Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Efficacy of a Topical Product Containing Purified Omental Lipids and Three Anti-Itching Compounds in the Treatment of Chronic Pruritus/Prurigo Nodularis in Elderly Subjects: A Prospective, Assessor-Blinded, 4-Week Trial with Transepidermal Water Loss and Optical Coherence Tomography Assessments
Authors Ardigò M, Franceschini C, Campione E , Cosio T , Lanna C, Bianchi L, Milani M
Received 23 November 2020
Accepted for publication 17 December 2020
Published 30 December 2020 Volume 2020:13 Pages 1051—1058
DOI https://doi.org/10.2147/CCID.S292636
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Marco Ardigò,1 Chiara Franceschini,1 Elena Campione,2 Terenzio Cosio,2 Caterina Lanna,2 Luca Bianchi,2 Massimo Milani3
1Clinical Dermatology, San Gallicano Dermatological Institute, Rome, Italy; 2Dermatology Institute, Università Tor Vergata, Rome, Italy; 3Medical Department, Cantabria Labs Difa Cooper, Caronno Pertusella, VA, Italy
Correspondence: Massimo Milani
Medical Department, Cantabria Labs Difa Cooper, Caronno Pertusella, Italy
Email [email protected]
Purpose: To investigate the efficacy of a cream containing purified omental lipids 10% and three anti-itching substances (polidocanol/stimutex/palmitoylethanolamine) in elderly subjects with chronic pruritus/prurigo nodularis (CP/CPN).
Patients and Methods: Thirty-five subjects (6 men; mean age 67± 4 years) with CP/CPN were enrolled in a prospective, assessor-blinded, 4-week study. The cream was applied twice daily in the most affected body area. The primary endpoints were the evolution of the 10-cm visual analogue itch severity scale (VAS) and the 4-point verbal itching rating scale (VRS) (from 0 to 3). Secondary endpoints were the evolution of optical coherence tomography (OTC) of four skin parameters (acanthosis/hyperkeratosis/scale/dermal vascular pattern), assessed in a target lesioned area, and the transepidermal water loss (TEWL). Study endpoints were evaluated at baseline and after 2 and 4 weeks by an investigator unaware of the type of treatment.
Results: All the enrolled subjects concluded the trial. At baseline, the mean±SD scores for VAS and VRS were 4.9± 2.2 and 1.7± 0.7, respectively. The treatment was associated with a significant reduction (p=0.0001) of VAS score of 60% at week 2 and of 86% at week 4. VRS score was significantly reduced by 49% after 2 weeks and by 81% after 4 weeks, in comparison with baseline. TEWL (expressed as g/m2/h) mean values were 18± 5.4 at baseline and 12.7± 4.4 at week 2 and 9.8± 4.7 at week 4 (P=0.0001 vs baseline). All the OCT parameters evaluated improved during active treatment; acanthosis grade was 0.22 mm at baseline, 0.19 mm at week 2 and 0.17 mm at week 4 (p=0.0005), representing a 23% reduction in comparison with baseline. The product was very well tolerated.
Conclusion: This purified omental lipid with three anti-itching components cream reduces significantly itch intensity in subjects with chronic pruritus/prurigo nodularis, improving the skin barrier function and skin structure.
Trial Number: ISRCTN869561669.
