Back to Journals » Infection and Drug Resistance » Volume 11
Efficacy of a 14-day quadruple-therapy regimen for third-line Helicobacter pylori eradication
Authors Huang HT , Wang HM, Yang SC, Tai WC , Liang CM , Wu KL , Lee CH, Chuah SK
Received 28 August 2018
Accepted for publication 3 October 2018
Published 30 October 2018 Volume 2018:11 Pages 2073—2080
DOI https://doi.org/10.2147/IDR.S185511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Hsiang Tso Huang,1 Hsin-Ming Wang,1 Shih-Cheng Yang,1 Wei-Chen Tai,1 Chih-Ming Liang,1 Keng-Liang Wu,1 Chen-Hsiang Lee,2 Seng-Kee Chuah1
1Division of Hepato-Gastroenterology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Niao-Song District, Kaohsiung 833, Taiwan; 2Division of Infectious Diseases, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Niao-Song District, Kaohsiung 833, Taiwan
Purpose: To assess the efficacy of amoxicillin, tetracycline, high-dose metronidazole, and a proton-pump inhibitor for third-line Helicobacter pylori eradication.
Methods: We enrolled 70 consecutive patients who had registered, failed to respond to two rounds of H. pylori eradication, and undergone endoscopy for H. pylori culture. Seven patients were lost to follow-up. Patients were treated according to the results of antibiotic-susceptibility testing reports (cultured group, n=39). Those who failed the H. pylori culture were prescribed 14-day quadruple therapy containing esomeprazole 40 mg twice daily, amoxicillin 1 g twice daily, tetracycline 500 mg four times daily, and metronidazole 500 mg three times daily (empirical group, n=24). A follow-up urea breath test was performed 8 weeks later.
Results: Antibiotic-resistance rates were 79.5% (clarithromycin), 94.9% (levofloxacin), 66.7% (metronidazole), 2.6% (amoxicillin), and 0 (tetracycline). Eradication rates attained by the cultured and empirical group were 89.7% (95% CI 72.7%–97.1%) and 58.3% (95% CI 36.6%–77.9%) in per-protocol analysis (P=0.004) and 81.4% (95% CI 66.6%–91.6%) and 51.8% (95% CI 31.9%–71.3%) in intention-to-treat analysis (P=0.014), respectively. Culture-guided therapy was the only clinical factor influencing the efficacy of H. pylori eradication (OR 0.16, 95% CI 0.04–0.60; P=0.006). Despite the high metronidazole-resistance rate (66.7%) after two treatment failures, the eradication rate in patients with this condition was 84%.
Conclusion: Empirical 14-day modified quadruple therapy is not acceptable as an alternative third-line rescue H. pylori treatment. The success rate of third-line susceptibility-guided treatment was near 90%. This report is valuable as a reminder to medical practitioners that rather than a try-and-see approach, susceptibility-guided therapy should always be considered whenever possible for patients who have undergone several treatment failures.
Keywords: amoxicillin-resistance, metronidazole-resistance, empirical quadruple therapy, culture-guided therapy
Introduction
Helicobacter pylori infection is an extremely common bacterial infection that is prevalent worldwide and has been classified as a grade I carcinogen.1–3 H. pylori infection can induce chronic gastritis, which progresses through the premalignant stages of atrophic gastritis, intestinal metaplasia, and dysplasia, before finally leading to gastric cancer.4,5 Successful eradication of H. pylori has greatly reduced the recurrence of peptic ulcers.6 Globally, the standard triple therapy consists of one proton-pump inhibitor (PPI), amoxicillin, and clarithromycin. Metronidazole is an alternative to amoxicillin for patients who are allergic. However, H. pylori may develop resistance to the prescribed antibacterials, such as clarithromycin as a standard first-line therapy, and may acquire resistance through the acquisition and recombination of genes from other bacteria.7 The resistance battle against H. pylori continues, and many people still self-medicate with antibiotics, such as levofloxacin, which helps H. pylori to develop drug resistance.8–10
After the failure of second-line treatment, culture with susceptibility testing or molecular determination of genotype resistance is recommended by the Maastricht V/Florence Consensus Report.11 Secondary resistance to clarithromycin and levofloxacin is common in patients who fail to respond to regimens containing these antibiotics.12,13 Therefore, the reuse of clarithromycin and levofloxacin should be prevented on an empirical basis in third-line regimens. However, there is a major limitation to this therapeutic option, because of the low sensitivity of culture-based guidance. Moreover, few hospitals and clinics have a proper facility to perform cultures, test for antibiotic susceptibility, or perform molecular genotype-resistance testing. More effective third-line regimens for H. pylori are needed in the future. The need for an ideal, empirical third-line H. pylori rescue therapy has been raised. The Toronto consensus recommended that the choice of antibiotics be made empirically according to medication history.14 However, earlier studies have generally shown poor eradication rates with rifabutin-based15 or rifaximin-based16 third-line rescue (63%–66%), regardless of combination with amoxicillin, levofloxacin, or clarithromycin.
