Back to Journals » Journal of Inflammation Research » Volume 16
Efficacy of a Novel Pleiotropic MMP-Inhibitor, CMC2.24, in a Long-Term Diabetes Rat Model with Severe Hyperglycemia-Induced Oral Bone Loss
Authors Bhatt HD , Golub LM, Lee HM, Kim J , Zimmerman T , Deng J , Hong H, Johnson F , Gu Y
Received 25 November 2022
Accepted for publication 3 February 2023
Published 23 February 2023 Volume 2023:16 Pages 779—792
DOI https://doi.org/10.2147/JIR.S399043
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Heta Dinesh Bhatt,1 Lorne M Golub,1 Hsi-Ming Lee,1 Jihwan Kim,2 Thomas Zimmerman,3 Jie Deng,4 Houlin Hong,5 Francis Johnson,6 Ying Gu7
1Department of Oral Biology and Pathology, School of Dental Medicine, Stony Brook University, Stony Brook, NY, USA; 2Department of Pediatric Dentistry, University of Buffalo School of Dental Medicine, Buffalo, NY, USA; 3Division of Laboratory Animal Resources (DLAR) at Stony Brook, Stony Brook University, Stony Brook, NY, USA; 4Department of Orthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing, People’s Republic of China; 5Department of Community Health & Social Sciences, Graduate School of Public Health & Health Policy, City University of New York, New York City, NY, USA; 6Department of Chemistry and Pharmacological Sciences, School of Medicine, Stony Brook University, Stony Brook, NY, USA; 7Department of General Dentistry, School of Dental Medicine, Stony Brook University, Stony Brook, NY, USA
Correspondence: Heta Dinesh Bhatt, Department of Oral Biology and Pathology, School of Dental Medicine, Stony Brook University, Stony Brook, NY, 11794, USA, Tel +1631820-5311, Email [email protected]
Purpose: CMC2.24, a novel 4-(phenylaminocarbonyl)-chemically-modified-curcumin, is a pleiotropic MMP-Inhibitor of various inflammatory/collagenolytic diseases including periodontitis. This compound has demonstrated efficacy in host modulation therapy along with improved resolution of inflammation in various study models. The objective of current study is to determine the efficacy of CMC2.24 in reducing the severity of diabetes, and its long-term role as an MMP-inhibitor, in a rat model.
Methods: Twenty-one adult male Sprague-Dawley rats were randomly distributed into three groups: Normal (N), Diabetic (D) and Diabetic+CMC2.24 (D+2.24). All three groups were orally administered vehicle: carboxymethylcellulose alone (N, D), or CMC2.24 (D+2.24; 30mg/kg/day). Blood was collected at 2-months and 4-months’ time-point. At completion, gingival tissue and peritoneal washes were collected/analyzed, and jaws examined for alveolar bone loss by micro-CT. Additionally, sodium hypochlorite(NaClO)-activation of human-recombinant (rh) MMP-9 and its inhibition by treatment with 10μM CMC2.24, Doxycycline, and Curcumin were evaluated.
Results: CMC2.24 significantly reduced the levels of lower-molecular-weight active-MMP-9 in plasma. Similar trend of reduced active-MMP-9 was also observed in cell-free peritoneal and pooled gingival extracts. Thus, treatment substantially decreased conversion of pro- to actively destructive proteinase. Normalization of the pro-inflammatory cytokine (IL-1ß, resolvin-RvD1), and diabetes-induced osteoporosis was observed in presence of CMCM2.24. CMC2.24 also exhibited significant anti-oxidant activity by inhibiting the activation of MMP-9 to a lower-molecular-weight (82kDa) pathologically active form. All these systemic and local effects were observed in the absence of reduction in severity of hyperglycemia.
Conclusion: CMC2.24 reduced activation of pathologic active-MMP-9, normalized diabetic osteoporosis, and promoted resolution of inflammation but had no effect on the hyperglycemia in diabetic rats. This study also highlights the role of MMP-9 as an early/sensitive biomarker in the absence of change in any other biochemical parameter. CMC2.24 also inhibited significant activation of pro-MMP-9 by NaOCl (oxidant) adding to known mechanisms by which this compound treats collagenolytic/inflammatory diseases including periodontitis.
Keywords: matrix metalloproteinases, diabetes mellitus, resolvin, anti-oxidant activity, host modulation therapy
Introduction
Diabetes mellitus, which affects approximately 21 million people in the United States, is characterized by a group of metabolic disorder, which are marked by defects in insulin production, insulin action or both resulting in hyperglycemia.1,2 Long-term hyperglycemia leads to the formation of advanced glycation end products (AGEs) and is associated with the activation of immune responses that lead to various macro- and micro-vasculature diabetic complications.3,4 This latter activation initiates local responses characterized by the release of pro-inflammatory cytokines (such as TNF-α, IL-1β and IL-6), free radicals, increase tissue oxidant stress and collagenolytic MMPs, all of which are similar to the complications seen in the tissues of the classic diabetic complications.5–8 AGEs contribute to tissue destruction (as a result of up-regulation of inflammatory collagenolytic mediators, eg, MMP-8 and MMP-9) and cause a loss of attachment, which is a hallmark of periodontitis seen more in diabetic compared to non-diabetic individuals.4,9
Recent literature has highlighted the role of MMPs, particularly MMP-9, in the pathogenesis of various diseases accompanied by acute and chronic inflammation, including diabetes and periodontitis.10–15 Matrix Metalloproteinase-9 (MMP-9, 92 kDa) is produced by polymorphonuclear neutrophils (PMNs), macrophages, and fibroblasts.16,17 It can be activated to a lower molecular weight protein (82 kDa), the pathologically active form of MMP-9,18,19 which when present in excessive levels, has been implicated in contributing to a variety of inflammatory and malignant disorders, including periodontitis, periimplantitis, and pericoronitis, and a number of cancerous tumors.16,20,21 Various mechanisms are described in the literature on how MMP-9 pro-enzyme (92 kDa) may be activated to lower molecular weight active form (82 kDa) of the protein.22–26 Studies have recently indicated that oxidative stress and pro-inflammatory cytokines contribute to the elevation of gelatinase MMPs in inflammatory diseases like diabetes.
