Back to Journals » Infection and Drug Resistance » Volume 16
Efficacy of Active Rapid Molecular Screening and IPC Interventions on Carbapenem-Resistant Enterobacterales Infections in Emergency Intensive Care Units without Enough Single-Room Isolation
Authors Yang S , He L, Li K, Yu X, Ni L, Hu L, Guo J, Biskup E, Tang L, Wu W
Received 24 November 2022
Accepted for publication 8 February 2023
Published 20 February 2023 Volume 2023:16 Pages 1039—1048
DOI https://doi.org/10.2147/IDR.S396331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Simin Yang,1,* Lihua He,2,* Ke Li,3,* Xiaoyu Yu,1 Lijun Ni,1 Liang Hu,1 Jian Guo,1 Ewelina Biskup,4,5 Lunxian Tang,3 Wenjuan Wu1
1Department of Laboratory Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Department of Hospital Infection Management, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 3Department of Emergency Intensive Care Unit, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 4Department of Basic and Clinical Medicine, Shanghai University of Medicine and Health Sciences, Shanghai, People’s Republic of China; 5Department of Internal Medicine, University Hospital of Basel, Basel, Switzerland
*These authors contributed equally to this work
Correspondence: Lunxian Tang; Wenjuan Wu, Tel +86-15921155750 ; +86-13386057159, Email [email protected]; [email protected]
Purpose: To investigate whether rapid active molecular screening and infection prevention and control (IPC) interventions can reduce colonization or infection with carbapenem-resistant Enterobacterales (CRE) in a general emergency intensive care unit (EICU) without enough single-room isolation.
Methods: The study was designed as a before-and-after quasi-experiment. Before the experimental period, the ward was rescheduled and the staff were trained. From May 2018 to April 2021, active screening was performed by seminested real-time fluorescent polymerase chain reaction (PCR) detection with rectal swabs from all patients on admission to the EICU, and the results were reported in 1 hour. Other IPC interventions including hand hygiene, contact precautions, patient isolation, environmental disinfection, environment surveillance, monitoring, auditing and feedback were conducted under strict supervision. The patients’ clinical characteristics were collected simultaneously.
Results: In this 3-year study, 630 patients were enrolled and 19.84% of the patients were initially colonized or infected with CRE as shown by active molecular screening. The average drug resistance ratio to carbapenem shown by clinical culture detection of Klebsiella pneumoniae (KPN) before the study was performed was 71.43% in EICU. The drug resistance ratio decreased significantly from 75%, 66.67% to 46.67% in the next 3 years (p< 0.05) during which active screening and IPC interventions were strictly executed. While the ratio gaps between EICU and the whole hospital were narrowed from 22.81%, 21.11% to 4.64%. Patients with invasive devices, skin barrier damage, and the recent use of antibiotics on admission were found to have a higher risk of being colonized or infected with CRE (p< 0.05).
Conclusion: Active rapid molecular screening and other IPC interventions may significantly reduce CRE nosocomial infections even in wards without enough single-room isolation. The key to reduce the spread of CRE in the EICU is the strict execution of IPC interventions by all medical staff and healthcare workers.
Keywords: carbapenem-resistant, Enterobacterales, active screening, infection prevention and control intervention, emergency intensive care unit, nosocomial infection
Introduction
Carbapenem-resistant Enterobacterales (CRE) represent a serious therapeutic problem due to their pronounced multidrug resistance, with the main pathogens being Klebsiella pneumoniae, Escherichia coli, and other Enterobacterales.1 The worldwide spread of CRE is a global burden and has become one of the priorities of the Centers for Disease Control (CDC).2 In recent decades, along with the advent of extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, carbapenems have been used more frequently due to their broad antibacterial spectrum, thus contributing greatly to the drastic increase in the CRE problem.3,4 In some parts of Asia, especially eastern China, CRE have become endemic and CRE infections have reached epidemic dimensions.5,6
Recent studies have shown that the majority of CRE infections in China are caused by carbapenem-resistant Klebsiella pneumoniae (CR-KP), mostly leading to serious infections in ICUs.7,8 In our institution, the detection rates of CR-KP (included infected and colonized) from 2015 to 2017 ranged between 41.3% and 45.5%. These rates were much higher than the reported average in Shanghai (27.3%, data collected by CHINET, China Antimicrobial Surveillance Network) and can be mainly attributed to CR-KP infections in the ICU, where the carbapenem resistance rate reached 71.43% in 2017.
