Back to Journals » Journal of Pain Research » Volume 15
Efficacy of Dry Needling Under EMG Guidance for Myofascial Neck and Shoulder Pain: A Randomized Clinical Trial
Authors Liu Q , Huang Q , Liu L, Nguyen TT
Received 24 April 2022
Accepted for publication 23 July 2022
Published 8 August 2022 Volume 2022:15 Pages 2293—2302
DOI https://doi.org/10.2147/JPR.S372074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Qingguang Liu,1 Qiangmin Huang,2,3 Lin Liu,4 Thi-Tham Nguyen5
1International College of Football, Tongji University, Shanghai, People’s Republic of China; 2Department of Sport Rehabilitation, School of Kinesiology, Shanghai University of Sport, Shanghai, People’s Republic of China; 3Shanghai Ciyuan Rehabilitation Hospital, Sinopharm Healthcare, Shanghai, People’s Republic of China; 4Department of Rehabilitation, School of Sport and Health, Nanjing Sport Institute, Nanjing, People’s Republic of China; 5Faculty of Sport Science, Ton Duc Thang University, Ho Chi Minh City, Vietnam
Correspondence: Qiangmin Huang, Keyanlou 4-408, Hengren Road No. 188, Yangpu District, Shanghai, 200438, People’s Republic of China, Tel +8602155135072, Fax +8602155135076, Email [email protected]
Purpose: To determine the difference in maintenance of improvement of pain and disability for dry needling (DN) under needle electromyography (EMG) guidance technique in myofascial neck and shoulder pain patients, compared with DN alone.
Patients and Methods: In this randomized single-blind clinical trial, 30 participants with myofascial pain in the neck and shoulder were randomly allocated to two groups: myofascial trigger points (MTrPs) DN with EMG guided (DN-EMG) group and MTrPs DN without EMG (DN) group. Needling treatment lasted for 2 weeks, twice a week. The primary outcome was pain intensity as assessed by visual analogue scale (VAS) and neck disability index (NDI). A number of mappings referred to pain and spontaneous muscle activity (SEA) were considered secondary outcomes. VAS and NDI were measured before treatment, after 2 weeks of intervention and at 4-, 6- and 12-week follow-up periods after the intervention. Secondary outcomes were assessed before each treatment (T1–T4). Data were analysed using mixed-model analyses of variance (ANOVA) with time as a within-subject variable and groups as between-subject variables followed by Bonferroni’s post-hoc test.
Results: Mixed-model ANOVA revealed significant time-by-group interaction effects (F = 3.49, P = 0.01) for VAS. Post-hoc analysis showed a significant decrease in VAS and NDI after 2 weeks of intervention and at all follow-up periods compared with baseline in both groups (p < 0.01). The DN-EMG group exhibited higher improvements in VAS at 6- and 12-week follow-up period than the DN group (p < 0.05). In the SEA of MTrPs, we found positive sharp waves, fibrillation and fascicular potentials. DN-EMG group exhibited lower amplitudes at T2–T4 and frequencies at T2 and T3.
Conclusion: DN under needle EMG guidance technique exhibited greater improvements in maintenance of improvement of pain and lower SEA value than the DN group due to sufficient MTrPs inactivation.
