Back to Journals » Journal of Inflammation Research » Volume 14
Efficacy of a Standardized Turmeric Extract Comprised of 70% Bisdemothoxy-Curcumin (REVERC3) Against LPS-Induced Inflammation in RAW264.7 Cells and Carrageenan-Induced Paw Edema
Authors Gouthamchandra K, Sudeep HV, Chandrappa S, Raj A, Naveen P, Shyamaprasad K
Received 17 November 2020
Accepted for publication 29 January 2021
Published 12 March 2021 Volume 2021:14 Pages 859—868
DOI https://doi.org/10.2147/JIR.S291293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Kuluvar Gouthamchandra,1 Heggar Venkataramana Sudeep,1 Siddappa Chandrappa,2 Amrith Raj,1 Puttaswamy Naveen,3 Kodimule Shyamaprasad1
1Department of Biomedicinal Research, R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, Bangalore, Karnataka, 560105, India; 2Department of Phytochemistry, R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, Bangalore, Karnataka, 560105, India; 3Department of Analytical Development Research, R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, Bangalore, Karnataka, 560105, India
Correspondence: Kuluvar Gouthamchandra
Department of Biomedicinal Research, R&D Centre for Excellence, Vidya Herbs Pvt. Ltd, #14A, Jigani I Phase, Bangalore, Karnataka, 560105, India
Tel +91 80 42094158
Email [email protected]
Objective: It is well known that regular turmeric extract with 95% curcuminoid is comprised of curcumin (70.07%), desmethoxycurcumin (20.28%), and bisdemethoxycurcumin (BDMC) (3.63%). In the current study for the first time, we have enriched about 3% of bisdemethoxycurcumin (BDMC) to 70% as well as named it as REVERC3 and compared anti-inflammatory activity with regular turmeric extract using in vitro and in vivo models of inflammation.
Methods: To reveal the potential anti-inflammatory mechanism of action, we investigated nitric oxide (NO) scavenging, xanthine oxidase, and lipoxygenase inhibitory activity, further determined the level of pro-inflammatory cytokines, such as interleukin 6 (IL-6), tumor necrosis factor (TNF-α) and major inflammatory mediators like cyclooxygenase (COX-2) and inducible nitric oxide synthase (iNOS), inhibition in lipopolysaccharide (LPS) induced inflammation in RAW macrophage cells. In the other hand, a carrageenan-stimulated inflammatory rat model was carried out.
Results: Our study findings exhibited a significant anti-inflammatory activity of REVERC3 together with nitric oxide (NO), xanthine oxidase, and lipoxygenase inhibition. Further, we attenuated the levels of cyclooxygenase (COX-2), inducible nitric oxide synthase (iNOS), interleukin (IL-6) and tumor necrosis factor (TNF-α) expressions in the LPS-elicited RAW macrophage cells. REVERC3 showed a potential anti-inflammatory activity by inhibiting carrageenan induced paw edema after 4 hr at the dose of 100mg/kg body weight.
Conclusion: Thus, our findings collectively indicated that the REVERC3 could efficiently inhibit inflammation compared to regular turmeric extract. Since bisdemethoxycurcumin is a stable molecule it could be effectively used in the applications of health care and the nutraceutical industry, indeed which deserves further investigations.
