Back to Journals » OncoTargets and Therapy » Volume 15
Efficacy of Disitamab Vedotin in Treating HER2 2+/FISH- Gastric Cancer
Authors Dai L , Jin X, Wang L, Wang H , Yan Z, Wang G, Liang B, Huang F, Luo Y, Chen T, Wang Q
Received 22 November 2021
Accepted for publication 15 February 2022
Published 16 March 2022 Volume 2022:15 Pages 267—275
DOI https://doi.org/10.2147/OTT.S349096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Li Dai,* Xiangren Jin,* Liuxing Wang, Haibin Wang, Zhiqiang Yan, Guanghai Wang, Baichuang Liang, Fu Huang, Yuling Luo, Taichun Chen, Qian Wang
Department of Gastrointestinal Surgery, Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, 550001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qian Wang, Tel +8615885004333, Email [email protected]
Abstract: Currently, effective therapies for advanced gastric cancer with systemic metastasis are lacking. Pharmacological research has been slowly progressing over the past decades. Here, we report the case of a 56-year-old female with human epidermal growth factor receptor 2 (HER2) expression (IHC 2+/FISH-) in gastric cancer with systemic metastasis. The first-line therapeutic regime consisted of systemic administration of camrelizumab, local arterial infusion of oxaliplatin and arterial embolization, oral apatinib, and PS scheme (oral tegafur-gimeracil-oteracil (S-1) and paclitaxel (PTX), which was administered both intraperitoneally and systemically). After the treatment, a 3-month progression-free survival (PFS) was observed. Due to the occurrence of CTCAE grade 4 adverse reactions, the patient could not tolerate chemotherapy. In the second line of treatment, we replaced the PS scheme with disitamab vedotin and continued the use of carrilizumab and apatinib. After four cycles, efficacy evaluation showed that it was stable disease (SD), only CTCAE 1/2 grade adverse reactions occurred, and endoscopy examination showed local tumor control with a reduction in the ulcer lesion. At the time of submission of the current manuscript, a 6-month PFS was achieved and the treatment was continued. Due to the safety and efficacy of disitamab vedotin observed in our case, we propose that disitamab vedotin could be a promising drug for the treatment of advanced gastric cancer patients with HER2 expression.
Keywords: gastric cancer, human epidermal growth factor receptor 2, disitamab vedotin
Introduction
Due to the lack of safety and effectiveness of the treatment of advanced gastric cancer, research on the same has attracted much attention. Due to the unresectability of the gastric tumor, the focus is on neoadjuvant therapy, including systemic chemotherapy, radiotherapy, targeted therapy, immunotherapy, and traditional Chinese medicine.
Disitamab Vedotin is a novel HER2-ADC drug developed independently in China. It was approved by the State Drug Administration of China on June 9, 2021 and listed on the market (approval number: national drug standard S20210017). It is mainly used to treat patients with locally advanced or metastatic gastric cancer with HER2 expression (IHC2+ or 3+), regardless of whether a FISH test is positive or not. Disitamab Vedotin, an antibody-conjugated drug (ADC), consists of three parts: anti-human epidermal growth factor receptor 2 extracellular domain (HER2 ECD) antibody, connectors (MC-Val-Cit-PAB, Linker), and cytotoxin (Monomethyl Auristatin E MMAE). As for the action mechanism of Disitamab Vedotin, after part of its antibody binds to the extracellular domain of HER2 on the cell surface, the ADC complex is swallowed by the cell and transported to the lysosomes, and the connectors are digested with enzymes to release microtubule inhibitor MMAE, which destroys the intracellular microtubule network and leads to mitotic cell cycle arrest and apoptosis. In addition, in vitro studies have shown that Disitamab Vedotin can inhibit HER2 receptor signaling and shows antibody-dependent cell-mediated cytotoxicity (ADCC).1 Its common adverse reactions include hematological abnormalities (decreased white blood cell and neutrophil counts) and elevated transaminase (aspartate aminotransferase and alanine aminotransferase). Clinical symptoms include alopecia, fatigue, and hypoesthesia. For detailed adverse reactions and pharmacokinetic parameters, see Supplementary Material 1.