Keywords: chronic pruritus, prurigo nodularis, purified omental lipids, itch, antipruritic
Introduction
Itch is commonly defined as an unpleasant sensation that induces the desire to scratch.1 Chronic pruritus (CP), defined as pruritus lasting more than 6 weeks as defined by the International Forum for Study of Itch,2 is a frequent symptom in the general population, especially in older subjects with a relevant negative effect on the quality of life.3 Chronic pruritus could be a symptom of overt skin diseases like psoriasis, atopic dermatitis, lichen and urticaria or could be associated with systemic diseases, mainly chronic renal diseases, haematological conditions, and diabetes.4 However, quite commonly in subjects with CP is difficult to identify the underlying disease (the so call pruritus of unknown origin).5 Chronic itch is commonly observed in subjects aged 65 years or older.6 In more detail, CP is a very common and debilitating skin problem in the geriatric population with a reported prevalence ranging from 7% to 37%.6 CP is associated with secondary skin lesions due to prolonged scratching, like lichenification, skin fissuring and nodules.7 Prurigo nodularis or chronic prurigo (CPG), as suggested by European prurigo project,8 is a less common clinical form of CP and it is characterized by signs of repeated scratching and multiple, often symmetrical, pruriginous skin lesions such as papules, nodules and plaques.9 In elderly subjects, CP/CPN is very often associated with severe skin xerosis.10 A decrease in sebaceous and sweat gland activity is the primary cause of xerosis in the elderly.11 So far, there is not an accepted standard treatment for chronic itch.12 Purified porcine omental lipids (POL) are used in topical products in different textures (cream, fluid, emulsions, and cleanser) and at different concentrations (10–25%) for the treatment of fragile skin or other skin conditions at risk of ulcer formation (the so-called dermatoporosis of the elderly, severe skin xerosis in diabetic subjects, and skin at risk of pressure ulcers).13 Topical POL is very effective in the treatment of skin xerosis.14 Polidocanol is a non-ionic substance that possesses anaesthetic properties and moisturizing effects.15 Stimutex is a mixture of different compounds (spent grain wax, butyrospermum parkii extract, Argania spinosa kernel oil) with an antihistamine effect.16 Palmitoylethanolamine (PEA) is a lipid compound with anti-inflammatory, anti-allodynic and anti-hyperalgesic effects.17 Interestingly, PEA has transient receptor potential cation channels (TRPV) and peroxisome proliferator-activated receptors (PPAR) antagonism action.18 Both TRPV and PPAR receptors are involved in itch physiopathology.19,20 Furthermore, PEA could exert an anti-itching action through the modulation of cannabinoid receptor types 1 and 2 (CB1, CB2).21 A new cream formulation containing 10% of POL, polidocanol, Stimutex and palmitoylethanolamine, has been commercialized with the indication of moderate-severe itch relief. However, so far, no clinical data are available regarding its clinical efficacy and tolerability.
Study Aim
To investigate, clinically and instrumentally, the efficacy of a topical product containing purified omental lipids 10% and three anti-itching substances (polidocanol, stimutex and palmitoylethanolamine) in elderly subjects with chronic pruritus/prurigo nodularis (CP/CPN).
Patients and Methods
Subjects and Study Design
A total of 35 subjects (6 men and 29 women; mean age 67±4 years) with a positive history of chronic pruritus/prurigo nodularis (papular or nodular types) were enrolled in a prospective, assessor-blinded, 4-week study after written informed consent. Inclusion criteria were men and women with an age >60 years and a positive history of chronic (>6 weeks) pruritus with or without secondary skin lesions or the presence of prurigo nodularis lesions or severe skin xerosis. Exclusion criteria were active skin diseases such as psoriasis, lichen or chronic urticaria; known systemic diseases associated with CP such as chronic kidney diseases, haematological diseases, chronic liver diseases, endocrinological diseases, or diabetes mellitus. Additional exclusion criteria were the use of antihistamines, steroids, other systemic antipruritic therapies, or immunosuppressants or phototherapy 1 to 2 weeks before the baseline visit and during the entire study duration. The study was carried out in two third-level dermatology clinics from June and November 2020. The study was designed in agreement with the principles of the Declaration of Helsinki (2014 up-date).22 There was no change to the trial protocol after it commenced. The tested cream was applied twice daily (morning and evening) in the most affected body area (mainly arms and legs) using at least 4 Fingertip Units (2 g of cream) per treated limbs and application.