Both diabetes and periodontitis are associated with increased levels of markers of inflammation, namely MMP-9, pro-inflammatory cytokines, and oxidative stress.13,27–31 Although diabetes has long been recognized as a key risk factor for severe periodontitis (a well-known complication of diabetes) the latter can also increase the severity of diabetes (bi-directional).32,33 Increased levels of MMPs (a hallmark of both diseases) degrade the insulin receptors and this results in decreased insulin sensitivity and so promotes hyperglycemia which in turn leads to severe periodontitis and an increased level of cytokines, in a self-perpetuating manner.34 To break this self-promoting cycle of local and systemic inflammation, newer therapies such as host modulation therapy (HMT) are being developed.
The novel chemically modified curcumin (CMC2.24), a 4- (phenylaminocarbonyl) derivative, has been identified as a potent HMT and has down-regulated both the inflammatory and oxidative stress markers in various disease models.28,35–44 It acts as a strong (tri-ketonic cation-binding) MMP-inhibitor45 through various pleiotropic mechanism along with potent antioxidative,44 anti-inflammatory, and resolvin-generating activity with no obvious cytotoxicity or antimicrobial activity.28,35–38,40,42,46–48 The aim of the present study is to assess over a period of 4 months, the effect of this novel pleiotropic HMT on hyperglycemia and associated inflammation and bone loss, in the absence of antibiotic therapy. Consequently, the objectives of the current study are to determine whether CMC2.24, a potent inhibitor (in vitro and cell culture) of MMPs, also effective in vivo i) can reduce the severity of long-term diabetes and diabetes-induced periodontitis and ii) inhibits the activation of the MMP-9 proenzyme.
Materials and Methods
Chemical Reagents
CMC 2.24 was synthesized and provided by Chem-Master Intl. Inc. (99.5% pure, Stony Brook, NY, USA). Carboxymethyl cellulose as a vehicle was purchased from Sigma (C-4888, Sigma, USA). All cell culture reagents and chemical reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Animal Studies
Twenty-one adult male Sprague-Dawley (SD) rats (Strain code: CD001, Body Weight: 276–400 grams; viral antibody free; Charles River Laboratories International, Inc., Wilmington, MA, USA) were acclimatized to the study environment one week prior to the initiation of the experiment, then were randomly distributed into 3 experimental groups: non-diabetic normal controls (N), diabetics (D), diabetics with CMC2.2.4 treatment (D+2.24) (n = 6–8 rats per group).
After the rats had been acclimatized, those in the N group (n = 6), were given an intraperitoneal (i.p) injection of 10 mM citrate saline buffer, pH 4.5 (vehicle-alone). Those in the D and D+2.24 groups (n = 16) were given an i.p. injection of streptozotocin (STZ, 55mg/kg body weight; ENZO Life Sciences, Inc., Plymouth Meeting, PA, USA) to induce type I diabetes. All rats were hosed singly, the ambient temperature being maintained at 18–24°C, while the lights were turned on/off automatically at 06:00 AM/18:00 PM. There was access to unlimited food and water. After 1-week, all of the STZ-injected rats (D and D+CMC2.24 groups) exhibited pathologically elevated glucose levels in their urine. The diabetic status (hyperglycemia; glucosuria) of the rats was monitored weekly by means of nonenzymatic test strips (CTMI 4 LN; Cole-Tayler Marketing Inc., Redsa, CA, USA), which regularly can read >2% glucose in urine (~500 mg/dL glucose in serum).28 The hyperglycemic rats in D+CMC2.24 groups were then orally administered CMC2.24 daily (Monday-Friday) (30 mg/kg) for a period of 16 weeks. The untreated diabetic rats (D group) and the non-diabetic controls (N group) were administered vehicle-alone (1 mL of a 2% carboxymethylcellulose suspension in water) by oral gavage for the same period of time. The CMC2.24 (or placebo) treatment was initiated 1-week after STZ administration.
After all rats were confirmed as hyperglycemic their body weights were measured at day 1 (ie, baseline), 1 week, 8 week and 16 weeks. Blood plasma and serum were collected through tail vein to measure blood glucose, HbA1c, MMPs and inflammatory cytokines at 8 weeks and through cardiac puncture at 16 weeks. Blood was collected only at 2 time-points for the entire duration of the 4-month study, to avoid the formation of non-healing ulcers (as observed in previous studies) which are a known diabetic complication.28,46 At the end of the 16-weeks period (4-months) of therapy, all rats in each experimental group (N, D, and D+CMC2.24 groups, n = 6–8 rats/group) were fasted overnight and then euthanized by CO2 inhalation. The heads were collected and stored at −80°C until processed and used for alveolar bone analysis by micro-computerized tomography (μCT).49
Animals were housed in the Division of Laboratory Animal Resources (DLAR) at Stony Brook University with care provided by the Center’s personnel and all experimental procedures having been approved by Stony Brook University’s Institutional Animal Care and Use Committee (IACUC # 230617) at the same location. This facility follows the Animal Welfare (USDA enforced) Act, the Public Health Service Act (OLAW enforced), and NY State law (DOH enforced), and is an AAALAC International accredited facility.
Resident Peritoneal Washes (rPW)
Non-elicited resident peritoneal washes (rPW) were collected by peritoneal lavage following injection of 15mL of sterile-cold phosphate-buffered saline (PBS)/3mM EDTA into the peritoneal cavity. After gently massaging the peritoneum for 1 min, resident peritoneal washes (rPW) were collected (~12mL), then stored on ice (4°C), and centrifuged at 1000 rpm, 40°C, 5 min to separate the supernatant cell-free peritoneal fractions (CFPFs) from the mixed cells in the peritoneal cavity.