Compared to the traditional HER2 antibody, the antibody part of Disitamab Vedotin is a humanized monoclonal antibody, whose affinity for the HER2 receptor is greater. Also, Disitamab Vedotin shows a better rate of tumor cell endocytosis, and it is easier to transport small molecule toxins into the tumor cells. Disitamab Vedotin uses cleavable cathepsin to connect to the small molecular toxic drug MMAE. Compared to ADC drugs that use non-cleavable connectors (T-DM1), Disitamab Vedotin has a bypass killing effect, overcomes tumor spatial heterogeneity, enhances the killing effect on adjacent tumor cells, and can kill tumor cells with a low expression of HER2. Also, due to the precision of the targeted drugs, the adverse reactions of molecular toxic drugs are significantly lower than those of systemic chemotherapeutic drugs.2 With the C008 trial, it was confirmed that Disitamab Vedotin is effective and safe for treating locally advanced or metastatic gastric cancer patients with both HER2-positive and low HER2 expression.3
Here, we report a case of systemic multiple metastatic advanced HER2 2+/Fish- gastric cancer. After the first-line treatment, tumor progression was controlled. However, because of its adverse reactions, the treatment could not be continued according to plan. In the second line, the patients benefited significantly after switching to Disitamab Vedotin.
Case Presentation
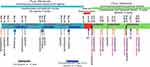
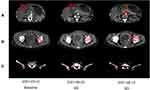
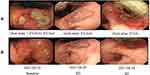
The patient, a 56-year-old female, was admitted to the hospital because of “4+ months of double hydronephrosis found during a physical examination”. Computed tomography (CT) assessment indicated ulcerative gastric cancer with an abdominal implant, bladder, and bone metastases. An endoscopic examination revealed that the ulcer of the gastric angle was huge, and advanced gastric cancer was speculated. Diseased tissues were sampled during endoscopy and subjected to pathological examination and genetic analysis, results of which are shown in Figure 1 and Table 1.Based on the results of the endoscopic biopsy and imaging, the following diagnosis was made: 1. HER2 2+/FISH- and low-adhesion adenocarcinoma of the gastric antrum with peritoneal implantation, bladder, and bone metastases, cT4bN3M1 (IV stage); 2. Bilateral kidney and bilateral ureteral hydronephrosis. After the first and second lines of treatment, the patient showed stable disease and only CTCAE 1/2 grade adverse reactions. Figures 2–4 illustrate the entire treatment process, the changes in the lesions as observed using CT, and the changes in the tumor and the wall of the gastric antrum observed using endoscopy.
 |
Table 1 Genetic Test Results of Patients |
The First Line of Treatment
Based on the diagnosis and the results of the genetic test, a treatment plan was formulated after consultation with the Multiple Disciplinary Team (MDT) and Professor Zhu Zhenggang, chairman of the Chinese gastric Cancer Committee. Camrelizumab (IVGTT 200 mg Q3wks) was administered on March 15, 2021. Transurethral cystoscopy and pigtail ureteral stent implantation in the right ureter was performed on March 17. During the surgery, the left ureteral orifice was found to show atresia and the pigtail ureteral stents could not be placed. Therefore, digital subtraction angiography (DSA)-guided percutaneous left pyelography and left pigtail ureteral stent implantation was performed the next day. On March 18, the left gastric artery and the right gastroepiploic artery were infused with 100 mg Oxaliplatin via a transarterial interventional microcatheter, and then the two arteries were embolized using lipiodol. Apatinib (PO 0.5 g, qd, for 14 days Q3wks) was administered on March 21. On March 26, laparoscopic exploration, intraperitoneal chemotherapy tube implantation, and chemotherapy infusion port implantation in the subcutaneous abdominal wall were performed under general anesthesia (Figure 5). During the process, 500 mL yellow and turbid ascites and scattered planting tumor nodules were found in the peritoneum of subphrenic, pelvic, and abdominal walls, and abdominal organs. On March 29, Oral S-1 and Paclitaxel were administered intraperitoneally and systemically, which was the PS regimen. In detail: S-1 PO, 40 mg, for 14 days, Q3wks; PTX IVGTT 50 mg/m2 on days 1 and 8, intraperitoneal chemotherapy port (IP) PTX 20 mg/m2 on days 1 and 8, Q4wks. PTX was diluted in 1000 mL saline and administered intraperitoneally via the intraperitoneal chemotherapy infusion port for 1 h, and PTX was infused intravenously at the same time. Along with the antineoplastic therapy, nutritional support, traditional Chinese medicine, and other supporting treatments were provided.
