Back to Journals » Hepatic Medicine: Evidence and Research » Volume 11
Efficacy of direct-acting antiviral therapy for hepatitis C viral infection. Real-life experience in Bahrain
Authors Abdulla M , Ali H , Nass H , Khamis J, AlQamish J
Received 22 October 2018
Accepted for publication 30 March 2019
Published 13 May 2019 Volume 2019:11 Pages 69—78
DOI https://doi.org/10.2147/HMER.S190967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gerry Lake-Bakaar
Maheeba Abdulla,1 Hamed Ali,2 Hafsa Nass,2 Jawad Khamis,2 Jehad AlQamish1
1Gastroenterology and Hepatology, Salmaniya Medical Complex, Manama, Bahrain; 2Medical Department, Salmaniya Medical Complex, Manama, Bahrain
Purpose: The introduction of direct-acting antivirals (DAAs) has revolutionized the treatment of chronic hepatitis C viral (HCV) infection. This study aims to establish real-world treatment efficacy of Sofosbuvir-based (SOF-B) and Ombitasvir/Paritaprevir/Ritonavir-based (OPR-B) regimens.
Patients and methods: This prospective, non-randomized observational real-life study was conducted in Salmaniya Medical Complex, Bahrain, and included consecutive patients with chronic HCV infection (genotypes 1–4) who were treated with direct-acting antivirals. Sustained virologic response to therapy was assessed at week 12 post end of treatment (SVR12).
Results: Of the 167 patients included, 60.5% (n=101) were treated with SOF-B and 39.5% (n=66) with OPR-B regimens for 12 weeks (n=148; 88.6%) or 24 weeks (n=19; 11.4%). SVR12 was achieved in 156 (93.4%) patients, 4 patients failed to achieve SVR despite completion of treatment, and 7 patients discontinued treatment due to non-compliance and were included in the analysis on an intention-to-treat basis. There was no difference between SOF-B and OPR-B regimens (95/101; 94.1%) and (61/66; 92.4%), respectively (p=0.68). However, SVR12 rates were significantly higher in patients without liver cirrhosis (103/104; 99.0%) compared to patients with cirrhosis (53/63; 84.1%; p<0.001), and in patients who received 12-week-regimen (141/148; 95.3%) compared to those who received 24-week regimen (15/19; 78.9%; p<0.024). However, logistic regression analysis identified cirrhosis at baseline to be the only independent predictor of non-SVR12 (OR: 16.1, 95% confidence interval 1.96–131.91, p=0.01). Apart from Hb, INR, and ALP, all other laboratory parameter improved following treatment (p<0.05).
Conclusion: Both SOF-B and OPR-B regimens achieved high SVR12 rates in this real-life cohort of patients with chronic HCV infection, similar to what is reported in other real-world studies. Cirrhosis was the only independent predictor of poor response.
Keywords: HCV, DAAs, treatment, sustained viral response, cirrhosis, liver disease
Introduction
Hepatitis C virus (HCV) infection represents a serious challenge to global health, with an estimated worldwide prevalence of 71.1 million1 even though it has dropped significantly from the estimated 170 million a decade ago.2 A substantial percentage of patients with chronic HCV infections develop significant complications, mainly chronic hepatitis C (CHC), liver cirrhosis, liver cell failure, and hepatocellular carcinoma (HCC).3–7
The primary goal of HCV treatment is to achieve a sustained virologic response (SVR), which is defined as undetectable HCV RNA levels at 12 weeks (SVR12) or 24 weeks (SVR24) after the completion of treatment.8 The achievement of SVR in patients with HCV infection is associated with infection eradication, improvement in their quality of life, and a reduced risk of complications including cirrhosis and HCC.9,10
Pegylated interferon-based therapy was the standard-of-care (SOC) therapy for HCV infection for nearly 2 decades. However, the introduction of 2nd generation direct-acting antivirals (DAAs) and interferon-free regimens represents the beginning of a new era and a revolution in the treatment of HCV. In a systematic review and a network meta-analysis of 27 randomized controlled trials (RCTs) involving 3415 patients with CHC treated with different DAA regimens, the SVR ranged from 94% to 99%, the greatest rates for patients without cirrhosis being estimated for those receiving sofosbuvir + velpatasvir with ribavirin for 12 weeks (99%; 95% Credible Intervals, 98–100%), and those with cirrhosis receiving sofosbuvir + velpatasvir for 24 weeks (96%; 95% CrI, 92-–99%). Ribavirin increased efficacy in patients with and without cirrhosis (Odds Ratio, 2.6–4.5).11 However, real-life results concerning the efficacy of such therapies for HCV are still scarce. In fact, efficacy rates reported in RCTs can be lower in community-based practice settings due to concomitant diseases or constitutional factors. Knowledge of these factors is valuable for the future management of affected patients.12
In this study, we share our clinical experience in treating patients with chronic HCV infection and evaluate treatment efficacy on a real-life practical ground. The objectives were to ascertain the SVR12 in consecutive patients treated at our facility and identify which factors are associated with better sustained virologic rates.