It is better to prevent CRE infections rather than try and control them after they start to spread. The WHO published guidelines for infection prevention and control (IPC) of CRE, carbapenem-resistant Acinetobacter baumannii (CRAB), and carbapenem-resistant Pseudomonas aeruginosa (CRPsA), including hand hygiene, patient surveillance, contact precautions, patient isolation (single isolation or concentrated isolation), environmental disinfection, environment surveillance, and monitoring, auditing and feedback.9 However, in China, compliance with these guidelines has not been satisfactory. One of the reasons is that the available literature mostly reported active screening based on bacterial culture tests that take at least 24 h to obtain the results. In addition, compared with those reported in the literature, the hospital settings were quite different in China, in which ICUs were not equipped with enough 1-bed rooms or isolation rooms.10 In China, IPC interventions mostly only include hand hygiene and contact precautions, and the number of isolation rooms are usually inadequate in ICUs to accommodate every infected or colonized patient.11
We conducted a prospective, multi-stage, long-term study to investigate whether a combination of screening and interventions can provide effective, efficient, long-lasting results with regard to reducing CRE colonization/infection rates. We aimed to establish whether IPC together with detection and monitoring of CRE colonization/infection (active screening at admission) of EICU patients by rapid semi-nested real-time fluorescent PCR without enough single-room isolation are effective with regard to the reduction of the infection, mortality and morbidity rates.
Materials and Methods
Settings
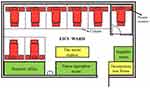
The study was conducted in a comprehensive teaching hospital. Patients were prospectively recruited from May 2018 through April 2021 in the EICU, where approximately 200–280 critically ill patients are treated every year. The EICU consists of 8 beds; one bed is located in a private room, and two others are in a relatively isolated area (Figure 1). Ethical approval was obtained from Shanghai East Hospital (Tongji University School of Medicine) in April 2018. The approval No. is [2018] (07).
Infection-Control Interventions and Data Collection
Before the initiation of the program, routine culture data were collected from May 2017 to April 2018, and this period was defined as the baseline period during which almost no isolation measures or patient surveillance were conducted for the patients with CRE. Although most nurses and doctors regularly disinfect their hands and adhere to contact precautions, the cleaners in this department did not strictly obey the standard routines, including hand hygiene, contact precautions and environmental disinfection, according to our investigation. Clinical specimens were regularly collected for culture. The data from this baseline period served as a reference for comparison with the data from our experimental period.
The program was performed in the next 3 years. Initially, EICU professional training at the practical and theoretical levels was conducted based on the IPC model, including hand hygiene, patient surveillance, contact precautions, patient isolation (single or aggregated), environmental disinfection, environmental surveillance, monitoring, auditing and feedback, and explanation of the goals, objectives, significance and exact methodology of the project. The entire ward was emptied, and the health care workers disinfected the environment with sodium hypochlorite disinfectant, ultraviolet-light radiation and Clinell Universal Wipes, which is a combination of benzalkonium chloride, didecyl dimethyl ammonium chloride and polyhexamethylene biguanide (PHMB). The hygiene control group examined and confirmed that the ward was contamination-free prior to the study onset. An inspection of hand hygiene was also conducted for all health care workers, followed by intensive training of the study guidelines for sampling and transportation.