After the third cycle of PS treatment on June 6, there were CTCAE 3/4 grade adverse reactions. Among them, myelosuppression, gastrointestinal bleeding, and secondary bacterial and fungal infections are life-threatening. Symptoms of urinary incontinence, which is considered to be related to bladder metastasis, were also observed. The ECOG (Eastern Cooperative Oncology Group) score decreased to 4, and the QOL (Quality Of Life) score was 18. All antineoplastic therapies had to be suspended on June 15. The patient was in poor condition and could not bear the PS regimen in the next cycle, so the curative effect was evaluated. CT scan results showed that the abdominal-retroperitoneal lymph nodes were smaller than before, the abdominal and pelvic effusion was significantly reduced, the gastric antrum wall and bladder wall thickened but not more than 20% and the range of bone metastasis was larger than before (Figure 3). Endoscopy results showed that the area and depth of the ulcer were smaller than before (Figure 4). The result of the efficacy evaluation was SD.
The Second Line of Treatment
After the first line of treatment, the tumor did not progress, which proved that our first line of treatment was effective. However, because of serious adverse reactions threatening the patient’s life, we had to stop the treatment. Since the patient was HER2 2+/FISH-, it was not advisable to administer Trastuzumab. As far as we know, there is no drug available for such patients. A new HER2-ADC drug, Disitamab Vedotin, entered the market in China on June 9, 2021, which could be used in the treatment of advanced gastric cancer of HER2 2+ and 3+, irrespective of whether a FISH test was positive or not. It brought new hope for the patients in desperate condition. Considering this development, we formulated a new treatment plan.
Considering that CTCAE 3/4 grade adverse reactions may be mainly due to the PS scheme, we changed the PS scheme to Disitamab Vedotin and continued to use Apatinib and Camrelizumab. According to the drug usage instructions (Supplementary Material 1), Disitamab Vedotin is administered via intravenous drip throughout the body, once every two weeks, and the recommended dose is 2.5 mg/kg. The specific treatment plan after the change was as follows: On June 24, Disitamab Vedotin (IVGTT 100 mg Q2wks) and Apatinib (PO, 0.5 g, qd, for 14 days, Q3wks), and on June 25, Camrelizumab (IVGTT 200 mg q2wks) were administered. On June 27, 3 days after the first use of Disitamab Vedotin, the patient’s urinary incontinence disappeared and the serious adverse reactions gradually alleviated. After three cycles, the adverse reactions and quality of life of the patient significantly improved compared to those after the first-line treatment, as shown in Table 2. After four cycles of Disitamab Vedotin treatment, a CT scan was performed on August 12. Abdominal and pelvic effusion decreased again. No progress was observed in multiple osteogenic metastases and the thickening of the gastric antrum wall and bladder wall. The enhancement of the gastric mucous surface was lesser than before. The curative effect evaluation suggested SD. On August 25, after five cycles, endoscopy results showed that the area of gastric angle ulcer was reduced by about 70%, the local gastric wall was softer, gastric peristalsis was visible, and the biopsy was soft. At the time of submission of this case report, the patient was generally in good condition and the second-phase treatment regimen was continued.