Keywords: lipopolysaccharide, nitric oxide, xanthine oxidase, TNF-α, IL-6, iNOS, COX2
Introduction
Inflammation is an essential part of a body self-defence mechanism to fix an injury that occurred by trauma, microbial invasion, or any chemical substance. Inflammation is a precise process during which the body’s immune system identifies and eliminates toxic as well as external stimuli and starts the restorative process, there are two types of Inflammation it could be either acute or chronic. Acute inflammation begins very quickly, turns into severe within a brief period and therefore the signs may continue for a couple of days, chronic inflammation lasting for prolonged intervals of more than a few months to years.1–3 The distinctive features of inflammation are redness, swelling, unusual heat, pain, and discomfort for physical tasks. The body’s inflammatory response to chronic inflammation subsequently starts deteriorating body’s cells, tissues, and organs, this might end in DNA damage, tissue mortality, and internal scars. All of those are linked to the development of various illnesses such as diabetes, obesity, cardiovascular diseases, and cancer. There are several medications for regulating and suppressing the inflammatory crisis; Primarily, nonsteroidal anti-inflammatory drugs, steroids, and immunosuppressants are pragmatic. Nevertheless, which have been linked to adverse effects like gastrointestinal (GI) and cardiovascular complications. Hence, we need to explore natural anti-inflammatory factors within medication therapy to attain the deepened pharmacological response and subsided adverse side effects.4
Curcuma longa is a rhizomatous plant that belongs to the family Zingiberaceae. It is widely recognized for its bright yellow powdered form which is known as turmeric. Turmeric extracts containing curcumin, demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC) are often identified as curcuminoids. Curcumin is reported to possess beneficial properties due to its strong anti-inflammatory and antioxidant properties.5,6 Most of the studies have been concentrated on curcumin, it is interesting to note that the stability of curcumin is less and it could be simply reduced in in vivo system and at the same time there are reports that BDMC the other curcuminoids is stable and have encouraging pharmacological and biological effects. BDMC is described to have an improved stability and enhanced cellular absorption in comparison with the curcumin.7,8 Curcumin and its analogs such as bisdemethoxycurcumin, demethoxycurcumin, tetrahydrocurcumin and turmerones regulate anti-inflammatory and anti-proliferative effects selectively.9 Recent research has shown that the BDMC suppresses the proliferation and persistence of numerous types of tumor cells.10,11 BDMC has been investigated to have antioxidant and anti-inflammatory activities12. However, the underlying molecular processes are largely undiscovered. In this study, for the first time, about 3% of Bisdemethoxycurcumin present in turmeric extract is enriched to 70% and investigated for anti-inflammatory action.
Materials and Methods
Reagents
Dulbecco’s modified eagle medium (DMEM) and Fetal bovine serum (FBS) were purchased from Gibco-BRL®. Penicillin– streptomycin, LPS (Escherichia coli 0111: B4), sodium nitrite (NaNO2), Griess Reagent and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was procured from Sigma-Aldrich® (St Louis, MO, USA).
Extraction and Isolation of Bisdemothoxy-Curcumin (BDMC) Rich Fraction from Rhizomes of Curcuma longa
Finely powdered rhizomes of Curcuma longa (500gm) were extracted with 95–98% v/v ethyl acetate (2L) with the use of round bottom flask by refluxing at 65 °C for about 2–3 hrs, extract was filtered, and the process was repeated twice using fresh ethyl acetate solvent. Filtered extract was combined and evaporated under vacuum to get a curcuminoid-rich extract. The resulting crude extract was loaded to the column containing silica gel 60–120 mesh size and eluted with chloroform followed by chloroform: methanol with increasing polarity. All the column fractions were subjected to TLC silica gel (Merk-60 F254,0.25mm thick) plate using chloroform: ethanol: glacial acetic acid (94:5:1) as the developing solvent system and detected as yellow spots. The three curcuminoids were nicely separated (with Rf values of Curcumin, DMC, and BDMC being 0.78, 0.53, and 0.25, respectively). The last fraction recovered from column chromatography known as BDMC fraction with Rf value 0.25 and the organic solvent was removed by distillation in a rotary evaporator (Heidolph-Laboarat 4000). Finally, a brown-coloured extract (6.5gm) was obtained.
High-Performance Liquid Chromatography Analysis of REVERC3
Sample Preparation
Initially, standard stock concentration of 200 ppm was prepared by dissolving 10.0 mg of Bisdemethoxycurcumin in 50.0 mL acetone by using standard volumetric flask, then by dispensing 5mL of standard stock solution to 50.0 mL standard volumetric flask and make up the volume with 60% citric acid in water and 40% tetrahydrofuran of diluent mix to get a final concentration of 20 ppm. REVERC3 solution was prepared by dissolving 20mg of sample in acetone in 50mL volumetric flask, subsequently by pipetting 5mL sample stock to 50mL volumetric flask and make up the volume with 60% citric acid in water and 40% tetrahydrofuran of diluent mix to get a final concentration of 40 ppm. Both standard and sample solutions were passed through 0.2 µ nylon syringe filter.