In Taiwan, H. pylori culture with susceptibility testing or molecular determination of genotype resistance is recommended in third-line rescue therapy by the Taiwan Consensus Report.17 However, patients who fail H. pylori culture or refuse to undergo further endoscopy can be encountered, and molecular determination of genotype resistance is sometimes not available. Liou et al reported that secondary antibiotic resistance to amoxicillin and tetracycline is uncommon in Taiwanese patients who fail in at least two eradications.18 The addition of a PPI helps to overcome metronidazole resistance, and administration of a double dose (twice per day) is recommended.19 In our previous studies, we observed that high-dose metronidazole may overcome metronidazole resistance.20 The present study aimed to evaluate the efficacy of a simple, modified, empirical 14-day quadruple therapy containing esomeprazole, amoxicillin, metronidazole, and tetracycline as a third-line regimen for H. pylori eradication.
Methods
Study population
Between January 1, 2015, and 31, December 31, 2017, 1,318 naïve patients infected by H. pylori were treated at Kaohsiung Chang Gung Memorial Hospital, Taiwan according to the registration system. We enrolled a total of 70 registered consecutive H. pylori-infected patients who had failed on standard first-line triple therapy (PPI twice daily, amoxicillin 1 g twice daily, clarithromycin 500 mg twice daily for 7 days) and second-line levofloxacin-based triple therapy (PPI twice daily, amoxicillin 1 g twice daily, levofloxacin 500 mg once daily for 14 days) were recruited in this study. Based on international guidelines, these patients underwent endoscopy for H. pylori culture and consequent antibiotic-susceptibility testing. Seven patients were lost to follow-up. All patients were at least 18 years old and underwent endoscopic examinations that showed peptic ulcers or gastritis. Confirmation of H. pylori-eradication failure was defined when the patient had either one positive result on a 13C urea breath test (UBT) or any two positive results on a rapid urease test, histology, or culture after second-line eradication therapy. Exclusion criteria were use of antibiotics, bismuth, or PPIs within the previous 4 weeks, patients with a history of allergies to the medications used, patients with a history of gastric surgery, and pregnant women. Patients were then treated according to the antibiotic-susceptibility testing reports (cultured group [n=39], esomeprazole 40 mg twice daily, amoxicillin 1 g twice daily, tetracycline 500 mg four times daily, metronidazole 500 mg three times daily, or levofloxacin 500 mg once daily). Those who failed the H. pylori culture and did not want to undergo a repeat endoscopy for H. pylori culture were also prescribed with a simple empirical 14-day quadruple therapy containing esomeprazole 40 mg twice daily, amoxicillin 1 g twice daily, tetracycline 500 mg four times daily, and metronidazole 500 mg three times daily (empirical group, n=24). The UBT was used to confirm the status of the H. pylori 8 weeks later. The absence of H. pylori after eradication therapy was defined as a negative result on the UBT.9,21,22
The primary outcome variables were eradication rate, presence of adverse events, and level of patient compliance. Demographic information, including age, sex, history of smoking (defined as one or more cigarettes per day), alcohol consumption (defined as up to one drink per day for women and two drinks per day for men). History of peptic ulcers and medical history were collected via electronic medical records. Poor compliance was defined as taking <80% of the total medication.20,21
Ethics approval and informed consent
Data collection in this study was based on reviewing computerized medical charts. This study was approved by both the institutional review board and ethics committee of Chang Gung Memorial Hospital, Taiwan (IRB 201800034B0). The ethics committee waived the requirement for informed consent, and each patient’s medical records were anonymized and deidentified prior to access. All patients provided written informed consent for endoscopic examinations before the procedure. None of our patients were minors/children. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution’s human research committee.