 |
Table 2 Comparison of Adverse Reaction Grades and ECOG and QOL Scores |
Discussion
The incidence of gastric cancer in China accounted for 43.9% of that of the world in 2020, and nearly 50% of the patients were diagnosed at an advanced stage.4 At present, the main treatments recommended by the Chinese Society of Clinical Oncology (CSCO) guidelines for advanced gastric cancer are Apatinib, anti-PD-1, HER2-targeted therapy, and chemotherapy. As far as ≥2-line chemotherapy is concerned, the common scheme is Irinotecan or PTX. The objective remission rate (ORR) of the disease ranged from 3% to 10.9%.5–7 The incidence of adverse reactions of grade 4 of CTCAE 3 was as high as 57%.8 The curative effect of chemotherapy was limited and adverse reactions were common. The 2-year survival rate of patients treated with Apatinib was close to that of the placebo group and there was no significant improvement in the ORR. The incidence of adverse reactions in CTCAE 3/4 grade was more than 50%.9 In the study of anti-PD-1 -targeted therapy for advanced gastric cancer, the median Overall Survival (OS) was less than 6 months, the median PFS was less than 2 months, and the ORR was about 12%.10 After the first-line treatment of Trastuzumab, the second-line and above treatments of HER2 -positive patients with advanced gastric cancer were not as successful as that of breast cancer, which may be related to the high heterogeneity of HER2 expression in gastric cancer and the change of pathway.11 The research and development of therapeutic drugs for advanced gastric cancer is slow, and most of the targeted drugs do not, as of yet, provide the expected anti-tumor effect.12 Currently, the 5-year survival rate of advanced gastric cancer in China is less than 10%,13 and the overall survival time is less than 1 year.14 Generally speaking, the treatment of advanced gastric cancer is not satisfactory and new treatment options need to be explored.
Our patient has advanced gastric cancer with multiple systemic metastases. We have made a personalized treatment plan according to the patient’s condition. If we had strictly followed the first-line treatment recommended by the CSCO guidelines, once we failed, there would have been no chance of second-or third-line medication. Also, for advanced gastric cancer with multiple metastases, the guidelines are mostly unavailable or they recommend the participation of the patient in clinical trials. Our treatment plan combined the latest relevant clinical research in China and abroad, the opinions of authoritative experts in the field of treatment of gastric cancer in China, and our own clinical experience. The most important thing is that the patients trust us and agree with the treatment plan. The reasons for using various treatments are: 1. PDL1 gene is negative and there is Microsatellite Stability (MSS). We used Camrelizumab, a PD-1 inhibitor developed in China, since it has been reported that PD-1 inhibitors can benefit PDL1-negative advanced gastric cancer patients,15 and our experience supported this. The mechanism may be related to unknown immune checkpoints. Moreover, we used Carrilizumab before other treatments. We think that the mechanism of the PD-1 inhibitor is to reactivate the immune response of T cells towards the tumor by inhibiting the binding of PD-1 of T cells to PD-L1 expressed by the tumor cells. Targeted therapy and chemotherapy lead to myelosuppression, thus, reducing the quality and quantity of T cells in the body. The efficacy of PD-1 inhibitors might be weakened. 2. The reason for the first-line use of Apatinib was that in related studies, Camrelizumab + Apatinib, as first-line therapy for advanced gastric cancer, demonstrated encouraging antitumor activity and manageable toxicity.16 3. Since the tumor was located in the gastric antrum and was larger in size, it was easier to lead to pyloric obstruction. Oxaliplatin was infused through the left gastric artery and the right gastroepiploic artery, and then, we embolized the two arteries. Local administration and cutting off of the blood supply to the tumor can prevent the progression of the primary tumor to a certain extent. 4. The latest meta-analysis of neoadjuvant intraperitoneal chemotherapy for gastric cancer showed that among the different regimens of neoadjuvant intraperitoneal and systemic chemotherapy (NIPS), the PS regimen showed the best prognosis for gastric cancer patients with peritoneal metastasis.17 Therefore, the PS regimen is adopted for patients with extensive intraperitoneal metastasis. 5. The use of Disitamab Vedotin is the last treatment resort. The patient achieved a PFS of 6 months with fewer adverse reactions, justifying our treatment choices. However, for patients with advanced gastric cancer with multiple systemic metastases, the next step is not known. How long does it take to use Vidixitumab? Is it possible to operate? When should the surgery be performed? The answers to these questions are not known. The only thing we can do is continue the therapy and pray that Disitamab Vedotin works again.