Study Outcomes
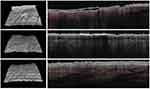
The primary endpoints were the evolution of the 10-cm visual analogue 24 h itch severity scale (VAS) and the 4-point verbal itching rating scale (VRS) (from 0 to 3). Secondary endpoints were the evolution of optical coherence tomography (OTC) of four skin parameters (grade of acanthosis, hyperkeratosis and presence of plaque/scale, with the evaluation of the dermal vascular pattern), assessed in a target area, and the transepidermal water loss (TEWL). All the study endpoints were evaluated at baseline and after 2 and 4 weeks of treatments by an investigator unaware of the type of treatment. In more detail, subjects participating in our trial were evaluated, at each visit, regarding itch intensity and skin barrier function, by a health-care physician unaware of the treatment used. The severity of itching was assessed by VAS 0–10 cm scale and VRS using a 4-point score (from 0: no itch to 3: severe itch). The VAS is a scale consisting of a 10-cm long line and a single question (“on a scale of ‘no-itch’ to ‘worst imaginable’, how was your itch, on average, in the past 24 hours?”). The left endpoint of the scale (0) represents “no itch” and the right end point (10) the “worst imaginable itch”. VAS is the most used tool in clinical trials for measuring itch intensity and features high reliability and concurrent validity.23 The VRS is a 4-point scale and consists of a list of adjectives describing various levels of symptom intensity (0= no itch, 1= mild itch, 2= moderate itch and 3= severe itch). Also, the VRS is a validated and sensitive tool for itch intensity evaluation.24 VAS and VRS recordings were performed at baseline and after 2 and 4 weeks. At the same time-points, TEWL measurements were performed in standardized conditions (temperature- and humidity-controlled room 22°C and 40% of relative humidity and an acclimatization time of 30 minutes) and according to published guidelines25 using a validated vapometer (Tewameter TM 300; Courage + Khazaka Electronic GmbH). Optical coherence tomography (OCT) is an in vivo imaging technique that offers visualization of the epidermis and upper dermis.26 For this study, OCT images were acquired using a commercially available 20-kHz-swept source laser OCT system (VivoSight1 Dx, Michelson Diagnostics Ltd., Kent, UK) with a handheld probe and 1305 nm wavelength laser. The OCT images are created by differences in the refractive indices of different skin layers and structures. OCT provides cross-sectional scans with a 5–7.5 mm resolution and a penetration depth of 1–2 mm.27 In this study, a multi-slice modality consisting of 250 B-scans was used. OCT skin images were presented in both cross-sectional and face mode and images were analysed with the built-in VivoSight software tool. We evaluated four protocol specified OCT parameters: acanthosis, hyperkeratosis, scale/crust thickness and the vascular signal at baseline and after 2 and 4 weeks. Self-reported side effects were recorded at each visit to assess the safety and tolerability of the tested product.
Statistical Analysis and Sample Size Calculation
Statistical analysis was performed using GraphPad statistical software ver. 13.0 (La Jolla, CA, USA). Continuous variables were expressed as mean ±Standard Deviation (SD). The paired T-test and the ANOVA test were used for normally distributed variables (ieTEWL values, OTC parameters); the Wilcoxon test was used for variables not normally distributed (ie VAS and VRS values). The Shapiro–Wilk test was used to check the normal distribution. We calculated also the 95% Confidence intervals of the difference in all the variables evaluated. The primary endpoint of the trial was the evolution of VAS and VRS mean scores from baseline and after treatment. We have hypothesized that the tested treatment could reduce the VAS score of at least 1.5 points (ie from 5.0 to 3.5) in comparison with baseline. With an effect size (Cohen’s d value) of 0.55, with an alpha value of 0.05 and a power of 95%, a total of at least 30 subjects should be enrolled to detect this difference. The sample size was calculated using G-Power statistical software version 3.9 (Kiel, Germany). A p-value of <0.05 was considered significant.
Ethics Approval and Consent to Participate
This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with the guidelines of Good Clinical Practice. All subjects provided signed informed consent. The study protocol was approved by the local investigator review board (University Tor Vergata, Rome) in June 2020.
Results
All the enrolled subjects concluded the trial. Table 1 shows the subjects’ baseline demographic characteristics. In line with other trials in CP/CPN subjects,28 women are more represented (83% of the evaluated group). Chronic pruritus (CP) only was present in 9 (40%) subjects and CPN in 21 (60%). At baseline, the mean±SD score for VAS and VRS were 4.9±2.2 (range: 3–9.8) and 1.7±0.7 (range 1–3), respectively. The treatment with the cream was associated with a significant reduction (p=0.0001) of VAS score of 60% at week 2 and 86% at week 4. The absolute difference of VAS score at week 4 in comparison with baseline was −4.2 cm (95% CI: from −3.3 to −5.2 cm). VRS score was significantly reduced by 49% after 2 weeks and by 81% after 4 weeks, in comparison with baseline (p=0.0001). Figure 1A and B shows the evolution of VAS and VRS scores during the study. TEWL mean values were 18.0±5.4 g/m2/h at baseline and 12.7±4.4 at week 2 and 9.8±4.7 at week 4 (P=0.001 vs baseline) (Figure 2). All the OCT parameters evaluated improved during active treatment: acanthosis grade was 0.22 mm at baseline, 0.19 mm at week 2 and 0.17 mm at week 4 (p=0.0005; ANOVA test), a 27% reduction at week 4 in comparison with baseline. Similar improvements were observed for the other OCT parameters. Figure 3 shows OCT images of one subject. Table 2 reports all the OCT skin parameters evaluated in the trial and the evolution during the treatment. The product was very well tolerated.