Keywords: myofascial trigger point, muscle pain, needling
Introduction
Neck and shoulder pain (NSP) is a common musculoskeletal pain syndrome, affecting more than 2/3 of the population at some stage over lifespan.1 NSP is mainly manifested as pain in the neck and shoulders, sometimes accompanied by upper limb pain or persistent tension headache, dizziness and limited range of motion with neck and shoulder dysfunction. Myofascial trigger points (MTrPs) are defined as a hyperirritable spot in a palpable taut band of skeletal muscle fibers; they could give rise to characteristic-referred pain, motor dysfunction and autonomic phenomena.2 MTrPs may be provoked by trauma, overuse, postural faults, nutritional deficiencies, emotional psychological stress, degeneration or endocrine and metabolic deficiencies.2
Numerous studies have shown that MTrPs are prevalent in patients with chronic musculoskeletal pain.3–8 In the sedentary lifestyle in today’s societies with too much time in static postures, phasic muscles become progressively inhibited and lax, while postural muscles gradually become tighter. A muscle imbalance between dynamic and postural muscles may lead to MTrPs in the cervical region.9 The upper trapezius (UT) muscle has been found to be often affected by MTrPs.10–12 Considering the role of the UT muscle in the scapulohumeral rhythm during shoulder movement, the MTrPs in the UT muscle could result in altered scapulohumeral rhythm and shoulder dysfunction and disability. The MTrPs at the UT muscle are the important factors that cause NSP.13
Conservative interventions for MTrPs include dry needling (DN), wet needling (eg lidocaine injection and some local anesthetic injections), ischemic compression, laser and oral drugs.14 Of these therapies, DN has been widely used in clinical practice because of its simple operation and good efficacy.15 The objectives of DN include inactivating the MTrPs, normalizing the chemical environment of MTrPs, releasing muscle shortening, removing the source of muscle irritation, normalizing peripheral nerve sensitization, promoting self-healing of the injured tissue, and decreasing spontaneous muscle activity (SEA).16
The diagnosis of MTrPs has always been a difficult problem; the main methods for detecting MTrPs are as follows: thumb palpation, tenderness threshold detected by pain detector, ultrasound imaging, electromyography (EMG), magnetic resonance elastography and infrared thermography.17 Needle EMG studies have found that abnormal SEA in MTrPs18,19 and DN treatment could effectively reduce SEA at MTrPs,20,21 while DN at the exact MTrPs seems more effective than needling at non-MTrPs.22 The use of EMG could help find MTrPs more accurately and observe the inactivation status of MTrPs in real time.
A systematic review recommended DN for relieving MTrPs pain in the NSP in the short term.23 It is not clear whether the poor long-term effect of DN is caused by incomplete inactivation of MTrPs. At the same time, there is no unified conclusion on how many times of DN at MTrPs will have better curative effect. The use of EMG could help find MTrPs more accurately and observe the inactivation status of MTrPs in real time. Therefore, this study aimed to investigate whether DN under needle EMG guidance treatment techniques leads to a better long-term treatment effect due to sufficient MTrPs inactivation.
Patients and Methods
A total of 30 participants were recruited from the Shangti Orthopedic Hospital and the Pain Department in Hudong Hospital between 2017 September and May 2018. The participants were randomly divided into two groups: MTrPs DN (DN) group and MTrPs DN with EMG guided (DN-EMG) group. The research was performed by five independent researchers. Randomisation was performed using a computerised random number generator by researcher one. Individual and sequentially numbered index cards with random assignment were prepared. The index cards were folded and placed in sealed opaque envelopes. Another researcher (researcher two), opened the envelope and proceeded with allocation. Researcher three will be in charge of recruitment of patients and collecting the EMG data. Researcher four will administer the treatment. Researcher five will be in charge of the statistical analysis of the VAS, NDI and EMG data. The participants were blinded to the type of treatment as they could not observe the collection of EMG. Researchers three and five were also blinded to both the group allocation and type of treatment. Researcher four was not blinded as to observe the changes in EMG in real time in the DN-EMG group.
The characteristics of the participants in the study groups were assessed at baseline, and no significant differences were found in age, gender, height, weight and body mass index between the two groups at baseline (Table 1).
 |
Table 1 Demographic Data of the Subjects (Mean ± SD) |
Inclusion Criteria
The MTrPs of the UT were diagnosed in accordance with the criteria initially established by Travell and Simons 2: (1) a palpable, hypersensitive tender spot in a taut band, (2) local twitch response (LTR) on muscle palpation and (3) referred and spontaneous pain elicited by firm compression. The participants were examined and treated by a clinician with more than 20 years of experience in the diagnosis and management of MTrPs.
The inclusion criteria included the following:
- NSP of at least 3 cm on a visual analogue scale (VAS) from 0 cm (no pain) to 10 cm (worst imaginable pain) and more than 3 months;
- At least three MTrPs in the UP muscle;
- Having no treatment with acupuncture previously and having poor response to previous conservative and non-invasive treatments, such as oral medicine or physical therapy.