Gingival Tissue Extraction
The gingival tissues from the maxillary jaws were excised, weighed, and pooled for each experimental group (n = 3 rats per group) as described previously.38 The pooling of gingival tissues for each group is necessary because individual rats do not yield sufficient gingiva for enzyme analyses. The gingival tissues were extracted and the MMP content of the extract was partially purified as described previously.50,51 In brief, the samples were homogenized (all procedures at 4°C) with a glass grinder (Kontes, Glass Co., Vineland, NJ) attached to a T-Line Lab stirrer (Model 106 Taboys Engineering Corp., NJ) in 50 mM Tris-HCl buffer (pH 7.6) containing 5 M urea, 0.2 M NaCl and 5 mM CaCl2, then extracted overnight and centrifuged at 15,000 rpm for 1 hour. The supernatants were collected and dialyzed exhaustively against 50 mM Tris buffer (pH 7.8) containing 0.2M NaCl and 5 mM CaCl2. Ammonium sulfate was added to the dialysate (to produce 60% saturation) which was then allowed to stand overnight. The resulting precipitate containing the MMPs was collected by centrifugation at 15,000 rpm for 90 min, then the pellets were dissolved in the Tris buffer (pH 7.8) containing NaCl, CaCl2 and 0.05% Brij and exhaustively dialyzed against the same buffer. Protein content of the extracts was determined by Bio-Rad Protein Assay.
Blood Glucose and HbA1c Analysis
Blood was collected from the rat tail vein after 8 weeks and by cardiac puncture at 16 weeks, for analysis of glucose contents (One Touch Ultra Glucometer; Johnson & Johnson, New Brunswick, N.J.) and HbA1c (Bayer A1CNow Self check, Sunnyvale, CA) levels.
Gelatin Zymography for MMPs Analysis
Assays for MMP-2 (pro-form: 72 kDa; activated-form: 63 kDa) and MMP-9 (pro-form: 92 kDa; activated-form: 82 kDa) in CFPFs in rPW, blood plasma and gingival tissue extract were conducted by gelatin zymography as described previously.28,45,52,53
ELISA Assay
The CFPFs in rPW, blood plasma and gingival tissue extracts were analyzed for cytokines (IL-1β, IL-6, and TNF-α) and resolvins (RvD1). Quantikine® ELISA kits for IL-1β (Catalog# RLB00, SRLB00, PRLB00), IL-6 (Catalog# R6000B, SR6000B, PR6000B), and TNF-α (Catalog# RTA00, SRTA00, PRTA00) were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). RvD1 (Catalog# 500380) is purchased from Cayman Chemical (Ann Arbor, Michigan, USA). All measurements of cytokines and resolvins were performed according to manufacturer’s instructions.
Micro Computerized Tomography (μCT) Imaging and Morphometric Analyses
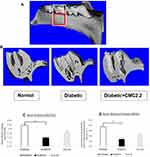
In this experiment, the heads were thawed at 4 °C, fixed and stored in 70% ethanol at room temperature. The maxillae were scanned with a Scanco MicroCT (μCT40; Scanco Medical, Bassersdorf, Switzerland) at 20.0 μm of voxel size, followed by reconstruction of the μCT images. Results were analyzed on the HP open platform (OpenVMS Alpha Version 1.3–1 session manager) using μCT V6.0 software. The volume of interest was defined on the axial planes between the distal root surface of the first molar and the mesial root surface of the third molar (Figure 1A, white box). A three-dimensional volume of interest was generated using the contours delineated continuously from every plane of the distal root surface of the first molar till the mesial root surface of the third molar.46,54 The relative bone volume of interest was calculated by first excluding the root and tooth volume from the total volume of interest, then using a bone volume fraction [bone volume/total volume (BV/TB)] to obtain the volume of mineralized bone per unit volume of the sample, based on counting voxels (Figure 1A, red box).46,54 Bone mineral density (BME) was calculated by mean voxel value in units of hydroxyapatite density (mg HA/cm3) as described previously.46,49,54
Activating Human Recombinant MMP-9
MMP-9 (Proenzyme, Human, Recombinant) (rh-MMP-9) was obtained from Calbiochem (EMD Millipore Corporation, CA, USA). This was diluted to 0.4ng/μL in 50 mM Tris, 10 mM CaCl2, 150 mM NaCl, 0.05% Brij-35 (w/v), pH 7.5 (TCNB) buffer and then activated by adding Sodium Hypochlorite (NaClO) solution from Sigma-Aldrich (St. Louis, MO, USA) to achieve a final concentration of 100 μM. Stock solutions of Doxycycline, Curcumin and CM2.24 were dissolved in DMSO (purchased from Sigma-Aldrich, St. Louis, MO, USA) at concentration of 1mM. Doxycycline and Curcumin were used as controls. The rh-enzyme was incubated for 24 hours at 37°C with NaOCl with or without Doxycycline, Curcumin or CM2.24 at a final concentration of 10 μM. The reaction mixture was then analyzed for pro- and activated-MMP-9 by gelatin zymography.
Statistical Analysis
For body weight, HbA1c, blood glucose, and MMP-9 in plasma, because time is introduced, the linear mixed model was used to evaluate the “value” between different experimental groups, with p < 0.05 taken as a statistically significant difference. Other MMP levels, cytokines, and bone assessment, for the different experimental groups were analyzed by ANOVA, with p < 0.05 taken as a statistically significant differences. Differences between groups were represented by *p < 0.05, **p < 0.01, ***p < 0.005, **** p < 0.001, #p < 0.0005, ##p < 5×10−5.
Results
Systemic Measurements: Plasma and Cell-Free Peritoneal Fraction (CFPFs)
Based on densitometric analysis of MMP-9 in the plasma, the levels of lower (MW) activated form of MMP-9 (82 kDa) increased significantly in Diabetic group at 4 months compared with those at 2 months (p value <0.05) (Figure 2). By comparison, this increase was significantly reduced in the D+CMC2.24 group at 4 months (p value <0.05) (Figure 2). The levels of total- and Pro- MMP-9 in the D+CMC2.24 group showed a significant increase at 4 months compared to its levels at 2 months (p value <0.05; p value <0.01) (Figure 2). Unlike MMP-9, there was no statistically significant difference in the levels of the MMP-2 in plasma between any of the three groups: normal (N), diabetic (D), and treatment (D+2.24) groups (data not shown).