The isolation guidelines were developed based on the spatial constraints of the ward. In the case of multiple patients presenting with infectious diseases, bedside screens, curtain dividers and isolation signage, with instructions and precautions (clothing, hand disinfection, etc), were utilized. Therapeutic supplies and household items were used separately for each patient and disposed of after use. Daily care provided by the health care workers was strictly directed and supervised, as were the measures for daily disinfection and the final disinfection after the discharge of the patients from the ward.12 A flowchart of workflow and IPC interventions is provided in Figure 2.
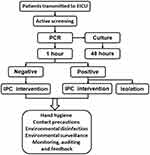 |
Figure 2 Flowchart of workflow and IPC interventions. When patients were transmitted to the ECIU, active molecular screening and other IPC interventions were conducted according the flowchart. |
From May 2018 to April 2021, rapid active screening by molecular detection and IPC interventions were conducted. At admission to the EICU, rectal swab sampling was performed by a trained nurse, and the swab was immediately sent to the clinical laboratory. Each sample was accompanied by a case report form (CRF) containing the clinical characteristics of the patients, including name, sex, age, admission time, temperature, diagnosis, invasive devices and/or skin barrier damage, and laboratory test results (Supplementary Table 1). The samples were tested with Gene Xpert, a semi-nested real-time fluorescent PCR method, and cultivated at the same time. The Gene Xpert results were reported within 1 hour. Isolation was implemented according to the results of the molecular detection test, even if the culture results did not exactly match (ie, the phenotype and genotype of CRE did not match exactly). Infection control interventions were undertaken to reduce the epidemic spread of CRE. During hospitalization, various types of specimens from patients were sent to the laboratory for culture, which was the same procedure that was followed during the baseline stage. Environmental check-ups and disinfection measures were performed at least twice a day, and environmental surveillance was performed twice a month. In addition, sink monitoring and dust avoidance measures during bed preparation were implemented.13 The correlations of the clinical characteristics of the EICU patients with the Gene Xpert results and mortality were analyzed.
Active Rapid Molecular Screening by GeneXpert Carba-R Assay
A pair of rectal swabs was obtained from each patient at admission to the EICU. One swab was placed into the sample reagent vial and vortexed at high speed for 10 seconds. The prepared sample was aspirated using the transfer pipette provided and transferred into the Xpert Carba-R Assay cartridge, which was then inserted into the GeneXpert instrument. The results are obtained in 45 minutes. Rapid active screening by molecular detection was carried out with the GeneXpert Carba-R Assay, which simultaneously detects three carbapenemase genes, including blaKPC, blaNDM, blaIMP, blaVIM and blaOXA-48.
Bacterial Isolation and Antimicrobial Susceptibility Testing
Surveillance cultures were sampled simultaneously with unused rectal swabs on McConkey agar with a 10 g meropenem disc (Oxoid). Further routine culture specimens were obtained, including sputum, endotracheal aspirate, urinary tract, blood, and infection sites (individually selected according to the patient’s symptoms/differential diagnosis). Culture sampling was repeated weekly or more often when necessary (eg, in cases of fever, elevated levels of inflammatory markers, etc) Thus, we were able to monitor the incidence of CRE colonization or infection once a week or more frequently if the patient had a fever or developed other symptoms of infection. All isolates were identified by MALDI-TOF MS (Autof ms1000), and routine antibiotic susceptibility tests were performed with the VITEK2 compact system (bioMérieux, France) to determine carbapenem resistance. The control strain was Escherichia coli ATCC 25922. Susceptibility breakpoints were interpreted as per the Clinical and Laboratory Standards Institute guidelines (CLSI M100).14
Multiplex PCR Detected Carbapenemase Genes
Carbapenemase genes of the isolates collected from the rectal swab and cultured on admission were verified by multiplex PCR with the primers referred in the previous study.15
Pulsed-Field Gel Electrophoresis (PFGE)
A retrospective analysis of the genetic correlations between the isolates from both rectal swabs on admission and the clinical cultures during hospitalization was performed by PFGE.16 The strains from different patients were interpreted as “related” if more than 80% of the bands matched, and “closely related” if more than 90% matched.