Exclusion Criteria
The exclusion criteria for the selection of patients included the following:
- Having conditions with contraindication for needling, such as local infection, malignancy, serious medical problems, taking anticoagulant medicine and pregnancy;
- Taking medicine that may change pain intensities, such as analgesics, sedatives and substance abuse (including alcohol and narcotics);
- Having previous surgery or trauma to the neck, upper back or upper limb;
- Having evidence of cognitive deficit.
Either the inclusion or exclusion criteria were assessed by the same doctor on the basis of detailed medical history and careful physical examination.
Study Design and Ethical Approval
This randomized single-blinded clinical trial was designed and reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement, approved by the Ethics Committee of Shanghai University of Sport (approval code: 2,015,026), and it was prospectively registered (www.chictr.org.cn; ChiCTR-IPR-17010764), and this study was carried out in accordance with the World Medical Association’s Declaration of Helsinki (1964). The purpose, protocol and procedures of the trial were demonstrated to all participants. Informed consent forms were signed.
Sample Size Calculation
The sample size estimation was based on the average pain scores of the pre-experiment, and it was performed by using the G*Power 3.1.9.7 software (Düsseldorf, Germany). Assuming a two-tailed Wilcoxon signed-rank test at the 0.05 significance level with a statistical power of 85%, this analysis showed that 12 subjects would be required in each group. Considering the risk of dropouts, an expected sample loss of 20% were considered. The sample size calculation resulted in a sample of 15 participants per group, totaling 30 participants.
Interventions
MTrPs were identified and marked on the skin with an indelible black marker. Before needling, the skin was swabbed with alcohol wipes. DN procedures were performed by the same acupuncturist by using Hong’s fast-in, fast-out technique until an LTR was achieved24 and a Ф0.35 mm × 0.75 mm sterilised stainless-steel needle (Wujiang Jiachen Acupuncture Equipment Co., Ltd., Jiangsu, China). Immediately after needling, compression was applied to the treated MTrPs for 15 s.
In the DN group, the needle was inserted into the skin at the MTrPs area until the first LTR was obtained, and then the needle was moved up and down at approximately 1 Hz until no more LTR was elicited. In the DN-EMG group, the grounding surface electrode and the reference electrode were placed similarly to those in the DN group. However, the recording electrode was clamped at the end of the sterilised stainless-steel needle for acupuncturists to observe the changes in EMG in real time and finish the treatment after the EMG waveform disappeared. The MTrPs in other parts of the trapezius muscle were treated in the same manner in each group. All patients received two treatment sessions every week for 2 weeks.
Outcome Measurements
The primary outcomes were pain intensity and neck disability. The numbers of mappings referred to pain and SEA were considered secondary. Primary outcome measures were performed at baseline, after 2 weeks of intervention, and 4, 6, and 12 weeks follow-up after the intervention (W0–W4), and secondary outcome measures were performed before each treatment (T1–T4). Outcomes were measured by an external person not directly involved in the trial.
1. Pain intensity was measured using the VAS created by Scott and Huskisson.25 The VAS is a simple, sensitive and reproducible instrument that is often considered as a measure of the efficacy of pain treatment. In this study, a 10 cm VAS for pain was used. The subjects were asked to state their pain level by placing a mark on a horizontal line of 10 cm. The score on the 10 cm scale ranged from 0 cm (no pain) to 10 cm (worst imaginable pain).
2. Neck disability was measured using the neck disability index (NDI), which is the most widely used instrument for assessing related disability in patients with neck pain.26 It consists of 10 questions with six possible responses, each of which was assigned a different number of points, from 0 (no disability) to 5 (complete disability). The total NDI score could range from 0 to 50 points, where higher scores represent higher levels of disability.27
3. In this study, the participants were asked to describe the referred pain pattern by using an anatomical map of the upper half of the human body. The designated areas within the head and neck, the upper back, the shoulder and the arm, which may all encompass pain referred there by MTrPs. The anatomical map was divided into 34 areas described by Travell and Simons.2 A number of areas of pain were evaluated.