HER2 is a member of the epidermal growth factor transmembrane receptor family that is overexpressed in several cancer types and contributes to tumor cell proliferation, adhesion, migration, differentiation, and apoptosis. Gene amplification or overexpression of HER2 is observed in 15–20% of breast cancer cases18–21 and HER2 overexpression is reported in about 20% of gastric tumors. According to the TOGA trial, which tried to evaluate the efficacy of anti-HER2-targeted therapy for advanced gastric cancer, the second-line (2L) and ≥2L treatments for HER2-positive advanced gastric cancer patients with drug resistance after the first-line treatment of Trastuzumab have not been as successful as those for breast cancer.11,22 This may be because HER2-positive advanced gastric cancer has been not only found to share some of the mechanisms of drug resistance with breast cancer but also to manifest specific mechanisms of resistance to trastuzumab, including tumor heterogeneity in HER2 positivity, loss of HER2 protein expression, alteration in HER2 downstream signaling, and activation of bypass pathways.23 On the other hand, trials involving Pertuzumab, Lapatinib, and T-DM1 (Ado-Trastuzumab Emtansine [Kadcyla]) have failed to show significant improvements in the outcomes of HER2-positive advanced gastric cancer patients.24 Therefore, a novel targeted drug like HER2-ADC might improve the outcome of advanced gastric cancer with HER2 expression.
There are only two new HER2-ADC drugs, Trastuzumab Deruxtecan developed by the Japanese and Disitamab Vedotin developed by the Chinese. In the treatment of advanced gastric cancer with HER2 expression, the results showed that the curative effect of Trastuzumab Deruxtecan in the Japanese population was better than that of Disitamab Vedotin in the Chinese population. However, the clinical and tumor characteristics of Chinese patients were significantly different from those of Japanese patients, as follows: 1. Histopathological classification - the proportion of patients with intestinal-type gastric cancer was 71.2% in those treated with Trastuzumab Deruxtecan, and was 29.1% in those treated with Disitamab Vedotin. 2. Baseline ECOG score – about 49.6% of the patients treated with Trastuzumab Deruxtecan had an ECOG score of “0”, and 22.8% of those treated with Disitamab Vedotin had an ECOG score of “0”. 3. Tumor burden - the percentage of patients receiving Trastuzumab Deruxtecan treatment with a total diameter of the target lesion at baseline ≥10 was 17.6%, and that receiving Disitamab Vedotin was 30.7%. 4. HER2 expression level - the proportion of HER2 3+ patients who received Trastuzumab Deruxtecan treatment was 76.8%, and that receiving Disitamab Vedotin was 55.1%. The HER2 2+/FISH+ patients who received Trastuzumab Deruxtecan treatment accounted for 23.2%, and those who received Disitamab Vedotin accounted for 10.2%. The percentage of HER2 2+/FISH- patients receiving Trastuzumab Deruxtecan treatment was 0% and that receiving Disitamab Vedotin was 34.7%. Since these aforementioned four baseline