13C UBT
The procedure of the 13C UBT was modified from a previously described method.23 After a 2-hour fast, a baseline sample of expired breath in a 20 mL Vacutainer from each patient was obtained before administration of 75 mg 13C-urea in 80–100 mL distilled water. At 20 minutes after ingestion of 13C-urea, expired breath was collected. All samples were taken in duplicate and analyzed by an automated breath 13C analyzer (isotope-ratio infrared spectrometer). Results were obtained using “delta over baseline”, which indicates change in the 13CO2:12CO2 ratio from metabolic activity induced by administering the labeled urea. Delta-over-baseline values >4% were considered positive on the UBT. This UBT for H. pylori has excellent sensitivity (98%, 95% CI 96%–99%) and specificity (95%, 95% CI 92%–97%) after clinical data analysis.24
Culture and antimicrobial susceptibility testing
One biopsy specimen each from the antrum and corpus were obtained for H. pylori isolation using previously described culture methods.10,11 The biopsy specimens were cultured on plates containing Brucella chocolate agar with 7% sheep blood and incubated for 4–5 days under microaerobic conditions. The minimal inhibitory concentration (MIC) was determined using the agar dilution test. The H. pylori strains were tested for susceptibility to amoxicillin, clarithromycin, levofloxacin, metronidazole, and tetracycline using the E-test method (AB Biodisk, Solna, Sweden). H. pylori strains had MIC values of ≥0.5, ≥1, ≥1, ≥4, and ≥8 mg/L, which were considered to be the resistance breakpoints for amoxicillin, clarithromycin, levofloxacin, metronidazole, and tetracycline, respectively.22
Statistical analysis
Using SPSS 23, χ2 tests with or without Yates’s correction for continuity and Fisher’s exact tests were used when appropriate to compare major outcomes between the groups. Eradication rates were analyzed using per protocol (PP) approaches. PP analysis excluded patients with unknown H. pylori status following therapy and those with major protocol violations. P<0.05 was considered statistically significant. To determine independent factors affecting treatment response, clinical parameters were analyzed using univariate and multivariate analyses. For multivariate variables, variables with P<0.3 on univariate analysis were used as covariates. Binary logistic regression was used with a forward conditional method to take into account only variables with P<0.05.
Results
Patient demographic and baseline characteristics
Figure 1 is a flowchart of the study. Seven patients were lost to follow-up. As such, 63 patients were included in the PP analysis. The characteristics of the patients are summarized in Table 1. The mean age was 58.9±10.1 years, and 36.5% were men. There was no significant difference between the cultured group and empirical group in terms of age, sex, smoking/alcohol habits, peptic ulcer history, or endoscopic findings.
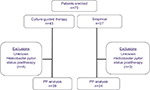  | Figure 1 Patient characteristics. |
  | Table 1 Demographic data and endoscopic findings of the two patient groups |
Eradication rate and susceptibility of H. pylori culture
Figure 2 shows the antibiotic resistance of strains after two H. pylori treatment failures. Clarithromycin-resistant strains were found in 79.5% (31 of 39) of patients. Levofloxacin-resistant strains were found in 94.9% (37 of 39) of patients. Metronidazole-resistant strains were found in 66.7% (26 of 39) of patients. Amoxicillin-resistant strains were found in 2.6% (one of 39) of patients. No strains developed resistance to tetracycline. Table 2 demonstrates the major outcomes in terms of eradication rate. The eradication rates attained by the cultured group and empirical group were 89.7% (95% CI 72.7%–97.1%) and 58.3% (95% CI 36.6%–77.9%), respectively, in PP analysis (P=0.004) and 81.4% (95% CI 66.6%–91.6%) and 51.8% (95% CI 31.9%–71.3%), respectively, in intention-to-treat analysis (P=0.014). Despite the high metronidazole-resistance rate after two treatment failures, the eradication rate was 84% in patients with this condition (Table 3).