Chromatographic Condition
The Bisdemethoxycurcumin quantification was performed on a Shimadzu LC2030 C Prominence-i (Japan), A separation was carried out in Kinetex C-18 column (100 Aº, 150 mm × 4.6 mm, 5 μm pore size). Mobile phase used was consists of isocratic elution with a low-pressure gradient using 1mg/mL citric acid in water: Tetrahydrofuran (60:40) with a flow rate of 1 mL/min and the injection volume of 20 μL. All solutions were degassed and filtered through 0.22 μm pore size filter. The column was maintained at 26°C throughout analysis, and the UV detector was set at 420 nm for Bisdemethoxycurcumin. 60% citric acid in water and 40% tetrahydrofuran of diluent mix were used as a diluent for HPLC analysis and the total liquid chromatography (LC) run time was 20 min. Using these chromatographic conditions, it has been possible to confirm the peak identification by retention time (RT) of Bisdemethoxycurcumin by injection of corresponding standard separately (Supplementary Figure S1)
Nitric Oxide Radical Scavenging Activity
Nitric oxide radical scavenging activity was determined by following method described.13 Briefly, to 1mL of different concentrations (5–160µg/mL) of REVERC3 and regular turmeric extract in phosphate buffer (0.025M, pH 7.4), 1 mL of sodium nitroprusside (10mM) was mixed and incubated at 25°C for 150 min subsequently the 1 mL of Griess reagent (1% sulphanilamide, 2% Orthophosphoric acid and 0.1% N-(1-naphthyl) ethylene diamine) was added and the absorbance was measured at 546nm. The reaction mixture without the sample was treated as control.
Lipoxygenase Enzyme (LOX) Inhibition Assay
LOX Inhibition was determined by applying the previously reported method.14 Briefly, sodium phosphate buffer 0.1M (pH 8.0) and soybean lipoxygenase (165 U/mL) and 10 µL of different concentrations of REVERC3 and regular turmeric extract were combined and incubated at 25°C for 10 min. 10 μL of 0.32 mM substrate in the form of sodium linoleic acid solution was added to initiate the reaction. The formation of (9Z, 11E)- (13S)-13-hydroperoxyoctadeca-9,11-dienoate from sodium linoleic acid by enzymatic conversion was determined by measuring the change in absorbance at 234 nm using UV-Vis spectrophotometer. Negative control was drawn up by replacing samples with sodium phosphate buffer and DMSO into the quartz cuvette. All the reactions were carried out in triplicates.
Xanthine Oxidase (XO) Inhibition Assay
The inhibition of XO activity was measured spectrophotometrically by adopting the method described by Noro et al.15 Briefly, the reaction mixture consists of enzyme solution (0.04 units/mL in 70mM phosphate buffer, pH 7.5), and different concentrations of REVERC3, regular turmeric extract and 70mM phosphate buffer (pH 7.5) were prepared freshly prior to use, subsequently pre-incubated at 25°C for 15 min. The reaction was instigated by adding xanthine substrate solution (150 mM). The assay mixture tubes were kept at 25°C for half an hour and the absorbance was read at 290 nm with UV-Vis spectrophotometer (Shimadzu). The percentage inhibition of XO in the above assay system was calculated using the formula: (1-B/A) X 100; where A and B are the activities of the enzyme without and with test material. In this experiment here we define XO as the amount needed to produce 1 mmol of uric acid/min at 25°C.
Cell Culture
RAW 264.7 cell line (murine macrophage) was purchased from NCCS Pune and cells maintained in 25 cm2 tissue culture flask in DMEM added with 100 units/mL penicillin, 100 μg/mL streptomycin and 10% FBS. Cultures were grown at 37°C in 5% CO2.
Cell Viability Assay
Cell viability assay was performed to determine the effect of REVERC3 and regular turmeric extract on RAW264.7. Briefly, cells (2×104cells/well) were seeded in 96-well microplates and allowed to adhere overnight and then treated with REVERC3 and regular turmeric extract separately at varying concentrations 2.5–30μg/mL, respectively, for 24 h. later, cells were stained with MTT solution for 4 hr. The formazan crystals formed were dissolved in 100 μL DMSO and the plates were mildly shaken, the OD was measured using Thermoscan EX reader at 570 nm.16
Measurement of Nitric Oxide (NO) Level
RAW 264.7 cells were treated with REVERC3 and regular turmeric extract at two different concentrations (2.5 and 5.0µg/mL) separately for 4 h, then washed with phosphate-buffered saline, and elicited with LPS (1µg/mL) for 24 h. Then, 100µL of cell culture supernatant was mixed with 100μL of Griess reagent. The absorbance (optical density) was read at 540 nm using a microplate reader and NO production was determined using sodium nitrite standard reference curve.