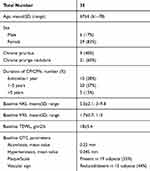 |
Table 1 Patient Demographics and Baseline Characteristics |
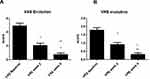 |
Figure 1 Evolution of VAS (visual analogue scale) (A) and VRS (verbal rating scale) (B) scores from baseline to 2 and 4 weeks. *P=0.0001 vs baseline; **P=0.001 week 4 vs week 2. |
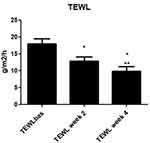 |
Figure 2 Evolution of transepidermal water loss (TEWL) from baseline to 2 and 4 weeks. *P=0.001 vs baseline; **P=0.003 week 4 vs week 2. |
 |
Table 2 Evolution of OCT Parameters at Baseline and After Treatment |
Discussion
An effective treatment of CP/NP could be problematic and burdensome.29 First, a correct diagnostic approach is mandatory to identify dermatological or systemic diseases associated with CP.30 On the other hand, a small number of studies have evaluated the efficacy of treatments on CP especially in the elderly.31 A multi-step approach including locally acting agents, phototherapy and systemic neuromodulating substances is suggested for the treatment of CP/CPN.32 However, topical treatments are commonly considered as the first-line strategy,33 even if the efficacy of this approach is considered limited.34 The clinical efficacy (reduction of VAS score) we observed in this trial was an absolute reduction of 4.2 points (0.7 vs 4.9) (95% CI of the difference: from −3.3 to −5.2) with an effect size of 1.9 (three time more than the effect size used for the sample size calculation). Reich et al35 stated that when VAS is used in evaluating an anti-pruritic effect of a treatment the minimal clinically important difference (MCID) in CP should be of at least 2–3 points; therefore, our results strongly support that this topical product has a relevant and clinically meaningful anti-itch effect. The percentage reduction we have observed at week 4 in comparison with baseline was almost 90%. In a trial using an NK1 Receptors antagonist (serlopitant), Stander et al36 demonstrated that this oral anti-itching drug, after 8 weeks of treatment, reduced the VAS score by 48%, in comparison with baseline, in subjects with CPN. In hemodialysis subjects with chronic pruritus, the use of a kappa-opioid receptor agonist difelikefalin reduced the itching score by 49%.37 In subjects with xerotic eczema, the use of emollient creams reduced itch intensity by 69%.38 This relevant clinical effect we have observed could be ascribed to the peculiar composition of the cream with an emollient component (purified omental lipids) and three different anti-itching molecules. The emollient component can improve the skin barrier function which is a pillar effect when we want to interrupt the skin-barrier-alteration-itch-scratch cycle.39,40 Furthermore, the PEA component of the cream, in addition to its anti-itching action, could have an anti-inflammatory and emollient effect.41 The mainstay therapy of CP in the elderly is commonly performed using topical products with a special emphasis on barrier repair and skin hydration.42 A recovered skin barrier function could have a positive effect on the well-known itch-scratch cycle.43 In our study, the use of the cream was associated with a significant and clinically relevant reduction in TEWL (−46%), supporting the improvement of the skin barrier function. The three anti-itching components of this cream share different potential mechanisms of action. Polidocanol is used as an antipruritic agent in galenic formulations and cosmetic products.15 Its anti-itching effect seems to be targeted to PAR-2/PAR-4 receptor signalling.44 Stimutex has an antihistamine effect and the third component, PEA, could exert an anti-itching effect through antagonism action toward receptors involved in the itching signal like TRPV and PPAR and modulating the skin endocannabinoid system.45 Some limitations should be taken into account in evaluating the results of this trial. This was a prospective open uncontrolled study. To improve internal validity, we adopted for the primary outcomes of the trial (VAS and VRS score evolution) an assessor-blinded evaluation study design. In addition, the study protocol has included an objective instrumental evaluation (OCT and TEWL) of other relevant clinical outcomes: skin anatomical modifications and skin barrier functions. Another study limit of our study is the fact that we have evaluated a relatively small sample size. However, it should be taken into account that we have decided to enrol 35 subjects after a specific sample size calculation with a pre-specified hypothesis on the clinical effect of this cream. In our study, subjects with CPN, which is a not so common skin condition, represented a relevant percentage (ie 60%) of the evaluated population. Therefore, in relation to the primary outcomes (VAS and VRS evolution), the sample size of this trial should be considered sufficiently powered.