Statistical Analysis
All discontinuous variables (ie, sex, invasive devices or skin barrier damage on admission, antibiotic use before admission, outcomes) were compared between the Xpert positive and negative groups using the χ2 test or Fisher’s test. The means of all continuous variables (ie, age, WBC, CRP, PCT, days in hospital, and ICU days) were compared between the Xpert positive and negative groups using the Mann–Whitney U-test. All statistical analyses were performed using SPSS software, version 20.0. All tests were 2-tailed, with P values <0.05 or a 95% CI excluding 1 considered statistically significant.
Results
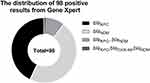
A total of 630 patients were enrolled in the study. According to the results of the rectal swabs of Gene Xpert detection on admission, 15.56% (98/630) of the patients carried carbapenemase genes, 8.25% had blaKPC (52/630), 0.16% had blaOXA-48 (1/630) and 8.89% had (56/630) blaNDM (Figure 3). A total of 1.43% (9/630) of the patients carried a combination of 2 different carbapenemase genes, and 0.16% (1/630) of the individuals carried a combination of 3 different carbapenemase genes. We collected 16 isolates of CRE (15 isolates of CR-KP and 1 isolates of carbapenem-resistant Escherichia coli) from 16 patients in the first year by direct culture on MacConkey agar of the positive rectal swabs obtained on admission. When the isolated strains were verified by multiplex PCR, only one gene (blaKPC or blaNDM) could be detected, even though Gene Xpert suggested the presence of 3 different genes in one sample from the swab. The drug susceptibility information of 16 CRE strains isolated from the rectal swabs on admission and 14 CRE strains isolated from the clinical cultures from various specimens are shown in Supplementary Table 2.
The pulsed-field gel electrophoresis (PFGE) patterns included isolates collected from 16 patients on admission and from the 13 clinical samples afterward (The clinical samples were all collected after 48h of admission.) (Figure 4). The PFGE results of the isolates from the admission screening rectal swabs suggested that 4 pairs of the strains were from the same clone of the pathogen, while 6 (75%) of the patients were transferred from emergency medical unit. The samples collected from a patient on admission and the clinical culture during his stay in the EICU showed same PFGE patterns. We compared the PFGE with other information, such as those for the bed in the ward, time of admission, collection time of the samples and where the patients were transferred from. Comparing the PFGE results of the 13 clinical samples afterward with the results of admission screening rectal swabs, except for the strains collected from the same patient, the PFGEs of 7 clinical strains were related to the isolates collected on admission, which suggested nosocomial infections.
We collected and analyzed the detailed clinical information of the 224 patients in the first year. The clinicopathological characteristics of the included patients, laboratory and Gene Xpert test results, duration of hospitalization and outcomes are recorded in Table 1. Patients with invasive devices or skin barrier damage on admission (p=0.002) and those who used antibiotics before admission (p<0.001) were more likely to be colonized or infected by CRE, as shown in Table 1. The latter observation confirms that previous antibiotics prescription increases the risk of CRE infections. There was no significant association between the Xpert test results and the outcome (p=0.412). The details of the clinicopathological/laboratory characteristics are shown in original data file.