ELISA Assay
RAW264.7 cells (0.5 x 105cells/well) were seeded in 96 well plates and left to adhere overnight. Cells were pre-treated with REVERC3 and regular turmeric extract (2.5 and 5.0µg/mL) discretely for 1 h, then elicited with LPS (1µg/mL) for 24 h. The cytokines (TNF-α and IL-6) in the supernatant were measured using an ELISA kit (Krishgen) according to the manufacturer’s instructions.
Western Blot Analysis
Briefly, RAW 264.7 cells were incubated with different concentrations of REVERC3 and regular turmeric extract separately for 4 h before a 24h stimulation period with LPS (1 μg/mL). Total protein was extracted from the harvested cells with RIPA buffer, protein concentration was measured using Bradford protein assay, equal amounts of total proteins were separated by SDS-PAGE (8%-12%) and electro transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk for 2 h and subsequently incubated with specific primary antibodies, anti-NOS2 antibody (1:1000, sc-7271), anti-NFκB p65 antibody (1;1000, sc-8008) and anti-Cox-2 antibody (1;1000, sc-19999) for overnight at 4°C. Membranes were later washed three times with Tris-Buffered Saline Tween-20 (TBST) and Tris-Buffered Saline (TBS) incubated further for 2 h with the corresponding secondary antibodies. The blot was visualized using an enhanced chemiluminescence kit.
In vivo Anti-Inflammatory Activity
Grouping of Animals
Wistar rats were randomly allotted into six groups (n=6) and kept for 7-day acclimatization. Group 1 was treated as control, group 2 received indomethacin 10 mg/kg.bw. Rats in groups 3 and 4 were treated with REVERC3 (100 and 200 mg/kg.b.w. p.o) group 5 and 6 with regular turmeric extract (100 and 200 mg/kg.b.w. p.o). This study was approved by Institutional Animal Ethics Committee of Vidya Herbs Pvt. Ltd, Bangalore, India, with the protocol number VHPL/PCL/IAEC/08/19. During animal experimentation guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experimentation on Animals) were followed.
Carrageenan-Induced Acute Inflammatory Model
The carrageenan-induced paw edema test was performed in accordance with the method17 with some modifications. Drugs were administered to all the respective groups. One hour later, the animals were injected with 0.01 mL of 1% (w/v) freshly prepared carrageenan solution into the right-hind paw sub plantar injection. Paw thickness and volume were measured after 30 min, 1, 2, 4, and 24 h after carrageenan injection using Vernier calipers and Plethysmometer, respectively. The percentage inhibition of inflammation was calculated.
Statistical Analysis
Data were analyzed using one-way analysis of variance (ANOVA) with Duncan multiple range test for means ± standard error, where *P < 0.001 was considered to indicate statistically significant difference between groups. All the experimental data were derived from three independent experiments.
Results
Nitric Oxide Scavenging Activity
REVERC3 and regular turmeric extract were investigated for NO scavenging activity. REVERC3 and regular turmeric extract inhibited 10–90% at 5–160µg/mL with an IC50 value of 69.5µg/mL and (Figure 1A) respectively. Excitingly REVERC3 showed strong NO scavenging activity compared to regular turmeric extract.
 |
Figure 1 Nitric oxide scavenging activity of REVERC3 and regular turmeric extract. Values are expressed as mean ± SEM of three experiments. |
Xanthine Oxidase Inhibition
Xanthine oxidase inhibitors have been testified from a wide medley of plants used in conventional plant-based medications for rheumatism and gout in Australia, North America, and China. In this study, the level of XO inhibition was evaluated for the REVERC3 and regular turmeric extract at different concentrations. As illustrated in Figure 2A. Both REVERC3 and regular turmeric extract suppresses the formation of uric acid catalyzed by xanthine oxidase significantly in a dose-dependent manner. The concentrations of REVERC3 and regular turmeric extract causing the half-maximal inhibition (IC50) were determined to be 27.93μg/mL and 32.83μg/mL, respectively, indicating that REVERC3 possessed a strong inhibitory activity on XO. Our results were consistent with previously published reports.