4. SEA records were measured with an EMG equipment (NeuroCare-E, NCC Medical Co., Ltd., Shanghai, China, sampling frequency at 50,000 Hz) with three fine-needle electrodes (Ф = 0.3 mm). A band with a filter was set at 20–3000 Hz. The gain was set at 10 μV/division. The sweep speed was 10 ms/division. The grounding surface electrode was attached to the acromial bone marker. The reference electrode was inserted into the taut band of the UT by palpation. A local twitch response indicated a possible MTrPs. Thus, the recording electrode was inserted longitudinally approximately 3−5 mm away from the second electrode. The examiner needed to move the needle in different directions to obtain the SEA with the highest amplitude. The EMG of the confirmed SEA was recorded for 2 min and the average peak-to-peak value within groups was calculated.
Data Analysis
SPSS version 22.0 (IBM, Armonk, NY, USA) for Windows was used for statistical analysis. Descriptive statistics (means ± standard deviation) were calculated for all parameters. Shapiro–Wilk test was used to test normality considering all participants were together, and each group was independently assessed. A mixed-design ANOVA, with time (W0–W4) as the within-subject factor and groups (DN and DN-EMG) as the between-subject factor, was used to determine the effects of the intervention on VAS and NDI. For SEA and the number of pain area parameters, a mixed-design ANOVA was applied, with time (T1–T4) as within-subject factors and groups (DN and DN-EMG) as a between-subject factor. In case of significant factors or interactions, the Bonferroni test was used for post-hoc comparisons. p-value <0.05 was considered to be statistically significant.
Results
The participant flow diagram presented in Figure 1 shows the numbers and timing of randomization assignment, interventions and measurements for each group.
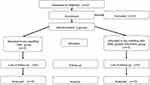 |
Figure 1 Flow diagram of patients throughout the course of the study. |
No statistically significant differences were found in the age, BMI, and duration of NSP and VAS between the groups before any intervention (P ≥ 0.05), as shown in Table 1.
Assessment of Pain Intensity and Neck Disability
The mixed-model ANOVA revealed significant time-by-group interaction effects (F = 3.49, P = 0.01) for VAS. However, no significant time-by-group interaction effects were observed for NDI (p > 0.05). Post-hoc analysis showed a significant decrease in VAS and NDI after 2 weeks of intervention and 4, 6, and 12-week follow-up compared with baseline in both groups (p < 0.01, Table 2). Post-hoc tests also revealed a significant increase in VAS in W4 compared with that in W1 (p < 0.01). The DN-EMG group exhibited higher improvements in VAS at 6- and 12-week follow-up periods than the DN group (p < 0.05, Table 2). No difference was observed for NDI between the two groups.
 |
Table 2 Mean ± SD of the Pre- and Post-Measurement Scores for All Groups |
Assessment of SEA and Referred Pain Area
SEA were found in both groups.
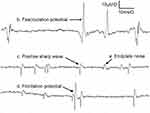
a. Endplate noise is a low-amplitude monophasic negative potential occurring in a dense steady pattern point with an amplitude of 10–20 μV, short duration (0.5–1.0 ms) (Figure 2). Graded non-propagated membrane potential is induced in the postsynaptic membrane of muscle fibers via release of acetylcholine from the presynaptic axon terminal in response to an action potential.28
b. Positive sharp wave starts with a positive direction, followed by a negative going wave (Figure 2). This wave is often present in the early stages of muscle denervation or myogenic damage.
c. Fibrillation potentials are triphasic, with an initial positivity followed by a long-duration negative phase (Figure 2). Fibrillation potentials strongly suggest denervation, but they have been observed in polymyositis, muscular dystrophy and botulism, which are all thought to be spontaneous discharges from acetylcholine-hypersensitive denervated muscle fibers or may result from muscle necrosis, muscle inflammation and focal muscle degeneration.29
d. The fasciculation potential shows a triphasic, small positive–negative–small positive potential. Any traumatic injury causing lasting compression or damage to nerves in or near muscles could increase a patient’s chances of having fasciculations (Figure 2).