characteristics were significantly different between the two groups, the results of the comparison of the curative effects of Trastuzumab Deruxtecan and Disitamab Vedotin were controversial. Disitamab Vedotin is superior to Trastuzumab Deruxtecan in safety since the incidence of adverse reactions with a CTCAE ≥3 is lower, and Disitamab Vedotin shows no incidence of interstitial lung disease (ILD), while the incidence of Trastuzumab Deruxtecan-related ILD is 9%-13.6%.3,25
Disitamab Vedotin was used to treat metastatic bladder urothelial carcinoma with HER2 overexpression. The C004 clinical trial results showed that the disease control rate (DCR) was 90% and the ORR was as high as 51%.26 Therefore, it was recognized as a “breakthrough ADC drug” by the National Food and Drug Administration (FDA). In the C008 clinical trial, Disitamab Vedotin was used to treat patients with locally advanced or metastatic gastric cancer or gastroesophageal junction adenocarcinoma who expressed HER2 (IHC2+ or 3 +) and had previously received more than 2 systemic chemotherapies. The results indicated that the primary outcomes of mOS of 7.9 months, mPFS of 4.1 months, and ORR 24.4% were significantly better than the results of similar clinical studies of Apatinib and anti-PD-1 therapy. The ORR was consistent in all subgroups, including patients with a low expression of HER2 (IHC 2+/ Fish-).3,10
In summary, we cannot deny the benefits of the first line of treatment, especially the effect of the PS regimen in the control of peritoneal metastasis. What surprised us is the new hope that Disitamab Vedotin brought to the patient in terms of efficacy and safety, highlighting its potential in the treatment of advanced gastric cancer with HER2 overexpression. Disitamab Vedotin could be a promising drug for the treatment of advanced gastric cancer with HER2 expression.
Abbreviations
HER2, human epidermal growth factor receptor 2; IHC, immunohistochemical; PS scheme, oral tegafur-gimeracil-oteracil, and paclitaxel, which was administered intraperitoneally and systemically; S-1, tegafur-gimeracil-oteracil; PTX, paclitaxel; PFS, progression-free survival; SD, stable disease; CTCAE, common terminology criteria for adverse events; ADC, antibody-conjugated drug; MMAE, monomethyl auristatin E; ADCC, antibody-dependent cell-mediated cytotoxicity; CT, computed tomography; NGS, next-generation sequencing; PD-L1, programmed cell death-ligand 1; VEGFR2, vascular endothelial growth factor receptor 2; MSI, microsatellite instability; MSS, microsatellite stability; ABCB1, ATP-binding cassette subfamily B member 1; AE, arterial embolization; PO, peros; IVGTT, intravenously guttae; Q3wks, one course of treatment every 3 weeks; Q4wks, one course of treatment every 4 weeks; Q2wks, one course of treatment every 2 weeks; ECOG, Eastern Cooperative Oncology Group; QOL, quality of life; ORR, remission rate; OS, overall survival; CSCO, Chinese Society of Clinical Oncology; ILD, lung disease; DCR, disease control rate; mOS, median overall survival; mPFS, median progression-free survival.