Material and methods
Study design
This was a prospective, non-randomized, observational single-center cohort study.
Patients
All consecutive patients who started treatment for HCV infection (genotypes 1–4) at the Salmaniya Medical Complex hospital were included in this study and followed up from January 2016 to September 2017. Patients who had not received any prior treatment (treatment-naïve) and those who had (treatment-experienced) were both included, as well as patients with hepatocellular carcinoma. Patients with concomitant hepatitis B virus and/or HIV infections were excluded.
Methods
All patients were subjected to thorough history talking (age, sex, history of diabetes mellitus, hypertension, liver transplantation, hyperlipidemia, hypothyroidism, end-stage renal disease, renal transplantation, sickle-cell disease, other systemic comorbidities) and full clinical examination.
At baseline (pre-treatment) and 12 weeks after the end of therapy, the following laboratory investigations were performed: HCV antibody, HCV RNA PCR quantitation, complete blood count, international normalization ratio (INR), partial thromboplastin time (PTT), serum creatinine, serum albumin, total serum bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (γ-GT). HCV genotype was performed only at baseline.
HCV genotyping and consolidated HCV viral load estimation were performed using a fully automated Abbott m2000 machine along with the manufacturer supplied reagent kits (Abbott Molecular, Abbott Park, IL, USA). This assay quantifies HCV RNA using in vitro reverse transcription-polymerase chain reaction (PCR) method, and it has a sensitivity of 12 IU/mL for 0.5 mL and 30 IU/mL for 0.2 mL sample volume with a detection range of 12 IU/mL (log 1.08 IU/mL) to 100 million IU/mL (log 8.0 IU/mL). It detects genotypes 1–6 with a specificity of ≥99.5%. Genotyping was performed using standard oligonucleotide-specific primers through PCR.
A baseline abdominal ultrasound was performed to evaluate the presence of cirrhosis and its complications (shrunken liver, coarse echotexture, irregular surface, dilated portal vein, ascites, splenomegaly).
Efficacy and safety assessment
Sustained virologic response to therapy was assessed at week 12 post the completion of treatment (SVR12) by HCV RNA PCR quantitation. Patients were followed up regularly for adverse events or abnormal findings on physical examination and clinical laboratory tests. They were seen fortnightly during the first 4 weeks of treatment, then every 4 weeks till the end of treatment, and 12 weeks after end-of-treatment.
Ethical considerations
The study was approved by the local institutional research ethics and scientific committees of Salmaniya Medical Complex Hospital. This work was conducted in accordance with the Declaration of Helsinki (2013) and the International Conference on Harmonization Guidelines for Good Clinical Practice (ICHG-GCP). A written informed consent was obtained from all participants, and their data sheets were coded to ensure anonymity and confidentiality.