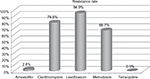  | Figure 2 Antibiotic resistance after two Helicobacter pylori-eradication attempts. |
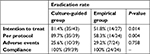  | Table 2 Major outcomes of eradication therapy |
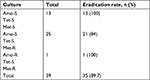  | Table 3 Antibiotic resistance and Helicobacter pylori-eradication rate Abbreviations: Amo, amoxicillin; Met, metronidazole; PP, per protocol; R, resistant; S, sensitive; Tet, tetracycline. |
Adverse events and compliance
Adverse events occurred in 17 patients (22.9%). There was no significant difference between the two groups in terms of the adverse-event rate (25.6% vs 29.2%, P=0.758l Table 2). The most common side effect from the use of medication was digestive-system problems, such as nausea, abdominal pain, and constipation (Table 4). All patients completed their course of treatment, so the rate of compliance was 100%.
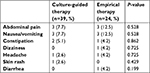  | Table 4 Adverse events |
Factors influencing the efficacy of anti- H. pylori therapy
Table 5 shows the univariate analysis of all demographic data and factors. There was only one clinical factor that affected efficacy (culture vs empirical therapy, P=0.004). In the multivariate analysis, culture vs empirical therapy was the only clinical factor that influenced H. pylori eradication (OR 0.16, 95% CI 0.04–0.60; P=0.006; Table 6).
  | Table 5 Univariate analysis of clinical factors influencing the efficacy of Helicobacter pylori eradication |
  | Table 6 Multivariate analysis of clinical factors influencing the efficacy of Helicobacter pylori eradication |
Discussion
According to the Maastricht V/Florence Consensus Report,11 after failure of second-line therapy for the eradication of H. pylori, the best therapeutic choice should be based on the cultivation of H. pylori to determine its susceptibility to medications. However, determination of H. pylori MIC is not practiced widely, because it is time-consuming, inconvenient, and relatively expensive. Furthermore, the successful culture rate ranges 75%–90%.25,26 There are many issues that can affect the results of culture, like H. pylori cultured from several biopsied samples not accurately representing bacteria of the whole stomach and also external factors, including incubation conditions, growth media, lack of experienced personnel, and examination technique, and thus the results of antimicrobial susceptibility testing may not be consistent.41 Genotypic resistance-guided testing is a quick and effective assay to determine to which antibiotics H. pylori will respond well. Liou et al18 published a study concerning genotypic resistance-guided testing as a third-line treatment in Taiwan. This method improved the overall eradication rate in patients who received clarithromycin-, levofloxacin-, and tetracycline-based sequential therapies: 78.9% (15 of 19), 92.2% (47 of 51), and 71.4% (25 of 35) for strains susceptible to clarithromycin, levofloxacin, and tetracycline, respectively. The eradication rate is optimal in patients who are receiving a levofloxacin-based regimen if the genotype is sensitive to levofloxacin. However, in the current study, patients who failed to achieve H. pylori eradication after first-line standard triple clarithromycin-based therapy and subsequent second-line levofloxacin-based therapy developed high resistance to clarithromycin 79.5% (31 of 39) and levofloxacin 94.9% (37 of 39). Conversely, amoxicillin- and tetracycline-resistance rates were as low as 2.6% (one of 39) and 0. Therefore, we used a simple, modified 14-day high-dose quadruple therapy with esomeprazole, amoxicillin, metronidazole, and tetracycline as the empirical third-line regimen for H. pylori eradication for patients who had failed the H. pylori culture and refused further endoscopic procedures. There were several reasons for the empirical use of this modified quadruple empirical therapy. First, the prevalence of tetracycline and amoxicillin resistance, even in patients who have failed multiple treatments, remains low worldwide.17,27 Our results confirmed that the prevalence of tetracycline resistance was 0 in patients who failed two eradication treatments, with an increase from 0 to up to 10% over the course of 5 years in our previous report.20 Second, the impact of tetracycline and metronidazole resistance regarding eradication rate remains controversial.25,28–31 Also, high-dose metronidazole was effective for eradication therapy despite a relatively high resistance rate. Third, several randomized controlled trials have compared two durations of eradication therapy and demonstrated that the longer duration is more effective.32,33 Therefore, we set the third-line treatment course at 14 days.