Ethics Approval and Informed Consent
The study was approved by the ethics committee of The Affiliated Hospital of the Guizhou Medical University. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Consent for Publication
We obtained consent to the publication of medical data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave approval to the final version to be published; have agreed on the journal to which the article has been submitted; and agree to be held accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li L, Xu MZ, Wang L, et al. Conjugating MMAE to a novel anti-HER2 antibody for selective targeted delivery. Eur Rev Med Pharmacol Sci. 2020;24(24):12929–12937. doi:10.26355/eurrev_202012_24196
2. Jiang J, Li S, Shan X. Preclinical safety profile of Disitamab Vedotin: a novel anti-HER2 antibody conjugated with MMAE. Toxicol Lett. 2020;324:30–37. doi:10.1016/j.toxlet.2019.12.027
3. Xu Y, Wang Y, Gong J. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–925. doi:10.1007/s10120-021-01168-7
4. Bray F, Ferlay J, Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
5. Zheng Y, Fang W, Mao C. Biweekly S-1 plus paclitaxel (SPA) as second-line chemotherapy after failure from fluoropyrimidine and platinum in advanced gastric cancer: a Phase II study. Cancer Chemother Pharmacol. 2014;74(3):503–509. doi:10.1007/s00280-014-2537-2
6. Zhong H, Zhang Y, Ma S. Docetaxel plus oxaliplatin (DOCOX) as a second-line treatment after failure of fluoropyrimidine and platinum in Chinese patients with advanced gastric cancer. Anticancer Drugs. 2008;19(10):1013–1018. doi:10.1097/CAD.0b013e328314b5ab
7. Sun Q, Hang M, Xu W. Irinotecan plus capecitabine as a second-line treatment after failure of 5-fluorouracil and platinum in patients with advanced gastric cancer. Jpn J Clin Oncol. 2009;39(12):791–796. doi:10.1093/jjco/hyp116
8. Lam JYC, Choo SP, Tai DW. What is the value of third-line chemotherapy in advanced gastroesophageal cancer? A 5-year retrospective analysis at a single center. Asia Pac J Clin Oncol. 2020;16(1):23–27. doi:10.1111/ajco.13285
9. Li J, Qin S, Randomized XJ. Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34(13):1448–1454. doi:10.1200/JCO.2015.63.5995
10. Chan WL, Lam KO, So TH. Third-line systemic treatment in advanced/metastatic gastric cancer: a comprehensive review. Ther Adv Med Oncol. 2019;11:1758835919859990. doi:10.1177/1758835919859990
11. Palle J. Human Epidermal Growth Factor Receptor 2 (HER2) in Advanced Gastric Cancer: current Knowledge and Future Perspectives. Drugs. 2020;80(4):401–415. doi:10.1007/s40265-020-01272-5
12. Hsu A, Chudasama R, Almhanna K. Targeted therapies for gastroesophageal cancers. Ann Transl Med. 2020;8(17):1104. doi:10.21037/atm-20-3265
13. Wang H, Guo W, Hu Y. Superiority of the 8th edition of the TNM staging system for predicting overall survival in gastric cancer: comparative analysis of the 7th and 8th editions in a monoinstitutional cohort. Mol Clin Oncol. 2018;9(4):423–431. doi:10.3892/mco.2018.1683
14. Zhao L, Li J, Bai C. Multi-Modality Treatment for Patients With Metastatic Gastric Cancer: a Real-World Study in China. Front Oncol. 2019;9:1155. doi:10.3389/fonc.2019.01155
15. Fuchs CS, Doi T, Jang RW. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4(5):e18001. doi:10.1001/jamaoncol.2018.0013
16. Peng Z, Wei J, Wang F. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2021;27(11):3069–3078. doi:10.1158/1078-0432.CCR-20-4691
17. Gong Y, Wang P, Zhu Z. Benefits of Surgery After Neoadjuvant Intraperitoneal and Systemic Chemotherapy for Gastric Cancer Patients With Peritoneal Metastasis: a Meta-Analysis. J Surg Res. 2020;245:234–243. doi:10.1016/j.jss.2019.07.044
18. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi:10.1126/science.3798106
19. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi:10.1126/science.2470152
20. Yan M, Parker BA, Schwab R, Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat Rev. 2014;40:770–780. doi:10.1016/j.ctrv.2014.02.008
21. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi:10.1200/JCO.2013.50.9984
22. Bang YJ, Van Cutsem E, Feyereislova A. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial.lancet. 2010;376(9742):687–697. doi:10.1016/S0140-6736(10)61121-X
23. Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance. Cancers. 2020;12(2):400. doi:10.3390/cancers12020400
24. Oh DY, Bang YJ. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48. doi:10.1038/s41571-019-0268-3
25. Shitara K, Bang YJ, Iwasa S. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382(25):2419–2430. doi:10.1056/NEJMoa2004413
26. Sheng X, Yan X, Wang L. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody-Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin Cancer Res. 2021;27(1):43–51. doi:10.1158/1078-0432.CCR-20-2488
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.