Statistical analysis
Data were collected, revised, coded, and analyzed with the statistical software SPSS (Statistical Package for Social Sciences) Version 16.0 (SPSS, Chicago, IL, USA). Descriptive analysis of data was in the form of percentages, mean, or medians, and data are expressed as mean±standard deviation (SD) or number and percentages (%) as appropriate. An intention-to-treat analysis was performed. For numerical data, univariate analysis was performed for all independent variables using two sample t-tests, Wilcoxon Signed Ranked, or Mann–Whitney U tests as appropriate. For categorical data, univariate binary logistic regression analysis was performed for all independent variables using Chi-Square or Fisher’s exact test as appropriate. Based on the variables that showed statistical significance in the univariate analysis, multiple logistic regression analysis with the forward stepwise variable selection was used to identify the independent predictors impacting response to treatment. A p-value of <0.05 was set as a level of significance.
Results
Baseline (pre-treatment) patients’ characteristics
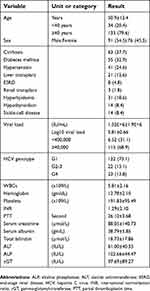
Baseline demographic, comorbidities, virologic, and laboratory characteristics of the cohort of the study are shown in Table 1. A total of 167 patients were included in this study. Their mean age was 50.9±12.4 years, 91 (54.5%) were males, 55 (32.9%) were diabetic, 41 (24.6%) were hypertensive, 63 (37.7%) were cirrhotic, 21 (12.6%) had liver transplantation, 14 (8.4%) had hypothyroidism, 14 (8.4%) had sickle cell disease, 31 (18.6%) had hyperlipidemia, 8 (4.8%) had end-stage renal disease, and 3 (1.8%) had renal transplant. One patient had HCC and he achieved SVR. High baseline (pre-treatment) viral RNA load ≥400,000 IU/L was detected in 115 (68.9%) of patients. Genotype 1, 2–3, and 4 were detected in 122 (73.1%), 22 (13.1%), and 23 (13.8%) patients, respectively.
 | Table 1 Baseline (pre-treatment) patients’ characteristics (n=167) |
Types, duration, and combinations of treatment regimens
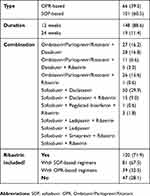
Ombitasvir/Paritaprevir/Ritonavir-based (OPR-B) and Sofosbuvir-based (SOF-B) regimens were given to 66 (39.5%) and 101 (60.5%) patients, respectively (Table 2). A total of 148 (88.6%) patients received therapy for 12 weeks and the remaining 19 (11.4%) patients were treated for 24 weeks. In addition, 120 (71.9%) patients were treated concomitantly with ribavirin, 81 (67.5%) of those received the SOF-B regimen and 39 (32.5%) the OPR-B regimen.
 | Table 2 Types, duration, and combinations of treatment regimes |
Response to therapy
SVR was achieved in 156 (93.4%) patients, and only 11 (6.7%) did not. The actual characteristics of the 11 patients with no response to therapy are shown in Table 3. Of them, 7 patients had adherence issues although they did not report any side effects, and 4 failed to achieve SVR despite completion of their regimens. Among the 7 who stopped therapy and did not complete their course, 4 were on SOF-B regimens and 4 were on a 24-week regimen. Two of the patients who completed treatment were on a SOF-B regimen while the other 2 were on an OPR-B regimen. A higher proportion of patients who achieved SVR12 had a genotype 1&4 infection (87.2%) compared to those who did not respond (81.8%); however, this was not statistically significant (p=0.611, Table 4).
 | Table 3 Baseline characteristics of 11 patients with failure to achieve SVR12 |
 | Table 4 Comparison between patients according to response to therapy |
Comparison between patients according to response to therapy
As shown in Figures 1 and 2 and Table 4, patients who achieved SVR (n=156) were similar to those who did not achieve SVR (n=11) regarding all demographics, comorbidities, virologic parameters, regimens used, and laboratory parameters apart from the rate of cirrhosis (p<0.001) and the duration of therapy (p=0.024). Using logistic regression analysis, only cirrhosis was found to independently predict SVR (OR: 16.09; 95% confidence interval 1.96–131.09; p=0.01) (Table 5).
 | Table 5 Results of the multivariate logistic regression analysis for the independent predictors of sustained virologic response |
Comparison between patients according to treatment regimen
As shown in Figure 3, patients who received SOF-based therapy (n=101) and those who received OPR-based (n=66) were similar regarding the rate of SVR (94.1% and 92.4%, respectively; p=0.754).