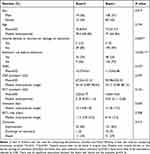 |
Table 1 Clinicopathological/Laboratory Characteristics and Outcomes in the First Year |
IPC interventions with active rapid molecular screening effectively reduced the colonization and infection of CRE in the EICU. We compared the drug resistance rate of CR-KP by regular clinical culture between baseline period and experimental period to reveal the effort of the interventions. The resistance rate of CR-KP colonization and infection identified by routine clinical culture detection significantly decreased from 71.43% to 46.67%. Comparing with the rate of the whole hospital, the drug resistance ratio gaps between EICU and the whole hospital were narrowed from 22.81%, 21.11% to 4.64% year by year (Table 2, Figure 5), supporting the primary hypothesis that rapid active screening by molecular detection in combination with other IPC interventions can reduce nosocomial CRE infections. To exclude the effect of any change in in-hospital patients from baseline period and experimental period, main diagnoses in both periods were compared, including sepsis, acute heart failure, severe pneumonia, cerebral infarction, and so on. There was no significant difference in diseases between baseline period and experimental period.
 |
Table 2 The Active Molecular Screening Results of Patients on Admission and Drug Resistance Rate of CR-KP Colonization/Infection of the Whole Hospital and the EICU |
Discussion
Carbapenem antibiotics are extensively used worldwide due to their strong and broad-spectrum antibacterial activity.17 CRE infections are an increasing challenge due to growing antimicrobial resistance and related high mortality.18,19 In this study, we conducted a series of planned and protocolized IPC interventions in an institution lack of enough isolation room. The aim of this program was to establish whether the combination of such interventions and measures of detection and monitoring of CRE colonization/infection (active screening at admission) by rapid, semi-nested real-time fluorescent PCR was effective in reducing the infection, mortality and morbidity rates in EICU patients. In our screening result, 15.56% (98/630) of the patients carried carbapenemase genes on admission to the EICU which suggested a big threat to CRE nosocomial epidemic. After our 3-years intervention, the resistance rate of CR-KP in EICU significantly decreased from 71.43% to 46.67%. We analyzed the clinical characteristics of the patients and found that there was no significant association between the Xpert test results and the outcomes. This may due to the reason patients were not followed up after they were discharged from hospital. The result in our study suggested that rapid active molecular screening and IPC interventions can reduce colonization or infection with CRE in the EICU without enough single-room isolation.
Carbapenemase-producing organisms (CPO) is a subset of Carbapenem resistant organisms (CRO) that produces an enzyme that destroys carbapenems. The production of carbapenemases is the main mechanism of carbapenem resistance. Carbapenem resistance genes spread rapidly among many bacterial species on multiple types of mobile genetic elements. Compared to traditional culture, the molecular detection takes a shorter time and is the most efficient method to screen for carbapenemase genes. The molecular detection is not affected when detecting organism with low carbapenem MICs, while not all carbapenem resistance genes give high MICs and some organisms will not grow on selective agar media. In our study, PCR test was implemented before the result of culture and Gene Xpert facilitates rapid results and active screening by detecting drug resistance genes and enables the clinical selection of antibiotics at the time point of CRE infection diagnosis.20 Distinguishing between serine (blaKPC, blaOXA-48) and metallo-carbapenemases (blaIMP, blaNDM, blaVIM) has important therapeutic importance.21 CRE producing serine retained β-lactam susceptibility to carbapenems such as ceftazidime–avibactam, meropenem–vaborbactam and imipenem–relebactam. Metallo-carbapenemases could be inhibited by metal-chelating agents. This molecular diagnostic test allows clinicians to diagnose CRE infections in a timely manner and to undertake appropriate treatment and management measures, isolating patients to avoid further spread of the bacteria.22
Active participation during the education phase of the intervention program was reported to be an important factor in decreasing the incidence of carbapenem-resistant pathogen infections.23 Feedback from screening services, a rapid turnaround time and efficient communication were all positively correlated with the overall success in outbreak control. In this study, we paid much attention to the education and training of medical professionals and health care workers, ensuring that they were familiar with the study-specific IPC intervention model, screening measures, and general hygiene guidelines. These educational approaches resulted in higher compliance and better adherence to study protocols, further resulting in the reduction of the infection rate, which highlight the importance of continuous education and specific training for these intervention processes.