Analysis of the data of the level of MMP-9 in the cell-free peritoneal fractions (CFPFs) from the non-elicited/resident peritoneal washes (rPW) of rats reveals that there is a significant increase in total MMP-9 for D group compared to the control group at end of the study (p < 0.05) (Figure 3A). This increase in the diabetic rats was 218% higher than in the non-diabetic control, compared to only a 62% increase in the treatment group compared to non-diabetic control. The activated-form of MMP-9 (82 kDa) showed a trend (not statistically significant) of increase in the D group (increased to 158%) compared to non-diabetic controls, whereas the D+2.24 group exhibited levels similar to normal group (Figure 3A), a pattern similar to blood plasma. Total MMP-2 levels were increased significantly in both the D and D+2.24 groups (p value < 0.005) (Figure 3B). However, the increase was 270% for D group, and only 151% for the D+CMC2.24 group compared to non-diabetic controls (N). However, the latter value was not statistically different from the placebo-treated diabetics.
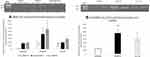
It is noteworthy that plasmas and CFPFs showed no difference in their levels of pro-inflammatory cytokines (IL-1ß, IL-6, and TNF-A) in the three experimental groups (Data not shown). We also measured resolvin (RvD1) levels in the CFPFs. CMC2.24 treatment significantly increased resolvin-RvD1 levels in CFPFs by 68% (p < 0.05), compared to the non-diabetic control (N) group, and by 44% (p < 0.05) compared to the diabetic (D) group (Figure 4). This indicates that CMC2.24 has a resolvin-stimulating ability and thus likely can prevent the prolongation (chronicity) of acute inflammation (Note: RvD1 was not measured in peripheral blood plasma).
Local/Oral Measurements: Gingiva and Alveolar Bone
Gingival tissues from 3 rats of each group were pooled, extracted, and partially purified as described above, and analyzed for both gelatinases, MMP-9, and MMP-2 using gelatin zymography (preliminary data not shown). These results show that the total amount of MMP-9 in both the D and D+2.24 group increased by 70% compared to that in gingival tissue extracts from non-diabetic controls. However, the Diabetic (D) group showed a 300% increase of activated form (82 kDa) of MMP-9 (a pattern similar to that was seen by systemic measurements) which was decreased to 52% upon treatment with CMC2.24 (preliminary data not shown). There was no difference seen in the levels of the MMP-2 in gingival tissue extract (3 rats pooled/group) in any of the three groups: N, D, and D+2.24 groups (data not shown).
Analysis of the pro-inflammatory cytokine, IL-1ß, in pooled gingival tissue extracts shows that Diabetes increases the levels of this pro-inflammatory cytokine compared to Control group by 371% (preliminary data not shown). Upon treatment with CMC2.24, IL-1ß was decreased by 59% compared to D group (Data not shown). Other pro-inflammatory cytokines (IL-6 and TNF-A) did not show any difference between the experimental groups at 4 months in pooled gingival tissue extracts.
Evidence of diabetes-induced osteoporosis was provided by μCT morphometric analysis of bone volume and bone mineral density in the maxilla, as well as visual evidence of increased porosity of the sectioned jaw bones in the untreated D group (Figure 1A–). Morphometric imaging analyses of maxilla, measured by micro-CT, showed a clear increase in porosity in the diabetic rats (D) compared to both non-diabetic controls and D+2.24 treated rats. Based on the analysis of bone volume fraction (BV/TV), the diabetic rats exhibited significantly lower values of BV/TV (p < 0.05), indicating lower relative bone volume in this group (Figure 1B). Moreover, analysis of bone mineral density (BMD) was consistent with results of relative bone volume. This showed a substantial reduction (p < 0.05) of the units of hydroxyapatite density (mg HA/cm3) in the diabetic jaw bones (Figure 1C). In contrast, in the D rats treated with CMC2.24, morphometric imaging analyses of maxilla showed almost the same lack of porosity as the non-diabetic normal control group (Figure 1A). In addition, based on bone-volume-fraction analysis, CMC2.24 treatment appeared to improve the relative bone volume although not back to levels seen in the normal rat jaws (Figure 1B). The reduced bone mineral density in the diabetic rats was also improved when they were treated with CMC2.24 (Figure 1C).
Blood Glucose and Glycated Hemoglobin
At 2 months and 4 months both the diabetic and treatment groups had a significant and similar increase in both the blood glucose and HbA1c protein compared to the normal group (p value < 0.0001, Figure 5A and Figure 5). There was no significant change in blood glucose or HbA1c levels in treatment group when compared to the diabetic group even after a period of 4 months (Figure 5A and Figure 5).
Diabetic Complications & Adverse Events
Three diabetic complications were observed in both the diabetic and treatment groups, namely, cataract, diabetic gastroparesis, and caries. The rats in the non-diabetic controls showed no complications. Although the treatment with CMC2.24 seemed to have a beneficial effect on these complications (data not shown), however, this and other complications would have to be the subject of another designed study. The rats in all the three groups, normal, diabetic and treatment, showed increases in the body weight at all time points during the 4-month study (data not shown). This indicates that treatment with CMC2.24 had no adverse effect on any of the rats to which it was administered.