In this study, PP analysis revealed that culture-guided therapy achieved a significantly higher eradication rate than empirical therapy (89.7% vs 58.3%, P=0.004). This finding suggested that empirical quadruple therapy was not acceptable as empirical third-line H. pylori eradication. However, there was no definite factor found in detailed logistic regression analysis to predict the successful rate, except for the culture-guided vs empirical group (P=0.004). Chen et al reported a >90% eradication rate using a bismuth-containing quadruple therapy with metronidazole and amoxicillin as an alternative to classical bismuth quadruple therapy for third-line H. pylori rescue therapy.34 At this point, we question why a similar regime with different doses and intervals had been reported as successful, but they did not use amoxicillin and tetracycline concomitantly. The possible explanation for our unacceptable empirical therapy report could be partly related to a drug–drug interaction between amoxicillin and tetracycline, which exhibits mild in vitro antibiotic resistance.35 The success in Chen et al’s study could have been due to the synergy between metronidazole and its hydroxymetabolite and between either the analogue or amoxicillin/tetracycline, both of which may contribute to the efficacy of both the amoxicillin–metronidazole and tetracycline–metronidazole combinations.36 It has been proposed that bacteriostatic drugs, such as tetracycline, might interfere with the bactericidal action of penicillin.37 The bactericidal action inhibits cell-wall formation, which is dependent on how quickly the bacteria are multiplying. Tetracycline may reduce the effectiveness of penicillin, because it is a bacteriostatic antibiotic and can inhibit the cellular protein synthesis required for cell division. Therefore, susceptibility-guided treatment is still recommended as a third-line H. pylori-eradication therapy as per the Maastricht V/Florence Consensus Report, because the drug–drug interaction alone could not fully explain the low eradication rates in the empirical group, as we did not know the culture sensitivity in the empirical group at all.
According to Table 3, the large majority of patients enrolled in the culture group were amoxicillin- and tetracycline-sensitive, with around 60% being metronidazole-resistant. Still, bias could have existed, as the empirical group also consisted of these three medicines. Nevertheless, we did not know the antibiotic resistance in the empirical group. It was possible that there could have been more highly resistant strains of H. pylori in the empirical group. On the other hand, it was not so surprising for the eradication of metronidazole-resistant strains, because evidence has shown that metronidazole resistance can be overcome by higher doses of metronidazole.20,32,33,37,38 A possible explanation was environmental changes in oxygen pressure in the stomach, as metronidazole-resistant H. pylori isolates can become susceptible to metronidazole under low oxygen conditions in vitro.31,39 In Spain, Puig et al published a systematic review and meta-analysis on therapy based on culture-guided susceptibility.40 They found that the eradication rate of H. pylori was only 72%. In our study, the eradication rate was near 90%. In real-world practice, despite the introduction of culture testing and molecular genotype resistance after failure of second-line treatment, these methods are not routinely available in most medical institutions. In this regional study, we noted that if patients failed to respond to clarithromycin-based standard triple therapy and levofloxacin-based therapy, the strains resistant to clarithromycin and levofloxacin were high. Our initial hypothesis was that the empirical modified 14-day high-dose quadruple therapy with amoxicillin, tetracycline, high-dose metronidazole, and esomeprazole could be an alternative third-line regimen for H. pylori eradication. Although the rate of drug compliance was high and it was easy for patients to adhere to treatment, the eradication-success rate did not fulfill our expectations.