In the DN and DN-EMG groups, the local twitch potentials of muscles with different amplitudes were detected when dry needling induced LTR (Figure 3).
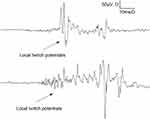 |
Figure 3 Local twitch response. |
The mixed-model ANOVA revealed significant time-by-group interaction effects (Famplitudes = 4.78, P = 0.009; Ffrequencies = 7.66, P < 0.001) for SEA. Post-hoc analysis showed a significant decrease in the amplitudes and frequencies of SEA in T2–T4 (p < 0.05, Figure 4). However, the DN-EMG group exhibited higher improvements in amplitudes at T2–T4 and frequencies at T2 and T3 (p < 0.05, Figure 4).
The mixed-model ANOVA revealed no significant time-by-group interaction effects (p > 0.05) for the number of referred pain regions. Post-hoc analysis showed that the number of referred pain regions significantly decreased in T4 in both groups (p < 0.01, Table 3).
 |
Table 3 Number of Body Regions for MTrPs-Referred Pain |
Discussion
Previous studies indicated that DN could be used to treat MTrPs in the upper trapezius muscle. A systematic review recommended DN for relieving MTrPs pain in the neck and shoulders in the short term.23 This study revealed that DN-EMG group exhibited greater improvements in VAS at 12-week follow-up period than the DN group and DN-EMG group exhibited lower amplitudes at T2–T4 and lower frequencies at T2 and T3, indicating better MTrPs inactivation and maintenance of pain relief.
In this study, the DN and DN-EMG groups resulted in a significant decrease in VAS, NDI and SEA amplitude after treatment and at all follow-up periods compared with baseline. The improved NDI may be a consequence of the decrease in pain and improvement in muscle tone and elasticity after treatment. Previous studies also indicated a decrease in NDI scores after DN.30 Although VAS and NDI increased compared with those at the end of treatment sessions, the measurement scores were significantly lower than before treatment. This finding suggested that improvements in pain and disability by using DN and DN-EMG could be maintained for 12 weeks after intervention.
However, the DN-EMG group exhibited higher improvements in VAS and SEA amplitudes at 12-week follow-up period than the DN group. Previous studies showed abnormal SEA in MTrPs.18,19 The use of EMG could help find MTrPs more accurately and observe the inactivation status of MTrPs in real time. Treatment with DN-EMG guidance is more accurate and specific, with the possibility of accessing deeper muscles and better inactivation of MTrPs. The DN-EMG group showed lower VAS and SEA amplitudes at 12-week follow-up than the DN group.
Investigators have attributed the therapeutic effects of DN to chemical effects and mechanical neurophysiology.31 Several studies have demonstrated changes in the chemical properties of MTrPs combined with eliciting LTR following dry needling.16 LTR has been thought to reduce the concentration of sensitizing substances in the MTrPs and they are considered as an important parameter in breaking the centrally mediated vicious cycle of the MTrPs phenomena.31 According to Hong,24 an LTR elicited during needling is the most definitive objective indication that the needle has been inserted exactly in the MTrPs. DN to considered to provide a mechanical localized stretch to the shortened sarcomeres and contracted cytoskeletal structures within the MTrPs.32 DN was also suggested to stimulate A-delta fibers and activate enkephalin-producing neurons in the dorsal horn, leading to the reduction and suppression of pain.33
The local twitch potentials of muscles with different amplitudes were detected when DN induced LTR. However, a better waveform analysis method has not been found. One study found that bilateral LTR has been recorded in the contralateral trapezius during unilateral DN of MTrPs in the ipsilateral and symptomatic trapezius.34 Audette et al concluded that LTR is generated by inducing a spinal reflex that involves sensory input to the spinal cord by mechanical irritation of the needle at sensitive loci in the MTrPs, which then results in a motor efferent response of the alpha motor neuron pool.35 The presence of bilateral LTRs during unilateral dry needling argues strongly for a central abnormality rather than a purely peripheral abnormality in patients with MTrPs. The persistence of MTrPs may lead to neuroplastic changes at the level of the dorsal horn, which results in amplification of pain sensation (ie central sensitization), with a tendency to spread beyond its original boundaries (ie expansion of receptive fields).35 In the current study, the participants described the referred pain pattern of NSP and showed pain distributed in the head and neck, the upper back, the shoulder and the arm. Changes in pain area decreased significantly at T4 in both groups, possibly indicating that DN could improve central sensitization.