 |
Figure 2 Dose-dependent inhibitory effects of REVERC3 and regular turmeric extract on xanthine oxidase (A) and Lipoxygenase (B). The results were expressed as mean ± SEM of three experiments. |
Inhibition of Lipoxygenase (LOX) Activity
High concentrations of leukotrienes (LTs) might be observed in the instance of allergic rhinitis, rheumatoid arthritis, asthma, psoriasis, and colitis ulcer which is formed from immune cells. Leukotrienes are a distinctive group of products of the lipoxygenase pathway, Lipoxygenases (LOX) enzymes are linked with allergic and inflammatory reactions. Hence, the LOX inhibition activity is the most important one. As depicted in Figure 2B REVERC3 was most effective and inhibited LOX activity up to 89.18% at the concentration of 50μg/mL with an IC50 value of 24.78μg/mL whereas regular turmeric extract IC50 value was 30.03μg/mL. Interestingly, LOX inhibitory activity of REVERC3 was strong compared to regular turmeric extract.
Effect of REVERC3 on RAW 264.7 Cells
The effect of REVERC3 and regular turmeric extract on RAW264.7 cell viability was demonstrated by MTT assay. As illustrated in Figure 3A, our results revealed that REVERC3 and regular turmeric extract at the concentration of 2.5–7.5µg did not induce any cytotoxic effect on RAW 264.7 cells, successive experiments were conducted with 2.5 and 5.0 µg/mL of REVERC3 and regular turmeric extract.
Effect of REVERC3 on the Production of Nitric Oxide (NO) in LPS-Elicited RAW264.7 Cells
It has been reported that excessive NO is a classical marker for inflammation in LPS stimulated macrophages. As demonstrated in Figure 3B, LPS induced the dramatic increase of NO in macrophages. Treatment with REVERC3 and regular turmeric extract significantly lessened the production of NO level. Interestingly, REVERC3 was better compared to regular turmeric extract.
Effect of REVERC3 on the Production of Cytokine in LPS-Elicited RAW264.7 Cells
Once inflammation occurs, stimulated macrophages secrete substantial amounts of pro-inflammatory proteins to exacerbate inflammation. As presented in Figure 4A and B, LPS treatment intensified the expression of TNF-α and IL-6 which could be reduced by the pre-treatment with REVERC3 and regular turmeric extract. Excitingly REVERC3 treatment strongly reduced TNF - α and IL-6 cytokine levels compared to regular turmeric extract.
Inhibition of iNOS and COX2 Expression in LPS Induced RAW 264.7 Cells
It is reported that COX-2 and iNOS play a significant role in the inflammatory process. Based on the significant inhibition of NO by REVERC3, we investigated the expression of COX-2 and iNOS. As shown in Figure 5A and B, LPS induced the statistically significant expression of COX-2 and iNOS compared to control. Treatment with REVERC3 and regular turmeric extract subsides the expression of COX-2 and iNOS dose-dependently. REVERC3 at the concentration of 5.0 µg/mL showed clear inhibition of iNOS and COX2 in the comparison to LPS treated cells, whereas regular turmeric extract at the concentration of 5.0 µg/mL showed moderate suppression compared to regular turmeric extract.
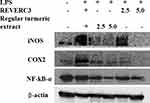 |
Figure 5 Effect of REVERC3 and regular curcumin on the protein expression of iNOS, COX-2 and NFkB- α in LPS stimulated RAW264.7 cells. Total proteins were isolated and analyzed by Western blot. |
Effect of REVERC3 on Carrageenan-Induced Paw
The carrageenan-induced paw edema test was used to assess the anti-inflammatory effects of REVERC3 (Supplementary Figure S2, Table 1). After 1 hour of induction, rats treated with REVERC3 at 100 mg and 200 mg/kg showed significant reduction in paw edema thickness as compared to the untreated vehicle control (p<0.001). Further at the tested doses, REVERC3 continued to exhibit a significant anti-inflammatory effect up to 24 h. The results were comparable to reference drug indomethacin. Interestingly, REVERC3 was superior in ameliorating the carrageenan-induced inflammation compared to the regular turmeric extract.