Effect of Oxidative Stress & CMC2.24 Treatment on Recombinant Human MMP-9
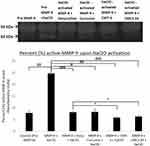
By means of zymography, it was found that incubating human recombinant 92kDa rh-MMP-9 (obtained from Calbiochem, EMD Millipore Corporation, CA, USA) with 100μM of Sodium Hypochlorite (NaClO) significantly increased the percentage of active-MMP-9 (82 kDa) from 7% to 23% (p value < 0.001, Figure 6). Moreover, treatment with 10μM of Doxycycline (p value < 0.001), Curcumin (p value < 0.01), and CMT-3 (p value <0.0001) all significantly decreased this activation to about 8%, 8% and 5.8%, respectively (similar to control values). The treatment with 10μM CMC2.24 appeared to be somewhat more effective since it decreased this activation of MMP-9 (82 kDa) to 6.3% (p value < 0.0001, Figure 6). This demonstrates that incubating rh-MMP-9 with oxidants like NaClO activates it to a lower molecular weight (82 kDa) active-form which is inhibited upon treatment with CMC2.24, demonstrating its antioxidant activity consistent with previous reports, by Kim et al44
Discussion
Earlier studies in our laboratory demonstrated the host modulatory activity of the novel CMC2.24 compound using an in vivo “short-term” rat model of experimental diabetes induced periodontitis and also in cell culture.28,39,46 Although these earlier studies were limited to 3 weeks – 4 weeks and used models of severe type I diabetes (induced with STZ administered intravenously (i.v) at 70 mg/kg), CMC2.24 treatment accomplished beneficial effects in rats with severe periodontal disease, even in the presence of continuing severe hyperglycemia. Therefore, based on the literature available, it was reasonable to hypothesize that longer-term studies (eg, of 4 months duration) of CMC2.24, a pleiotropic MMP-Inhibitor, in a less severe model of diabetes may reduce both blood glucose and HbA1c levels. This maybe because there is a reduction in non-enzymatic protein glycation together with a decreased inflammatory burden in the periodontal and other tissues. However, this did not happen! All of the beneficial effect on the inflammatory mediators and MMPs occurred in the ABSENCE of any significant improvement in hyperglycemia, at least during the 4-month time period of study.
In this current longer-term study, we describe a novel protocol to induce diabetes in rats using streptozotocin (STZ) at concentration of 55 mg/kg of body weight, intraperitoneally (i.p) in contrast to 70 mg/kg of intravenous (i.v) protocol used earlier. Consistent with earlier short-term studies, this longer-term study did not show any change in hyperglycemia due to treatment with CMC2.24 over the period of 4 months (Figure 5A and Figure 5). However, some pro-inflammatory cytokines (IL-1ß), and tissue-destructive enzymes like active- MMP-9 from various samples (plasma, gingival tissue extract, cell-free peritoneal fraction, cell supernatant) that were analyzed, did increase in the D group, but were decreased upon treatment with CMC2.24. Total MMP-9 in the treatment group increased significantly compared to the diabetic group (Figure 2). This increase in levels of total MMP-9 can be attributed to the increase in pro-MMP-9 levels in the treatment group (Figure 2). A similar trend of decrease, due to CMC2.24, in active MMP-9 was also seen in other systemic (CFPFs) (Figure 3) and local (gingival tissue extracts) tissue measurements (data not shown). We could not show data for gingival tissue extracts as even after pooling the gingiva from three rats, the sample size was small and we could not calculate any statistical significance for the same.
Consistent with previous studies36,37,42,46,47 on the resolvin-inducing property of CMC2.24, there was a significant increase in the resolvin (RvD1) in the group treated with CMC2.24 compared to the diabetic and non-diabetic controls (Figure 4). Derived from docosahexaenoic acid (DHA), this key pro-resolving lipid mediator belongs to a unique class of lipid mediators with anti-inflammatory.55,56 RvD1 helps regulate transmigration and infiltration of polymorphonuclear leukocytes (PMNs) during acute inflammation.55–57 Resolvins also have anti-inflammatory properties by reducing pro-inflammatory cytokines and preventing the prolongation of acute phase of inflammation.55–59
When the bone volume and bone mineral density were analyzed, we also observed the potent ability of CMC2.24 to reduce diabetes-induced osteoporosis in the upper jaws similar to previous short-term studies (Figure 1A–).36,37,39,46 This complication could be observed visually in the rats with severe and uncontrolled hyperglycemia, evidenced by increased roughness and porosity of maxillary jaws seen in a 3D-reconstructed image. Additionally, this was confirmed by micro-CT morphometric analysis of bone volume and bone mineral density. Although these decreased significantly in the diabetic group, there was no significant difference in these characteristics between the CMC2.24-treated diabetic rats and the non-diabetic controls. This demonstrates that CMC2.24 prevents systemic bone loss and osteoporosis even in the presence of persistent hyperglycemia. It is important to note that maxillary bone which mainly comprises trabecular bone is more prone to quick and severe osteoporosis when there is a demand for calcium as compared to the mandibular bone (made mostly of cortical bone).60,61 This strongly suggests that treatment of CMC2.24 could reduce the risk for pathologic fracture of skeletal bones. However, further studies are needed to confirm this hypothesis.