As early as 1995, Simons36 recorded a high-frequency repetitive spike from MTrPs of trapezius at rest. Liley37 identified low-amplitude continuous discharges as abnormal endplate potentials. Eventually, all those potentials were called SEA.2 A previous research showed that positive sharp waves, fibrillation and fascicular potentials were founded at MTrPs. These waveforms usually appeared in the early stage of muscle denervation or myogenic damage, or were found in muscle necrosis, muscle inflammation, local muscle degeneration and traumatic injury,38 and DN at MTrPs decreased the amplitude and frequency of SEA.22 Evidence showed that EMG provides increased accuracy for BoNT injections.39 The use of EMG could also help find MTrPs more accurately and observe the inactivation status of MTrPs in real time, suggesting enhanced attempts to inactivate MTrPs. In a clinical context, DN-EMG could be used as a tool to observe MTrPs, especially for therapists who lack experience in MTrPs diagnosis. This study revealed that DN under needle EMG guidance technique exhibited greater improvements in VAS at 12-week follow-up period and lower amplitudes at T2–T4 and lower frequencies at T2 and T3 than the DN group, indicating better MTrPs inactivation and maintenance of pain relief. However, the high cost of EMG equipment is a major problem that may limit the usage of this modality.
Conclusion
This study revealed that DN-EMG group exhibited lower scores in VAS at 12-week follow-up period and lower amplitudes at T2–T4 and frequencies at T2 and T3 than the DN group, indicating better MTrPs inactivation and better pain relief.
Data Sharing Statement
The authors confirm that the data underlying the findings described in this manuscript are available from the corresponding author upon reasonable request.
Funding
The present study is supported by the Fundamental Research Funds for the Central Universities (grant number: 22120190126) and the National Natural Science Foundation of China (NSFC), (grant number: 81470105).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Luime J, Koes B, Hendriksen I., et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33(2):73–81.
2. Simons D, Travell J, Simons L. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. Vol. 1. Williams & Wilkins; 1999.
3. Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989;151(2):157–160.
4. Do TP, Heldarskard GF, Kolding LT, et al. Myofascial trigger points in migraine and tension-type headache. J Headache Pain. 2018;19(1):84.
5. Fricton JR, Kroening R, Haley D, et al. Myofascial pain syndrome of the head and neck: a review of clinical characteristics of 164 patients. Oral Surg Oral Med Oral Pathol. 1985;60(6):615–623.
6. Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14(1):95–107.
7. Fernández-Lao C, Cantarero-Villanueva I, Fernández-De-Las-Peñas C, et al. Myofascial trigger points in neck and shoulder muscles and widespread pressure pain hypersensitivity in patients with postmastectomy pain: evidence of peripheral and central sensitization. Clin J Pain. 2010;26(9):798–806.
8. Malanga GA, Cruz Colon EJ. Myofascial low back pain: a review. Phys Med Rehabil Clin N Am. 2010;21(4):711–724.
9. Yap EC. Myofascial pain - An overview. Ann Acad Med Singapore. 2007;36(1):43–48.
10. Sarrafzadeh J, Ahmadi A, Yassin M. The effects of pressure release, phonophoresis of hydrocortisone, and ultrasound on upper trapezius latent myofascial trigger point. Arch Phys Med Rehabil. 2012;93(1):72–77.
11. Sciotti VM, Mittak VL, DiMarco L, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain. 2001;93(3):259–266.
12. Gemmell H, Allen A. Relative immediate effect of ischaemic compression and activator trigger point therapy on active upper trapezius trigger points: a randomised trial. Clin Chiropr. 2008;11(4):175–181.
13. Ribeiro DC, Belgrave A, Naden A, et al. The prevalence of myofascial trigger points in neck and shoulder-related disorders: a systematic review of the literature. BMC Musculoskelet Disord. 2018;19(1):252.
14. Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21(3):427–445.
15. Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil. 2001;82(7):986–992.
16. Dommerholt J. Dry needling in orthopedic physical therapy practice. Orthop Phys Ther Pract. 2004;16(3):15–20.
17. Mazza DF, Boutin RD, Chaudhari AJ. Assessment of myofascial trigger points via imaging: a systematic review. Am J Phys Med Rehabil. 2021;100(10):1003–1014.
18. Dommerholt J, Hooks T, Grieve R, et al. Myelinated afferents are involved in pathology of the spontaneous electrical activity and mechanical hyperalgesia of myofascial trigger spots in rats. Am J Phys Med Rehabil. 2013;3(1):1–8.
19. Huang QM, Lv JJ, Ruanshi QM, et al. Spontaneous electrical activities at myofascial trigger points at different stages of recovery from injury in a rat model. Acupunct Med. 2015;33(4):319–324.
20. Chen J, Chung K, Hou C, et al. Inhibitory effect of dry needling on the spontaneous electrical activity recorded from myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2001;80:729–735.
21. Hsieh YL, Yang CC, Liu SY, et al. Remote dose-dependent effects of dry needling at distant myofascial trigger spots of rabbit skeletal muscles on reduction of substance P levels of proximal muscle and spinal cords. Biomed Res Int. 2014;2014:982121.
22. Liu QG, Liu L, Huang QM, et al. Decreased Spontaneous Electrical Activity and Acetylcholine at Myofascial Trigger Spots after Dry Needling Treatment: a Pilot Study. Evid Complement Altern Med. 2017;2017:3938191.
23. Liu L, Huang QM, Liu QG, et al. Effectiveness of dry needling for myofascial trigger points associated with neck and shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(5):944–955.
24. Hong CZ. Considerations and recommendations regarding myofascial trigger point injection. J Musculoskelet Pain. 1994;2(1):29–59.
25. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175–184.
26. Vernon H. The neck disability index: state-of-the-art, 1991-2008. J Manipulative Physiol Ther. 2008;31(7):491–502.
27. Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–415.
28. Valls-Sole J, Castillo CD, Casanova-Molla J, et al. Clinical consequences of reinnervation disorders after focal peripheral nerve lesions. Clin Neurophysiol. 2011;122(2):219–228.
29. Jellinger KA. Handbook of Clinical Neurology. Eur J Neurol. 2009;16(2):e21–e21.
30. Itoh K, Katsumi Y, Hirota S, et al. Randomised trial of trigger point acupuncture compared with other acupuncture for treatment of chronic neck pain. Complement Ther Med. 2007;15(3):172–179.
31. Shah JP. Integrating Dry Needling with New Concepts of Myofascial Pain, Muscle Physiology, and Sensitization. Integrative Pain Medicine. Humana Pr Inc; 2008:107–121.
32. Southerst D. Myofascial trigger points: pathophysiology and evidence-informed diagnosis and management. Man Ther. 2011;16(3):e1.
33. Baldry P. Management of myofascial trigger point pain. Acupunct Med. 2002;20(1):2–10.
34. Audette JF, Wang F, Smith H. Bilateral activation of motor unit potentials with unilateral needle stimulation of active myofascial trigger points. Am J Phys Med Rehabil. 2004;83(5):368–374.
35. Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep. 2002;4(4):313–321.
36. Simons DG, Hong CZ, Simons LS. Prevalence of spontaneous electrical activity at trigger spots and control sites in rabbit muscle. J Musculoskelet Pain. 1995;3(1):35–48.
37. Liley AW. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956;132(3):650–666.
38. Liu QG, Huang QM, Liu L, et al. Structural and functional abnormalities of motor endplates in rat skeletal model of myofascial trigger spots. Neurosci Lett. 2019;711:134417.
39. Hong JS, Sathe GG, Niyonkuru C, et al. Elimination of dysphagia using ultrasound guidance for botulinum toxin injections in cervical dystonia. Muscle Nerve. 2012;46(4):535–539.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.