The results of our study suggest that environmental surveillance is a very important part of IPC interventions.24 Environmental monitoring was helpful in providing clear feedback to health care providers about disinfection processes. In our study, environmental monitoring was conducted twice a month. CRPsA was once detected in the sink of the ward, the ward underwent a total disinfection process with additional surveillance thereafter. Such an approach was efficient and effective in reducing the CRE infection rate and supported the hygiene awareness of the individual health care providers.
The existing evidence regarding the effectiveness of isolation and precautions with regard to controlling the spread of CRE is inconsistent. A 4-year prospective study in Israel showed that isolation precautions alone are ineffective as IPC measures.25 A similar 4-year quasi-experimental study in China suggested that bundled IPC interventions, including isolation precautions, rapidly decrease the incidence of ICU-acquired CR-KP colonization/infection.14 Due to limited space and the large number of patients in the EICU, the number of isolation rooms is limited in our institution, and this is often the case in Chinese general hospitals. Since not every CRE-positive patient at the time of admission could be hospitalized in a separate room, clinical isolation was achieved using the available resources. Our results confirm the hypothesis that combined IPC and isolation precautions are effective, even in real-world conditions with limited resources.
There are some limitations of our study. First, this was a single-center study with a limited number of included patients. Second, due to large discrepancies in different health care systems, the clinical relevance of the results may be limited to hospitals in Asia/China. Third, the assessment of how strictly the IPC interventions were adhered to be mainly subjective.
Conclusion
We report objective data on how IPC interventions with active rapid molecular screening appropriately implemented in a Chinese hospital can contribute to an actual reduction in the CRE infection rate. Our protocols can be used as a basis for practical real-world recommendations and implementation in Chinese hospitals without enough single-room isolation. Further prospective studies in multicenter settings are warranted to provide valid clinical research data for the development of multidrug resistance prevention and control guidelines for developing countries.
Abbreviations
IPC, infection-prevention and control; CRE, carbapenem-resistant Enterobacterales; CR, carbapenem resistance; EICU, emergency intensive care unit; PCR, polymerase chain reaction; CDC, Centers for Disease Control; ESBL, extended spectrum beta-lactamase; CR-GNB, carbapenem-resistant gram-negative bacilli; CRAB, carbapenem-resistant Acinetobacter baumannii; CRPsA, carbapenem-resistant Pseudomonas aeruginosa; KPN, Klebsiella pneumoniae; CR-KP, carbapenem-resistant Klebsiella pneumoniae; CRF, case report form; PFGE, pulsed-field gel electrophoresis.
Data Sharing Statement
All data analysed during this study are provided in the original data file.
Ethics Approval and Informed Consent
This study was conducted complying with the Declaration of Helsinki. Ethical approval was obtained from Shanghai East Hospital (Tongji University School of Medicine) in April 2018. The approval No. is [2018] (07). All study participants and/or their immediate guardians signed a detailed informed consent form prior to the study enrollment.
Funding
The study was sponsored by the National Natural Science Foundation of China (grant number 81971990) and Shanghai Municipal Health Commission Clinical Research (20194Y0386).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Suay-García B, Pérez-Gracia MT. Present and future of carbapenem-resistant Enterobacteriaceae (CRE) infections. Antibiotics. 2019;8(3):122. doi:10.3390/antibiotics8030122
2. Tamma PD, Aitken SL, Bonomo RA, et al. Infectious Diseases Society of America 2022 Guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2022;75(2):187–212. doi:10.1093/cid/ciac268
3. Zhanel GG, Lawrence CK, Adam H, et al. Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs. 2018;78(1):65–98. doi:10.1007/s40265-017-0851-9
4. Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4):e202899. doi:10.1001/jamanetworkopen.2020.2899
5. Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) Strains in China. EBioMedicine. 2017;19:98–106. doi:10.1016/j.ebiom.2017.04.032
6. Paveenkittiporn W, Lyman M, Biedron C, et al. Molecular epidemiology of carbapenem-resistant Enterobacterales in Thailand, 2016–2018. Antimicrob Resist Infect Control. 2021;10(1):88. doi:10.1186/s13756-021-00950-7
7. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2):e01882–17. doi:10.1128/AAC.01882-17
8. Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25(8):943–950. doi:10.1016/j.cmi.2019.04.013
9. World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. Geneva: WHO Guidelines Approved by the Guidelines Review Committee; 2017.