The impact of treatment on laboratory results
As shown in Table 6, successful completion of the treatment regimen with achievement of SVR in 93.4% of cases lead to a significant improvement in the mean total WBCs, platelets, and albumin levels, with significant reduction in the mean serum bilirubin, ALT, and γGT levels. However, Hb, INR, and ALP levels did not change.
 | Table 6 The impact of treatment on the laboratory results |
Safety
The 7 patients (4.2%) who could not adhere to treatment did not report any side effects. The reduction in Hb with ribavirin was insignificant and did not lead to need for transfusion, dose reduction, nor cessation of therapy. There were no mortalities.
Discussion
Even though phase III randomized controlled trials offer robust evidence of the efficacy of drug treatments, real-life therapeutic studies, like the present study, are invaluable. The conditions in RCTs are tightly controlled, and the results may not necessarily translate into real-world outcomes where there is inability to control over patients’ adherence, there is absence of inclusion and exclusion criteria, presence of variable comorbidities, and other factors which may affect the rate of SVR. In this study, we have shown that DAAs, irrespective of the type of or length of regimen used, are highly effective in achieving SVR, and the only independent predictor of response is presence of cirrhosis.
Although our experience indicates higher but not statistically significant SVR in patients infected with genotype 1&4 compared to those infected with other genotypes, another real-life study from Hawaii contradicts these findings with lower overall SVR rates for genotypes 1 and 4 (75%) compared to genotype 3 (81%).13 The authors attributed this to the inclusion of patients who may be excluded from clinical trials, such as those with prior treatment history, nonadherence issues, or with comorbidities that could result in discontinuation of treatment or loss to follow-up. Higher rates of noncompliance were noted among genotype 3 patients because of the longer duration of the regimen (24 weeks vs 12 weeks). In contrast, compliance was high in our cohort, and only 4 patients failed to achieve SVR despite completion of their course.
SVR of patients on SOF-based regimens was not affected by age, high viral load, or advanced fibrosis in the present cohort. Presence of comorbidities like diabetes mellitus, essential hypertension, hypothyroidism, hyperlipidemia, and sickle cell disease also did not affect the overall SVR, a similar conclusion to the Hawaii study;13 however, the authors identified that male gender was a statistically significant factor for failure to achieve SVR, something that did not hold true in our cohort.
In another large real-world cohort study (n=485), patients on sofosbuvir and daclatasvir therapy, with or without ribavirin, achieved high SVR12 (91%) and tolerated the treatment well, regardless of HCV genotype or cirrhosis, liver transplant or HIV/HCV coinfection status, and only 28 patients discontinued treatment.14 Our results are similar, with the exception of the influence of cirrhosis which appears to influence SVR. What is encouraging for patients with HCV is the large number of recent real-world studies from around the world that show similar results with high SVRs with different regimens, different genotypes, different durations of therapy (from 8 to 24 weeks), with or without ribavirin, whether patients are treatment-naïve or treatment-experienced, and whether the DAAs are original or generic.15–29
The present study failed to demonstrate a difference in SVR12 between treatment regimes, whether they were SOF-based or OPR-based. Although this may be interpreted as non-SVR being probably related to host, disease, or viral factors rather than regimen-related factors such as type and length of treatment, this is likely a type 2 error. In the literature, for example, ribavirin has a positive additional effect on SVR in certain regimens11 while in others, such as daclatasvir plus sofosbuvir, it does not.18
Patients with cirrhosis had significantly lower SVR12 compared to those without cirrhosis, and in multivariate analysis, cirrhosis was found to be the only independent predictor of non-SVR in our cohort. This is supported by a recent study from Egypt involving 2400 patients with HCV-related cirrhosis, where sofosbuvir and ribavirin therapy lead to SVR in only 71.2%, with more than 5% of patients discontinuing therapy due to adverse effects.21 In a Chinese study, patients with HCV-related decompensated cirrhosis achieved 90% SVR;23 however, this likely relates to the small number of patients (n=30).