There were some limitations to this study. First, the study sample was small, because large samples for multiple treatment failures are usually difficult to obtain in a single tertiary hospital. Second, we could not conduct a randomized trial, because we felt that it was not ethical to randomize patients with multiple H. pylori-eradication failures and available culture-susceptibility results.
Conclusion
Empirical 14-day modified quadruple therapy is not acceptable as an alternative third-line rescue H. pylori treatment, despite the low resistance rates of amoxicillin and tetracycline, including the fact that high-dose metronidazole may overcome antibiotic resistance. The success rate of third-line susceptibility-guided treatment was near 90%. This report is valuable as a reminder to medical practitioners that rather than a try-and-see approach, susceptibility-guided treatment should always be considered whenever possible for the treatment of similar patients, especially for those who have undergone several treatment failures.
Acknowledgment
The authors would like to acknowledge Miss Ching-Yi Lin for her assistance in this study.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Pellicano R, Ribaldone DG, Fagoonee S, Astegiano M, Saracco GM, Mégraud F. A 2016 panorama of Helicobacter pylori infection: key messages for clinicians. Panminerva Med. 2016;58(4):304–317. | ||
World Health Organization. International Agency for Research on Cancer (IARC). IARC monographs on the evaluation of carcinogenic risks to humans. Schistosomes, Liver Flukes and Helicobacter pylori. Lyon: IARC. 1994;61:177–240. | ||
Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113(6):1983–1991. | ||
Lu B, Li M. Helicobacter pylori eradication for preventing gastric cancer. World J Gastroenterol. 2014;20(19):5660–5665. | ||
Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis. 2012;13(1):2–9. | ||
Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110(4):1244–1252. | ||
Tursi A, Picchio M, Elisei W. Efficacy and tolerability of a third-line, levofloxacin-based, 10-day sequential therapy in curing resistant Helicobacter pylori infection. J Gastrointestin Liver Dis. 2012;21(2):133–138. | ||
Murakami K, Furuta T, Ando T, et al; Japan GAST Study Group. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013;48(10):1128–1135. | ||
Chuah SK, Tai WC, Hsu PI, et al. The efficacy of second-line anti-Helicobacter pylori therapy using an extended 14-day levofloxacin/amoxicillin/proton-pump inhibitor treatment--a pilot study. Helicobacter. 2012;17(5):374–381. | ||
Chuah SK, Tai WC, Lee CH, Liang CM, Hu TH. Quinolone-containing therapies in the eradication of Helicobacter pylori. Biomed Res Int. 2014;2014:151543. | ||
Malfertheiner P, Megraud F, O’Morain CA, et al; European Helicobacter and Microbiota Study Group and Consensus panel. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. | ||
Liou JM, Chang CY, Chen MJ, et al; Taiwan Gastrointestinal Disease and Helicobacter Consortium. The primary resistance of Helicobacter pylori in Taiwan after the national policy to restrict antibiotic consumption and its relation to virulence factors –A nationwide study. PLoS One. 2015;10(5):e0124199. | ||
Chang WL, Sheu BS, Cheng HC, Yang YJ, Yang HB, Wu JJ. Resistance to metronidazole, clarithromycin and levofloxacin of Helicobacter pylori before and after clarithromycin-based therapy in Taiwan. J Gastroenterol Hepatol. 2009;24(7):1230–1235. | ||
Fallone CA, Chiba N, van Zanten SV, et al. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151(1):51–69.e14. | ||
Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35(2):209–221. | ||
Perri F, Festa V, Clemente R, et al. Randomized study of two “rescue” therapies for Helicobacter pylori-infected patients after failure of standard triple therapies. Am J Gastroenterol. 2001;96(1):58–62. | ||
Sheu BS, Wu MS, Chiu CT, et al. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017;22(3):e12368. | ||