This new long-term study of less severe diabetic rats shows that active MMP-9 is an early sensitive molecular marker that reflects the chronic inflammatory state in vivo in the absence of any upregulation of other inflammatory cytokines, and before any increase in blood glucose, or HbA1c can be detected. A recent study by our group using a dog model of natural periodontitis also demonstrated that MMP-9 may be used as an early, specific, and sensitive biomarker in detecting early biochemical response to any proposed treatment, and an indication of future clinical changes.35 Excessive secretion of MMP-9 (and MMP-8) may contribute to the pathogenesis of tissue destructive processes in a wide variety of diseases. High levels of MMP-9 have been related to the pathogenesis of chronic inflammatory conditions such as diabetes,62 periodontitis,63 rheumatoid arthritis,64 cancer,65 and others. MMP-9 is involved in tissue damage and inflammation by degrading matrix proteins and activating cytokines/chemokines via proteolytic activation.22 Recent literature shows that MMP-8 and MMP-9 levels in biological fluids reflect the severity of chronic inflammatory diseases and certainly are diagnostically significant markers for the progression of inflammatory and destructive changes in periodontal disease.66–72
MMP-9 pro-enzyme can be activated by proteolytic removal of the propeptide or disruption of the zinc-cysteine bond by other proteases, other MMPs, heavy metals or organomercury compounds such as aminophenylmercuric acetate (APMA), oxidants (hypochlorous acid, etc) and alkylating agents.22–26 Activation of pro-MMP-9 can occur when the cysteine switch is disrupted. This disruption can occur, at physiological concentrations of reactive oxygen species (ROS), because of oxidation of the cysteine thiol group.22,24 The pathogenesis of many diseases, including diabetes and its complications, is often oxidative stress, a result of excessive ROS production and the suppression of the antioxidant defense system for ROS removal.73 Increased levels of blood glucose in vivo affects MMP-9 activity in various models.74,75 We hypothesize that MMP-9 may be activated in this experimental-diabetes model due to increased oxidative stress.73,74 One rational is that diabetes is characterized by high glucose concentrations that lead, via several mechanisms (such as glucose autoxidation, stimulation of the polyol pathway, activation of the reduced form of nicotinamide adenine dinucleotide phosphate oxidase, and production of advanced glycation end products), to an increased production of reactive oxygen species.27,73,74
The decrease in the levels of active MMP-9 in the treatment group when compared to the placebo-treated diabetic group suggests a possible mechanism by which the novel MMP-inhibitor CMC2.24 inhibits the activation of pro-enzyme form of MMP-9 in vivo. Since there are various mechanisms by which this novel MMP-inhibitor, CMC2.24, can prevent the activation of MMP-9, we examined the antioxidant activity. CMC2.24 has already demonstrated higher antioxidant activity than either resveratrol, vitamin C, or natural unmodified curcumin.44 In the current study, activation of MMP-9 was significantly decreased when rh-MMP-9 activated with NaClO (an oxidant) along with 10 µM of CMC2.24, Doxycycline, or Curcumin (Figure 6). The inhibition of the activation of MMP-9 was significantly greater when treated with CMC2.24 than with doxycycline. This highlights its potent antioxidant activity, but further studies are needed to outline the mechanism of action more clearly.
Conclusion
In conclusion, the current study indicates that long-term in vivo administration of CMC2.24 to diabetic rats can: (a) significantly suppress tissue-destructive, pathologically elevated levels of active MMP-9 (aMMP-9) both systemically and locally; (b) significantly enhance resolvin (RvD1) activity; and (c) reduce the severity of systemic bone loss (diabetes-induced osteoporosis). Moreover, these anti-collagenolytic, anti-inflammatory, and pro-resolving benefits occurred without any effect on severe hyperglycemia strongly indicating the therapeutic potential of this novel compound even in uncontrolled diabetics. This study also indicates that an increase of active MMP-9 even in the absence of changes in other biochemical parameters supports the role of a-MMP-9 as an earlier and more sensitive biomarker than cytokines in inflammatory/collagenolytic disease. This is consistent with, and supports the idea, currently clinically available as a diagnostic aid, a-MMP-8, a clinical diagnostic marker of active periodontal sites (as opposed to that are not active and respond to therapy).67–69,71,72 The significant antioxidant activity demonstrated by CMC2.24, which contributes to reduced levels of MMP-9, demonstrates the possible mechanism by which this novel compound therapeutic reduces the oxidative stress associated with periodontitis and other collagenolytic/inflammatory diseases.
Acknowledgments
This study is supported by Stony Brook University Research Foundation P1050308 T1 A37298, NIH grant R42DE024946, Traverse BioSciences Inc., and Chem-Master International. Inc.
Disclosure
Lorne M. Golub is listed as an inventor on several related patents, and these have been fully assigned to his institution, Stony Brook University, The State University of New York (SUNY). Francis Johnson is also listed as an inventor on several related patents which have been fully assigned to Stony Brook University and to Chem-Master Int. Inc. on a shared basis. In addition, both LMG and FJ are minor shareholders in Traverse BioSciences, Inc. All other authors declare that they have no conflicts of interest regarding the publication of this paper.
References
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement1):S81–S90. doi:10.2337/dc14-S081
2. International Diabetes Federation. IDF Diabetes Atlas.
3. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7(12):738–748. doi:10.1038/nrendo.2011.106
4. Grossi SG, Skrepcinski FB, DeCaro T, et al. Response to periodontal therapy in diabetics and smokers. J Periodontol. 1996;67(10S):1094–1102. doi:10.1902/jop.1996.67.10s.1094
5. Lalla E, Lamster IB, Feit M, et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. 2000;105(8):1117–1124. doi:10.1172/JCI8942
6. Schmidt AM, Hori O, Chen JX, et al. Advanced glycation end products interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96(3):1395–1403.
7. Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation end products (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1(1):1–15. doi:10.1016/S0047-6374(01)00366-9
8. Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15(2):135–141. doi:10.1097/MED.0b013e3282f824b7
9. Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65(3):260–267.
10. Shiau M-Y, Tsai S-T, Tsai K-J, et al. Increased circulatory MMP-2 and MMP-9 levels and activities in patients with type 1 diabetes mellitus. Mt Sinai J Med. 2006;73(7):1024–1028.
11. Smith PC, Muñoz VC, Collados L, Oyarzún AD. In situ detection of matrix metalloproteinase-9 (MMP-9) in gingival epithelium in human periodontal disease. J Periodontal Res. 2004;39(2):87–92. doi:10.1111/j.1600-0765.2004.00705.x
12. Lazăr L, Loghin A, Bud E-S, et al. Cyclooxygenase-2 and matrix metalloproteinase-9 expressions correlate with tissue inflammation degree in periodontal disease. Rom J Morphol Embryol. 2015;56(4):1441–1446.
13. Bastos MF, Tucci MA, de Siqueira A, et al. Diabetes may affect the expression of matrix metalloproteinases and their inhibitors more than smoking in chronic periodontitis. J Periodontal Res. 2017;52(2):292–299. doi:10.1111/jre.12394
14. Şurlin P, Oprea B, Solomon SM, et al. Matrix metalloproteinase-7,-8,-9 and-13 in gingival tissue of patients with type 1 diabetes and periodontitis. Rom J Morphol Embryol. 2014;55(3 Suppl):1137–1141.