One of the interesting findings in this real-world cohort is the high rate of response in the 21 patients who received treatment post-liver transplant (SVR12=100%). A recent Swedish study also identified that SVR12 was achieved in 91/93 (97.8%) of patients who relapsed post-liver transplantation, with 100% rates for genotype 2, 3, and 4, and a 96% rate for genotype 1.29
The main limitation of this study is its sample size, which limits any subgroup comparisons. In addition, since the allocation of patients to treatment was not randomized, direct comparisons between regimens, even as broadly as SOF-B and OPR-B, is limited. This is also confounded by the fact that there is great heterogeneity in the regimens used, where there is also one patient who received pegylated interferon in addition to sofosbuvir. Despite that, our results are comparable to other real-life studies of DAAs, where SVRs in excess of 90% are demonstrated. Finally, we did not evaluate the rapid viral response (RVR) with viral kinetics at 4 weeks. However, detecting a difference in RVR would be unlikely due to the high SVR.
Conclusion
Patients with chronic HCV irrespective of genotype, viral load, age, gender, and other medical comorbidities benefit greatly from SOF-B and OPR-B regimens. Both 12 and 24-week treatments are effective and well tolerated. However, cirrhosis influences the rate of SVR adversely.
Disclosure
The authors declare that no financial or any other conflicts of interest are associated with this work.
References
1.
2. Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi:10.1111/j.1478-3231.2008.01934.x
3. Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100.
4. Yoshida H, Tateishi R, Arakawa Y, et al. Benefit of interferon therapy in hepatocellular carcinoma prevention for individual patients with chronic hepatitis C. Gut. 2004;53:425–430.
5. Bruno S, Facciotto C. The natural course of HCV infection and the need for treatment. Ann Hepatol. 2008;7:114–119.
6. Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17–35.
7. Ascione A, Tartaglione T, Di Costanzo GG. Natural history of chronic hepatitis C virus infection. Dig Liver Dis. 2007;39(Suppl 1):S4–S7.
8. Formann E, Steindl-Munda P, Hofer H, et al. Long-term follow-up of chronic hepatitis C patients with sustained virologic response to various forms of interferon-based anti-viral therapy. Aliment Pharmacol Ther. 2006;23:507–511. doi:10.1111/j.1365-2036.2006.02785.x
9. Arase Y, Ikeda K, Suzuki F, et al. Long-term outcome after interferon therapy in elderly patients with chronic hepatitis C. Intervirology. 2007;50:16–23. doi:10.1159/000096308
10. Annicchiarico BE, Siciliano M, Avolio AW, Grillo RL, Bombardieri G. A 5-year prospective study of the late resolution of chronic hepatitis C after antiviral therapy. Aliment Pharmacol Ther. 2007;25:1039–1046. doi:10.1111/j.1365-2036.2007.03295.x
11. Berden FA, Aaldering BR, Groenewoud H, et al. Identification of the best direct-acting antiviral regimen for patients with hepatitis C virus genotype 3 infection: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2017;15:349–359. doi:10.1016/j.cgh.2016.10.034
12. Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–325. doi:10.1016/j.jhep.2011.05.032
13. Wu CJ, Roytman MM, Hong LK, et al. Real-world experience with sofosbuvir-based regimens for chronic hepatitis C, including patients with factors previously associated with inferior treatment response. Hawaii J Med Public Health. 2015;74:3–7.
14. Welzel TM, Petersen J, Herzer K, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virologic response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65:1861–1870. doi:10.1136/gutjnl-2016-312444
15. Marciano S, Haddad L, Reggiardo MV, et al. Effectiveness and safety of original and generic sofosbuvir for the treatment of chronic hepatitis C: a real world study. J Med Virol. 2018. Epub ahead of print. doi:10.1002/jmv.25033
16. El-Khayat HR, Kamal EM, El-Sayed MH, et al. The effectiveness and safety of ledipasvir plus sofosbuvir in adolescents with chronic hepatitis C virus genotype 4 infection: a real-world experience. Aliment Pharmacol Ther. 2018. Epub ahead of print. doi:10.1111/apt.14502
17. Thi Thu Thuy P, Bunchorntavakul C, Dat HT, et al. Sofosbuvir-ledipasvir with or without ribavirin for chronic hepatitis C genotypes 1 and 6: real-world experience in Vietnam. Antivir Ther. 2018;23(5):415–423. doi:10.3851/IMP3217.