 |
Table 1 Effect of REVERC3 on Carrageenan-Induced Paw Edema in Rats |
Discussion
Inflammation is a multifaceted process, it shows the response of organisms to several stimuli and is linked to many disorders such as arthritis, asthma, and psoriasis, which necessitate prolonged or repeated treatment. In this study, we investigated REVERC3 is having pronounced inhibitory activity against major pro-inflammatory targets. It has been stated that nitric oxide (NO) is produced from amino acid L-arginine by the enzymes that are present in the phagocytes, certain neuronal cells, and vascular endothelial cells.18 NO is implicated in several pathological diseases, such as chronic inflammation, rheumatoid arthritis, and autoimmune diseases.19 It has been reported that nitric oxide scavengers act out against oxygen, leading to reduced production of nitrite. The present study indicated that REVERC3 inhibited the nitrite formation by directly competing with oxygen in the reaction with nitric oxide, the NO scavenging activity was discovered to improve with increasing concentration REVERC3 and might be due to the presence of phenolics and flavonoids.
Xanthine oxidase (XO) is thought to be a vital biological source of free radicals during physical exercise, it is also involved in the pathogenesis of multiple diseases such as atherosclerosis, cancer, and gout.20–22 Thus, the inhibition of XO activity may have concurrently antiradical and inhibitory properties along with therapeutic interest. XO inhibitory activity of REVERC3 was evaluated for its effectiveness in reducing the production of uric acid. While the XO enzyme was repressed, it lowered the activity XO and this would subsequently prevent the catalysation of hypoxanthine to uric acid via to xanthine. XO inhibitory activity of REVERC3 was appreciable than regular turmeric extract which could be attributed to less curcumin content. Previous studies have demonstrated that luteolin, quercetin, silibinin inhibited xanthine oxidase but not curcumin.23
Lipoxygenases (LOX) are a family of iron-containing enzymes, they convert arachidonic acid, a component of membrane phospholipids into proinflammatory mediators called leukotrienes that play a key role in inflammation.24,25 Mounting evidence suggests that lipoxygenase catalyzed products have a profound impact on the progression and development of human cancers.26,27 It has been reported that agents that block lipoxygenase-catalyzed activity may be positive in blocking cancer.28 The lipoxygenase inhibition activity of REVERC3 was evaluated at different concentrations. REVERC3 showed dose-dependent lipoxygenase inhibitory activity, polyphenol and flavonoid presence might be responsible for the activity. Latest investigations have shown that BDMC to exert anti-tumour effects via a multimechanistic method of action,29 suppression of lipoxygenase could be one of them.
Macrophages are phagocytic cells that play a key role in immune reactions by releasing multiple pro-inflammatory cytokines and mediators to avoid invading pathogens. As a part of the defence mechanism macrophages express iNOS and stimulate the production of an enormous amount of NO. It has been reported that excessive NO is linked to inflammatory diseases.30 Since inhibition of NO is an important part of anti-inflammatory therapy, we investigated the effect of REVERC3 on NO production in RAW 264.7 macrophages. We discovered REVERC3 significantly subsided NO production in LPS elicited RAW 264.7 macrophages. As a quick response to inflammation, RAW264.7 macrophages could release inflammatory mediators (COX-2 and iNOS) and many pro-inflammatory cytokines (TNFα and IL-6), which is helpful to attract circulating immune effector cells, such as neutrophils, to fight against foreign invasion.31,32 Although, extreme inflammatory retorts could harm tissues and organs. Therefore, the expression of pro-inflammatory cytokines and inflammatory mediators needs to be strictly controlled throughout an inflammatory response.32–35 Lowering the levels of COX-2 and iNOS would represent an effective strategy for destroying inflammatory responses.