15. Ueland T, Holter J, Holten A, et al. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J Infect. 2020;81(3):e41–e43. doi:10.1016/j.jinf.2020.06.061
16. Sorsa T, Tjaderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38(5):306–321. doi:10.1080/07853890600800103
17. Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755(1):37–69.
18. Sang Q-X, Birkedal-Hansen H, Van Wart HE. Proteolytic and non-proteolytic activation of human neutrophil progelatinase B. Biochim Biophys Acta. 1995;1251(2):99–108. doi:10.1016/0167-4838(95)00086-A
19. Fridman R, Toth M, Peña D, Mobashery S. Activation of Progelatinase B (MMP-9) by Gelatinase A (MMP-2). Cancer Res. 1995;55(12):2548–2555.
20. Sorsa T, Tjäderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10(6):311–318. doi:10.1111/j.1601-0825.2004.01038.x
21. Escalona LA, Mastromatteo-Alberga P, Correnti M. Cytokine and metalloproteinases in gingival fluid from patients with chronic periodontitis. Investigación clínica. 2016;57(2):131–142.
22. Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48(3):222–272. doi:10.3109/10409238.2013.770819
23. O’Sullivan S, Medina C, Ledwidge M, Radomski MW, Gilmer JF. Nitric oxide-matrix metaloproteinase-9 interactions: biological and pharmacological significance: NO and MMP-9 interactions. Biochim Biophys Acta. 2014;1843(3):603–617.
24. Peppin GJ, Weiss SJ. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc Natl Acad Sci U S A. 1986;83(12):4322–4326. doi:10.1073/pnas.83.12.4322
25. Bannikov GA, Karelina TV, Collier IE, Marmer BL, Goldberg GI. Substrate Binding of Gelatinase B Induces Its Enzymatic Activity in the Presence of Intact Propeptide. J Biol Chem. 2002;277(18):16022–16027. doi:10.1074/jbc.M110931200
26. Paquette B, Bisson M, Therriault H, et al. Activation of matrix metalloproteinase-2 and −9 by 2- and 4-hydroxyestradiol. J Steroid Biochem Mol Biol. 2003;87(1):65–73. doi:10.1016/S0960-0760(03)00386-8
27. Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5(5):561–568. doi:10.1097/00075197-200209000-00016
28. Elburki MS, Moore DD, Terezakis NG, et al. A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. J Periodontal Res. 2017;52(2):186–200. doi:10.1111/jre.12381
29. Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y, Proposed Model A. Linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76(11S):2075–2084. doi:10.1902/jop.2005.76.11-S.2075
30. Moutsopoulos NM, Madianos PN. Low-grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Ann N Y Acad Sci. 2006;1088:251–264. doi:10.1196/annals.1366.032
31. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi:10.1001/jama.286.3.327
32. Löe H. Periodontal diseases: a brief historical perspective. Periodontology. 1993;2:7–12. doi:10.1111/j.1600-0757.1993.tb00215.x
33. Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329–334.
34. Frankwich K, Tibble C, Torres-Gonzalez M, et al. Proof of Concept: matrix metalloproteinase inhibitor decreases inflammation and improves muscle insulin sensitivity in people with type 2 diabetes. J Inflamm. 2012;9(1):35. doi:10.1186/1476-9255-9-35
35. Deng J, Golub LM, Lee H-M, et al. A novel chemically-modified curcumin 2.24: short-term systemic therapy for natural periodontitis in dogs. Front Dent Med. 2021;2(5). doi:10.3389/fdmed.2021.609795
36. Deng J, Golub LM, Lee HM, et al. Chemically-modified curcumin 2.24: a novel systemic therapy for natural periodontitis in dogs. J Exp Pharmacol. 2020;12:47–60. doi:10.2147/JEP.S236792
37. Deng J, Golub LM, Lee HM, et al. A novel modified-curcumin promotes resolvin-like activity and reduces bone loss in diabetes-induced experimental periodontitis. J Inflamm Res. 2021;14:5337–5347. doi:10.2147/JIR.S330157
38. Elburki MS, Rossa C, Guimaraes MR, et al. A novel chemically modified curcumin reduces severity of experimental periodontal disease in rats: initial observations. Mediators Inflamm. 2014;2014:959471. doi:10.1155/2014/959471
39. Elburki MS, Rossa C, Guimarães-Stabili MR, et al. A chemically modified curcumin (CMC 2.24) inhibits nuclear factor κB activation and inflammatory bone loss in murine models of LPS-induced experimental periodontitis and diabetes-associated natural periodontitis. Inflammation. 2017;40(4):1436–1449. doi:10.1007/s10753-017-0587-4
40. Bhatt HD, McClain, SA, Lee HM et al. The maximum-tolerated-dose and pharmacokinetics of a novel chemically-modified-curcumin in rats. J Exp Pharmacol. 2022;14:73–85. doi:10.2147/JEP.S341927
41. Golub LM, Lee HM. Periodontal therapeutics: current host-modulation agents and future directions. Periodontology. 2020;82(1):186–204. doi:10.1111/prd.12315
42. Gu Y, Deng J, Lee H, et al. Chemically-modified-curcumin: resolvin activity in experimental diabetes. J Dent Res. 2017;96(Special issue A):1173.
43. Gu Y, Lee HM, Napolitano N, et al. 4-methoxycarbonyl curcumin: a unique inhibitor of both inflammatory mediators and periodontal inflammation. Mediators Inflamm. 2013;2013:329740. doi:10.1155/2013/329740
44. Kim J, Lee HM, Golub LM, Gu Y. Antioxidant activity of a novel chemically-modified-curcumin compound (CMC 2.24). J Dent Res. 2019;11(Spec Iss 98):0912.
