Nordisk, Pfizer, and Shire. MAE participated in advisory boards, consultation, and educational talks and received personal fees from Sanofi, Novo Nordisk, Takeda and CSL Behring. DLC is an employee of Novo Nordisk Inc. GY reports honoraria and consultancy fees from Alnylam, Bioverativ, CSL Behring, Genentech/Roche, Grifols, Kedrion, Novo Nordisk, and Shire. He also reports personal fees from Bioverativ/Sanofi, CSL Behring, Genentech/Roche, Grifols, Kedrion, Novo Nordisk, Spark, Takeda, and UniQure, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Negrier C, Knobe K, Tiede A, Giangrande P, Moss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118(10):2695–2701. doi:10.1182/blood-2011-02-335596
2. Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124(26):3880–3886. doi:10.1182/blood-2014-05-573055
3. Den Uijl IE, Fischer K, Van Der Bom JG, et al. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44. doi:10.1111/j.1365-2516.2010.02383.x
4. Soucie JM, Monahan PE, Kulkarni R, Konkle BA, Mazepa MA. The frequency of joint hemorrhages and procedures in nonsevere hemophilia A vs B. Blood Adv. 2018;2(16):2136–2144. doi:10.1182/bloodadvances.2018020552
5. Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. doi:10.1111/j.1365-2516.2012.02909.x
6. Collins PW, Møss J, Knobe K, et al. Population pharmacokinetic modeling for dose setting of nonacog beta pegol (N9-GP), a glycoPEGylated recombinant factor IX. J Thromb Haemost. 2012;10(11):2305–2312. doi:10.1111/jth.12000
7. Buckner TW, Witkop M, Guelcher C, et al. Management of US men, women, and children with hemophilia and methods and demographics of the Bridging Hemophilia B Experiences, Results and Opportunities into Solutions (B-HERO-S) study. Eur J Haematol. 2017;98 Suppl 86(Suppl 86):5–17. doi:10.1111/ejh.12854
8. Buckner TW, Witkop M, Guelcher C, et al. Impact of hemophilia B on quality of life in affected men, women, and caregivers-assessment of patient-reported outcomes in the B-HERO-S study. Eur J Haematol. 2018;100(6):592–602. doi:10.1111/ejh.13055
Supplementary material
 |  |  |  |
Table S1 List of independent ethics committees or institutional review boards that approved the paradigm 2 trial (NCT01333111) |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