It has been reported that the carrageenan-caused paw edema model is valuable to examine the anti-inflammatory effect of herbal as well as synthetic products.36 The carrageenan induced inflammatory response consists of two phases. The early phase (first 2 h after carrageenan injection) is attributed to the release of proinflammatory mediators, such as histamine and serotonin; the second phase (3–5 h after carrageenan injection) is mainly mediated by kinins, prostaglandin, nitric oxide, cyclooxygenase, cytokines, and neutrophil derived free radicals.37 In the present study, we found that REVERC3 reduced carrageenan-induced paw oedema thickness from 1h post-carrageenan injection, implying that the anti-inflammatory action of REVVERC3 is associated with the inhibition of different inflammatory mediators.
Conclusion
In conclusion, our study showed that REVERC3 demonstrated significant anti-inflammatory activity by inhibiting xanthine oxidase and lipoxygenase enzymes. Further, inhibition of cytokines interleukin (IL-6) and tumor necrosis factor (TNF-α) and enzymes COX-2, expressions in LPS-elicited RAW macrophage cells. In the other hand, REVERC3 showed promising inhibitory effect in carrageenan induced Paw oedema (at 100 mg/kg.bw). Thus, our results together indicated that the REVERC3 could efficiently inhibit inflammation compared to regular turmeric extract due to its superior bioavailability compared to regular turmeric extract, the main concern which greatly limit the suitability and efficacy of regular turmeric extract is its low bioavailability. Therefore, it can be assumed that bioavailability of REVERC3 could contribute to anti-inflammatory activity. Overall results showed BDMC rich extract could be potent drug candidate.
Data Sharing Statement
The datasets used and/or analysed during the present study are accessible from the corresponding author on reasonable request. All data analysed during this study are included in this article.
Ethics Statement
All animal studies were approved by Institutional Animal Ethics Committee of Vidya Herbs Pvt. Ltd. Bangalore, India, with the protocol number VHPL/PCL/IAEC/08/19
Acknowledgments
We would like to thank Mr. Lingaraju and Dr. Vedamuthy B.M Department of Phytochemistry and Analytical development laboratory, Vidya Herbs Pvt. Ltd, Bangalore, India. Our lab members are also acknowledged for their useful ideas and suggestions.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The authors reveal that financial support was provided by Vidya Herbs Pvt Ltd. Bangalore. India.
Disclosure
The authors are all employees of Vidya Herbs Pvt Ltd. The authors declare that they have no other potential conflicts of interest for this work.
References
1. Zhang X, Wu X, Hu Q, et al. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019;236:116464.
2. Michels Silva D, Langer H, Graf T. Inflammatory and molecular pathways in heart failure-ischemia, HFpEF and transthyretin cardiac amyloidosis. Int J Mol Sci. 2019;20:2322.
3. Fritsch J, Abreu MT. The Microbiota and the Immune Response: what Is the Chicken and What Is the Egg? Gastrointest. Endosc Clin N Am. 2019;29:381–393.
4. Bagad AS, Joseph JA, Bhaskaran N, Agarwal A. Comparative evaluation of anti-inflammatory activity of curcuminoids, turmerones, and aqueous extract of curcuma longa. Adv Pharmacol Sci. 2013;80:
5. Panahi Y, Khalili N, Sahebi E, et al. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacol. 2017;25:25–31.
6. Karimian MS, Pirro M, Majeed M, Sahebkar A. Curcumin as a natural regulator of monocyte chemoattractant protein-1. Cytokine Growth Factor Rev. 2017;33:55–63.
7. Basile V, Ferrari E, Lazzari S, et al. Curcumin derivatives: molecular basis of their anti-cancer activity. Biochemical Pharmacol. 2009;78(10):1305–1315.
8. Ramezani M, Hatamipour M, Sahebkar A. Promising anti-tumor properties of bisdemethoxycurcumin: a naturally occurring curcumin analogue. J Cell Physiol. 2018;233(2):880–887.
9. Sandur SK, Pandey MK, Sung B, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773.
10. Luthra PM, Kumar R, Prakash A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun. 2009;384:420–425.
11. Boonrao M, Yodkeeree S, Ampasavate C, et al. The inhibitory effect of turmeric curcuminoids on matrix metalloproteinase-3 secretion in human invasive breast carcinoma cells. Arch Pharm Res. 2010;33:989–998.