10. Garpvall K, Duong V, Linnros S, et al. Admission screening and cohort care decrease carbapenem resistant Enterobacteriaceae in Vietnamese pediatric ICU’s. Antimicrob Resist Infect Control. 2021;10(1):128. doi:10.1186/s13756-021-00994-9
11. Li M, Wang X, Wang J, et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: a 4-year quasi-experimental before-and-after study. Antimicrob Resist Infect Control. 2019;8:8. doi:10.1186/s13756-018-0453-7
12. Martischang R, Buetti N, Balmelli C, Saam M, Widmer A, Harbarth S. Nation-wide survey of screening practices to detect carriers of multi-drug resistant organisms upon admission to Swiss healthcare institutions. Antimicrob Resist Infect Control. 2019;8:37. doi:10.1186/s13756-019-0479-5
13. Mathers AJ, Vegesana K, German Mesner I, et al. Intensive care unit wastewater interventions to prevent transmission of multispecies Klebsiella pneumoniae carbapenemase-producing organisms. Clin Infect Dis. 2018;67(2):171–178. doi:10.1093/cid/ciy052
14. Clinical and Laboratory Standards Institute (CLSI). Performance Standards For Antimicrobial Susceptibility Testing. CLSI Supplement M100.
15. Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–123. doi:10.1016/j.diagmicrobio.2010.12.002
16. Neoh HM, Tan XE, Sapri HF, Tan TL. Pulsed-field gel electrophoresis (PFGE): a review of the “gold standard” for bacteria typing and current alternatives. Infect Genet Evol. 2019;74:103935. doi:10.1016/j.meegid.2019.103935
17. Cerceo E, Deitelzweig SB, Sherman BM, Amin AN. Multidrug-resistant gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist. 2016;22(5):412–431. doi:10.1089/mdr.2015.0220
18. van Duin D, Arias CA, Komarow L, et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis. 2020;20(6):731–741. doi:10.1016/S1473-3099(19)30755-8
19. Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. doi:10.1016/S0140-6736(10)61458-4
20. Skodvin B, Wathne JS, Lindemann PC, et al. Use of microbiology tests in the era of increasing AMR rates- a multicentre hospital cohort study. Antimicrob Resist Infect Control. 2019;8:28. doi:10.1186/s13756-019-0480-z
21. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(suppl_1):S28–S36. doi:10.1093/infdis/jiw282
22. David S, Reuter S, Harris SR, et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat Microbiol. 2019;4(11):1919–1929. doi:10.1038/s41564-019-0492-8
23. Kousouli E, Zarkotou O, Politi L, et al. Infection control interventions affected by resource shortages: impact on the incidence of bacteremias caused by carbapenem-resistant pathogens. Eur J Clin Microbiol Infect Dis. 2018;37(1):43–50. doi:10.1007/s10096-017-3098-1
24. Pfrimmer DM, Johnson MR, Guthmiller ML, Lehman JL, Ernste VK, Rhudy LM. Surveillance: a nursing intervention for improving patient safety in critical care environment. Dimens Crit Care Nurs. 2017;36(1):45–52. doi:10.1097/DCC.0000000000000217
25. Cohen MJ, Block C, Levin PD, et al. Institutional control measures to curtail the epidemic spread of carbapenem-resistant Klebsiella pneumoniae: a 4-year perspective. Infect Control Hosp Epidemiol. 2011;32(7):673–678. doi:10.1086/660358
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.