Conclusion
This purified omental lipid with three anti-itching components cream reduces significantly itch intensity in subjects with chronic pruritus/prurigo nodularis, improving the skin barrier function and skin structure. (Trial Number: ISRCTN869561669).
Data Sharing Statement
The authors intend to share individual de-identified participant data upon request. Data available is the final database reporting per-subject single data of the collected variables during the trial. This data will be available for 1 year. Data can be obtained by contacting Dr Massimo Milani, MD; [email protected].
Disclosure
MM is the medical director of Difa Cooper, an Industrial Farmaceutica Cantabria group company that commercializes the tested product and reports no other potential conflicts of interest for this work. All the other authors report no conflicts of interest for this work.
References
1. Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7(7):535–547. doi:10.1038/nrn1950
2. Ständer S. Classification of itch. In: Itch-Management in Clinical Practice. Vol. 50. Karger Publishers; 2016:1–4.
3. Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147(10):1153–1156. doi:10.1001/archdermatol.2011.178
4. Ständer S, Zeidler C, Augustin M, et al. S2k Guidelines for the diagnosis and treatment of chronic pruritus–update–short version. J der Deutschen Dermatologischen Gesellschaft. 2017;15(8):860–872.
5. Pereira MP, Kremer AE, Mettang T, Staender S. Chronic pruritus in the absence of skin disease: pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2016;17(4):337–348. doi:10.1007/s40257-016-0198-0
6. Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32(3):201–215. doi:10.1007/s40266-015-0246-0
7. Liautaud B, Pape JW, DeHovitz JA, et al. Pruritic skin lesions: a common initial presentation of acquired immunodeficiency syndrome. Arch Dermatol. 1989;125(5):629–632. doi:10.1001/archderm.1989.01670170043005
8. Pereira MP, Steinke S, Zeidler C, et al. European academy of dermatology and venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol. 2018;32(7):1059–1065. doi:10.1111/jdv.14570
9. Zeidler C, Tsianakas A, Pereira M, Ständer H, Yosipovitch G, Ständer S. Chronic prurigo of nodular type: a review. Acta Derm Venereol. 2018;98(1–2):173–179. doi:10.2340/00015555-2774
10. Norman RA. Xerosis and pruritus in the elderly: recognition and management. Dermatol Ther. 2003;16(3):254–259. doi:10.1046/j.1529-8019.2003.01635.x
11. Harding R, Mayo C, Rawlings A. Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res. 1996;288(12):765–770. doi:10.1007/BF02505294
12. Patel T, Yosipovitch G. The management of chronic pruritus in the elderly. Skin Therapy Lett. 2010;15(8):5–9.
13. Romanelli M, Dini V, Milani M. Topical purified omental lipid formulations in the prevention of skin ulcers: a narrative review. J Wound Care. 2019;28(5):284–290. doi:10.12968/jowc.2019.28.5.284
14. Milani M, Federici A, Federici G. Purified omental lipids (pol) in the treatment of skin dryness in type 2 diabetic subjects with or without vascular or neurological complications: a prospective, controlled, assessor-blinded trial. J Clin Exp Dermatol Res. 2016;7(365):2. doi:10.4172/2155-9554.1000365
15. Freitag G, Höoppner T. Results of a post marketing drug monitoring survey with a polidocanol-urea preparation for dry, itching skin. Curr Med Res Opin. 1997;13(9):529–537. doi:10.1185/03007999709113326
16. Varothai S, Winayanuwattikun W, Phaitoonwattanakij S, Kasemsarn P, Boonchai W. An investigator‐blinded, randomized, prospective, comparative study of efficacy of four anti‐inflammatory and barrier hand moisturizers in patients with chronic hand dermatitis. Dermatol Ther. 2018;31(5):e12670. doi:10.1111/dth.12670
17. Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARγ receptors and neurotrophic factors. Pain. 2008;139(3):541–550. doi:10.1016/j.pain.2008.06.003
18. Ambrosino P, Soldovieri MV, Russo C, Taglialatela M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide. Br J Pharmacol. 2013;168(6):1430–1444. doi:10.1111/bph.12029
19. Steinhoff M, Bíró T. A TR (I) P to pruritus research: role of TRPV3 in inflammation and itch. J Invest Dermatol. 2009;129(3):531–535. doi:10.1038/jid.2008.440
20. Ostadhadi S, Nikoui V, Haj-Mirzaian A, Kordjazy N, Dehpour AR. The role of PPAR-gamma receptor in pruritus. Eur J Pharmacol. 2015;762:322–325. doi:10.1016/j.ejphar.2015.06.009
21. Kircik L. A nonsteroidal lamellar matrix cream containing palmitoylethanolamide for the treatment of atopic dermatitis. J Drugs Dermatol. 2010;9(4):334–338.