12. Ravindran J, Subbaraju GV, Ramani MV, et al. Bisdemethylcurcumin and structurally related hispolon analogues of curcumin exhibit enhanced prooxidant, anti-proliferative and anti-inflammatory activities in vitro. Biochem Pharmacol. 2010;79:1658–1666.
13. Sreejayan RMN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107.
14. Kemal C, Louis-Flamberg P, Krupinski-Olsen R, et al. Reductive inactivation of soybean lipoxygenase 1 by catechols: a possible mechanism for regulation of lipoxygenase activity. Biochemistry. 1987;26:7064–7072.
15. Noro T, Oda Y, Miyase T, et al. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull (Tokyo). 1983;31:3984–3987.
16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
17. Sulaiman MR, EPerimal K, Akhtar MN, et al. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia. 2010;81:855–858.
18. Nagmoti DM, Khatri DK, Juvekar PR, et al. Antioxidant activity, and free radical-scavenging potential of Pithecellobium dulce benth seed extracts. Free Radicals Antioxidants. 2012;2:37–43.
19. Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J Leukoc Biol. 2010;88:
20. Hong S, Joo T, Jhoo JW. Antioxidant, and anti-inflammatory activities of 3, 5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci Biotechnol. 2015;24:
21. Borges F, Fernandes E, Roleira F. Progress towards the discovery of xanthine oxidase inhibitors. Curr Med Chem. 2002;9:195–217.
22. Gomez-Cabrera MC, Borrás C, Pallardó FV, et al. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120.
23. Filha ZS, Vitolo IF, Fietto LG, et al. Xanthine oxidase inhibitory activity of Lychnophora species from Brazil (“Arnica”). J Ethnopharmacol. 2006;107:79–82.
24. Pauff JM, Hille R. Inhibition studies of bovine xanthine oxidase by luteolin, silibinin, quercetin, and curcumin. J Nat Prod. 2009;72:725–731.
25. Penrose JF, Austen KF, Lam BK. Leukotrienes: Biosynthetic Pathways, Release and Receptor-Mediated Actions with Relevance to Disease States. In Inflammation: Basic Principles and Clinical Correlates.
26. Martel-Pelletier J, Lajeunesse D, Reboul P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–509.
27. Timar J, Raso E, Fazakas ZS, et al. Multiple use of a signal transduction pathway in tumor cell invasion. Anticancer Res. 1996;16:3299–3306.
28. Steele VE, Holmes CA, Hawk ET, et al. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1999;8:467–483.
29. Yang H, Fan S, An Y, et al. Bisdemethoxycurcumin exerts pro-apoptotic effects in human pancreatic adenocarcinoma cells through mitochondrial dysfunction and a GRP78-dependent pathway. Oncotarget. 2016;7(50):83641–83656.
30. Chen L, Yin H, Lan Z, et al. Nephroprotective Effects of Smilax china L. J Ethnopharmacol. 2011;135:
31. Liu LM, Cheng SF, Shieh PC, et al. The methanol extract of Euonymus laxiflorus, Rubia lanceolata and Gardenia jasminoides inhibits xanthine oxidase and reduce serum uric acid level in rats. Food Chem Toxicol. 2014;70:179–184.
32. Fairweather D, Rose NR. Inflammatory heart disease: a role for cytokines. Lupus. 2005;14:646–651.
33. Porcherie A, Cunha P, Trotereau A, et al. Repertoire of Escherichia Coli agonists sensed by innate immunity receptors of the bovine udder and mammary epithelial cells. Vet Res. 2012;43:1–8.
34. Shuster DE, Jr KM, Rainard P, et al. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia Coli. Infect Immun. 1997;653:286–292.
35. Scheibel M, Klein B, Merkle H, et al. IκBβ is an essential co-activator for LPS-induced IL-1β transcription in vivo. J Exp Med. 2010;207:2621.
36. Terashima M, Kakuno Y, Kitano N, Matsuoka C. Antioxidant activity of flavonoids evaluated with myoglobin method. Plant Cell Rep. 2012;31:291–298.
37. Nile SH, Kim SH, Ko EY, Park SW. Polyphenolic contents and antioxidant properties of different grape (V. vinifera, V. labrusca, and V. hybrid) cultivars. Biomed Res Int. 2013;718065.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