18. Omar H, El Akel W, Elbaz T, et al. Generic daclatasvir plus sofosbuvir, with or without ribavirin, in treatment of chronic hepatitis C: real-world results from 18 378 patients in Egypt. Aliment Pharmacol Ther. 2018;47:421–431. doi:10.1111/apt.14428
19. Bichoupan K, Tandon N, Crismale JF, et al. Real-world cure rates for hepatitis C virus treatments that include simeprevir and/or sofosbuvir are comparable to clinical trial results. World J Virol. 2017;6:59–72. doi:10.5501/wjv.v6.i4.59
20. Buggisch P, Vermehren J, Mauss S, et al. Real-world effectiveness of 8-week treatment with ledipasvir/sofosbuvir in chronic hepatitis C. J Hepatol. 2018;68(4):663–671. doi:10.1016/j.jhep.2017.11.009. Epub 2017 Nov 11.
21. Abd-Elsalam S, Sharaf-Eldin M, Soliman S, Elfert A, Badawi R, Ahmad YK. Efficacy and safety of sofosbuvir plus ribavirin for treatment of cirrhotic patients with genotype 4 hepatitis C virus in real-life clinical practice. Arch Virol. 2018;163:51–56. doi:10.1007/s00705-017-3573-0
22. Nagaty A, Abd El-Wahab EW, Kanda T. Real-life results of sofosbuvir based therapy in chronic hepatitis C -naïve and -experienced patients in Egypt. PLoS One. 2017;12:e0184654. doi:10.1371/journal.pone.0184654
23. Ji F, Wang W, Dang S, et al. Outcomes after sofosbuvir-containing regimens for hepatitis C virus in patients with decompensated cirrhosis: a real-world study. Infect Agent Cancer. 2017;12:48. doi:10.1186/s13027-017-0158-1
24. Latt NL, Yanny BT, Gharibian D, Gevorkyan R, Sahota AK. Eight-week ledipasvir/sofosbuvir in non-cirrhotic, treatment-naïve hepatitis C genotype-1 patients with hepatitis C virus-RNA <6 million: Single center, real world effectiveness and safety. World J Gastroenterol. 2017;23:4759–4766. doi:10.3748/wjg.v23.i26.4759
25. Wehmeyer MH, Ingiliz P, Christensen S, et al. Real-world effectiveness of sofosbuvir-based treatment regimens for chronic hepatitis C genotype 3 infection: results from the multicenter German hepatitis C cohort (GECCO-03). J Med Virol. 2018;90:304–312. doi:10.1002/jmv.24903
26. Dalgard O, Weiland O, Noraberg G, et al. Sofosbuvir based treatment of chronic hepatitis C genotype 3 infections-A Scandinavian real-life study. PLoS One. 2017;12:e0179764. doi:10.1371/journal.pone.0179764
27. Curry MP, Tapper EB, Bacon B, et al. Effectiveness of 8- or 12-weeks of ledipasvir and sofosbuvir in real-world treatment-naïve, genotype 1 hepatitis C infected patients. Aliment Pharmacol Ther. 2017;46:540–548. doi:10.1111/apt.14204
28. Johnson SW, Ammirati SR, Hartis CE, et al. Effectiveness of ledipasvir/sofosbuvir in real-world patients with chronic hepatitis C: a collaborative treatment approach. Int J Antimicrob Agents. 2017;49:778–781. doi:10.1016/j.ijantimicag.2017.01.016
29. Maria C, Michael S, Susanne C, et al. INF-free sofosbuvir-based treatment of post-transplant hepatitis C relapse - a Swedish real life experience. Scand J Gastroenterol. 2017;52:585–588. doi:10.1080/00365521.2017.1283439
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.