22. General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14.
23. Reich A, Chatzigeorkidis E, Zeidler C, et al. Tailoring the cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97(6):759–760. doi:10.2340/00015555-2642
24. Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92:502–507. doi:10.2340/00015555-1246
25. Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis. 1990;22(3):164–178. doi:10.1111/j.1600-0536.1990.tb01553.x
26. Welzel J. Optical coherence tomography in dermatology: a review. Skin Research and Technology: review article. Skin Res Technol. 2001;7(1):1–9. doi:10.1034/j.1600-0846.2001.007001001.x
27. Gambichler T, Moussa G, Sand M, Sand D, Altmeyer P, Hoffmann K. Applications of optical coherence tomography in dermatology. J Dermatol Sci. 2005;40(2):85–94. doi:10.1016/j.jdermsci.2005.07.006
28. Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382(8):706–716. doi:10.1056/NEJMoa1908316
29. Boozalis E, Khanna R, Zampella JG, Kwatra SG. Tricyclic antidepressants for the treatment of chronic pruritus. J Dermatol Treatment. 2019;1–3.
30. Yosipovitch G, Greaves MW. Definitions of itch. Basic Clin Dermatol. 2004;27:1–4.
31. Berger TG, Steinhoff M. Pruritus in elderly patients—eruptions of senescence. Semin Cutan Med Surg. 2011;30(2):113. doi:10.1016/j.sder.2011.04.002
32. Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83(6):1567–1575. doi:10.1016/j.jaad.2020.04.182
33. Qureshi AA, Abate LE, Yosipovitch G, Friedman AJ. A systematic review of evidence-based treatments for prurigo nodularis. J Am Acad Dermatol. 2019;80(3):756–764. doi:10.1016/j.jaad.2018.09.020
34. Saraceno R, Chiricozzi A, Nisticò SP, Tiberti S, Chimenti S. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatol Treatment. 2010;21(6):363–366. doi:10.3109/09546630903386606
35. Reich A, Riepe C, Anastasiadou Z, et al. Itch assessment with visual analogue scale and numerical rating scale: determination of minimal clinically important difference in chronic itch. Acta Derm Venereol. 2016;96(7):978–980. doi:10.2340/00015555-2433
36. Ständer S, Kwon P, Hirman J, Perlman AJ, Weisshaar E, Metz M, TCP-102 Study Group. Serlopitant reduced pruritus in patients with prurigo nodularis in a Phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80(5):1395–1402. doi:10.1016/j.jaad.2019.01.052
37. Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F. A Phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med. 2020;382(3):222–232. doi:10.1056/NEJMoa1912770
38. Simon D, Nobbe S, Nägeli M, et al. Short‐and long‐term effects of two emollients on itching and skin restoration in xerotic eczema. Dermatol Ther. 2018;31(6):e12692. doi:10.1111/dth.12692
39. Cork MJ, Danby S. Skin barrier breakdown: a renaissance in emollient therapy. Br J Nurs. 2009;18(14):872–877. doi:10.12968/bjon.2009.18.14.43356
40. Yosipovitch G, Misery L, Proksch E, Metz M, Staender S, Schmelz M. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99(13):1201–1209. doi:10.2340/00015555-3296
41. Eberlein B, Eicke C, Reinhardt HW, Ring J. Adjuvant treatment of atopic eczema: assessment of an emollient containing N‐palmitoylethanolamine (ATOPA study). J Eur Acad Dermatol Venereol. 2008;22(1):73–82. doi:10.1111/j.1468-3083.2007.02351.x
42. Lawton S. Practical issues for emollient therapy in dry and itchy skin. Br J Nurs. 2009;18(16):978–984. doi:10.12968/bjon.2009.18.16.43964
43. Lawton S. Skin barrier function and the use of emollients in dermatological nursing. Br j Nurs. 2007;16(12):712–719. doi:10.12968/bjon.2007.16.12.23721
44. Hawro T, Fluhr JW, Mengeaud V, et al. Polidocanol inhibits cowhage‐but not histamine‐induced itch in humans. Exp Dermatol. 2014;23(12):922–923. doi:10.1111/exd.12555
45. Bíró T, Tóth BI, Haskó G, Paus R, Pacher P. The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol Sci. 2009;30(8):411–420. doi:10.1016/j.tips.2009.05.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.