45. Elburki MS. A Novel Chemically Modified Curcumin as A Pleiotropic MMP-Inhibitor: Therapeutic Potential in Locally- And Systemically-Induced Periodontal (And Other) Connective Tissue Breakdown [Doctoral]. Stony Brook: Stony Brook University; 2015.
46. Deng J. A Novel Chemically-Modified Curcumin: Pleiotropic Resolvin-Like Activity Reduces Inflammation/Collagenolysis During Periodontitis and Diabetes [Doctoral Thesis Dissertation]. Stony Brook: Stony Brook University; 2019.
47. Deng J, Gu Y, Lee H, et al. Novel chemically-modified-curcumin: resolution of cytokines and MMPs in cell culture. J Dent Res. 2018;97(Special Issue A):0129.
48. Zhang Y, McClain SA, Lee HM, et al. A novel chemically modified curcumin “Normalizes” wound-healing in rats with experimentally induced type I diabetes: initial studies. J Diabetes Res. 2016;2016:5782904. doi:10.1155/2016/5782904
49. Cengiz IF, Oliveira JM, Reis RL. Micro-CT - A digital 3D microstructural voyage into scaffolds: a systematic review of the reported methods and results. Biomater Res. 2018;22:26. doi:10.1186/s40824-018-0136-8
50. Ramamurthy NS, Golub LM. Diabetes increases collagenase activity in extracts of rat gingiva and skin. J Periodontal Res. 1983;18(1):23–30. doi:10.1111/j.1600-0765.1983.tb00331.x
51. Golub LM, Evans RT, McNamara TF, Lee HM, Ramamurthy NS. A non-antimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis-induced periodontitis in rats. Ann N Y Acad Sci. 1994;732:96–111. doi:10.1111/j.1749-6632.1994.tb24728.x
52. Golub LM, Ramamurthy NS, Llavaneras A, et al. A chemically modified nonantimicrobial tetracycline (CMT-8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra-oral bone loss in ovariectomized rats. Ann N Y Acad Sci. 1999;878(1):290–310. doi:10.1111/j.1749-6632.1999.tb07691.x
53. Lee HM, Golub LM, Cao J, et al. CMT-3, a non-antimicrobial tetracycline (TC), inhibits MT1-MMP activity: relevance to cancer. Curr Med Chem. 2001;8(3):257–260. doi:10.2174/0929867013373660
54. Park CH, Abramson ZR, Taba M, et al. Three-dimensional micro-computed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78(2):273–281. doi:10.1902/jop.2007.060252
55. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128(7):2657–2669. doi:10.1172/JCI97943
56. Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(S1):S200–S315. doi:10.1038/sj.bjp.0707489
57. Van Dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. 2017;58:21–36. doi:10.1016/j.mam.2017.04.006
58. Serhan CN. Controlling the resolution of acute inflammation: a new genus of dual anti‐inflammatory and proresolving mediators. J Periodontol. 2008;79:1520–1526. doi:10.1902/jop.2008.080231
59. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi:10.1038/nri2294
60. Roberts WE, Simmons KE, Garetto LP, DeCastro RA. Bone physiology and metabolism in dental implantology: risk factors for osteoporosis and other metabolic bone diseases. Implant Dent. 1992;1(1):11–21. doi:10.1097/00008505-199200110-00002
61. Melescanu-Imre M, Preoteasa E. Mandibular panoramic indexes predictors of skeletal osteoporosis for implant therapy. Curr Health Sci J. 2009;35:220–225.
62. Ryan M, Ramamurthy N, Sorsa T, Golub L. MMP-mediated events in diabetes. Ann N Y Acad Sci. 1999;878:311–334. doi:10.1111/j.1749-6632.1999.tb07692.x
63. Rai B, Kharb S, Jain R, Anand SC. Biomarkers of periodontitis in oral fluids. J Oral Sci. 2008;50(1):53–56. doi:10.2334/josnusd.50.53
64. Gruber BL, Sorbi D, French DL, et al. Markedly elevated serum MMP-9 (Gelatinase B) levels in rheumatoid arthritis: a potentially useful laboratory marker. Clin Immunol Immunopathol. 1996;78(2):161–171. doi:10.1006/clin.1996.0025
65. Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: a minireview. Eur J Med Chem. 2020;194:112260. doi:10.1016/j.ejmech.2020.112260
66. Domenyuk D, Samedov F, Dmitrienko S, et al. Matrix metalloproteinases and their tissue inhibitors in the pathogenesis of periodontal diseases in type 1 diabetes mellitus. Archiv Euro Medica. 2019;9(3):81. doi:10.35630/2199-885X/2019/9/3.25
67. Sorsa T, Alassiri S, Grigoriadis A, et al. Active MMP-8 (aMMP-8) as a grading and staging biomarker in the periodontitis classification. Diagnostics. 2020;10(2):61. doi:10.3390/diagnostics10020061
68. Sorsa T, Gursoy UK, Nwhator S, et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology. 2016;70(1):142–163. doi:10.1111/prd.12101
69. Sorsa T, Hernández M, Leppilahti J, et al. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral Dis. 2010;16(1):39–45. doi:10.1111/j.1601-0825.2009.01603.x
70. Sorsa T, Mäntylä P, Tervahartiala T, et al. MMP activation in diagnostics of periodontitis and systemic inflammation. J Clin Periodontol. 2011;38(9):817–819. doi:10.1111/j.1600-051X.2011.01753.x
71. Sorsa T, Sahni V, Buduneli N, et al. Active matrix metalloproteinase-8 (aMMP-8) point-of-care test (POCT) in the COVID-19 pandemic. Expert Rev Proteomics. 2021;18(8):707–717. doi:10.1080/14789450.2021.1976151
72. Sorsa T, Tervahartiala T, Leppilahti J, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. 2011;63(2):108–113.
73. Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37(101799):2213–2317.
74. Uemura S, Matsushita H, Li W, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity. Circ Res. 2001;88(12):1291–1298. doi:10.1161/hh1201.092042
75. Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. doi:10.1074/jbc.274.31.21491
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.