Liou JM, Chen CC, Chang CY, et al; Taiwan Helicobacter Consortium. Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother. 2013;68(2):450–456. | ||
Graham DY, Lee SY. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol Clin North Am. 2015;44(3):537–563. | ||
Chuah SK, Liang CM, Lee CH, et al. A Randomized Control Trial Comparing 2 Levofloxacin-Containing Second-Line Therapies for Helicobacter pylori Eradication. Medicine (Baltimore). 2016;95(19):e3586. | ||
Liang CM, Cheng JW, Kuo CM, et al. Levofloxacin-containing second-line anti-Helicobacter pylori eradication in Taiwanese real-world practice. Biomed J. 2014;37(5):326–330. | ||
Chuah SK, Hsu PI, Chang KC, et al. Randomized comparison of two non-bismuth-containing second-line rescue therapies for Helicobacter pylori. Helicobacter. 2012;17(3):216–223. | ||
Hsu PI, Hwang IR, Cittelly D, et al. Clinical presentation in relation to diversity within the Helicobacter pylori cag pathogenicity island. Am J Gastroenterol. 2002;97(9):2231–2238. | ||
Ling D. Carbon-13 urea breath test for Helicobacter pylori infection in patients with uninvestigated ulcer-like dyspepsia: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13(19):1–30. | ||
Gisbert JP. “Rescue” regimens after Helicobacter pylori treatment failure. World J Gastroenterol. 2008;14(35):5385–5402. | ||
Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology. 2007;133(3):985–1001. | ||
Wu IT, Chuah SK, Lee CH, et al. Five-year sequential changes in secondary antibiotic resistance of Helicobacter pylori in Taiwan. World J Gastroenterol. 2015;21(37):10669–10674. | ||
Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26(3):343–357. | ||
Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98(3):562–567. | ||
Nista EC, Candelli M, Cremonini F, et al. Levofloxacin-based triple therapy vs. quadruple therapy in second-line Helicobacter pylori treatment: a randomized trial. Aliment Pharmacol Ther. 2003;18(6):627–633. | ||
Gerrits MM, van der Wouden EJ, Bax DA, et al. Role of the rdxA and frxA genes in oxygen-dependent metronidazole resistance of Helicobacter pylori. J Med Microbiol. 2004;53(Pt 11):1123–1128. | ||
Treiber G, Wittig J, Ammon S, Walker S, van Doorn LJ, Klotz U. Clinical outcome and influencing factors of a new short-term quadruple therapy for Helicobacter pylori eradication: a randomized controlled trial (MACLOR study). Arch Intern Med. 2002;162(2):153–160. | ||
Kongchayanun C, Vilaichone RK, Pornthisarn B, Amornsawadwattana S, Mahachai V. Pilot studies to identify the optimum duration of concomitant Helicobacter pylori eradication therapy in Thailand. Helicobacter. 2012;17(4):282–285. | ||
Chen Q, Zhang W, Fu Q, et al. Rescue Therapy for Helicobacter pylori Eradication: A Randomized Non-Inferiority Trial of Amoxicillin or Tetracycline in Bismuth Quadruple Therapy. Am J Gastroenterol. 2016;111(12):1736–1742. | ||
Wu DC, Hsu PI, Tseng HH, et al. Helicobacter pylori infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine (Baltimore). 2011;90(3):180–185. | ||
Moellering RC. Rationale for use of antimicrobial combinations. Am J Med. 1983;75(2A):4–8. | ||
Sorice F, Ortona L, Pizzigallo E. [Further aspects of combination antibiotic therapy. Critical review and personal case studies]. Minerva Med. 1975;66(57):2805–2822. Italian. | ||
Kuo CH, Hsu PI, Kuo FC, et al. Comparison of 10 day bismuth quadruple therapy with high-dose metronidazole or levofloxacin for second-line Helicobacter pylori therapy: a randomized controlled trial. J Antimicrob Chemother. 2013;68(1):222–228. | ||
Moon JY, Kim GH, You HS, et al. Levofloxacin, Metronidazole, and Lansoprazole Triple Therapy Compared to Quadruple Therapy as a Second-Line Treatment of Helicobacter pylori Infection in Korea. Gut Liver. 2013;7(4):406–410. | ||
Puig I, López-Góngora S, Calvet X, et al. Systematic review: third-line susceptibility-guided treatment for Helicobacter pylori infection. Therap Adv Gastroenterol. 2016;9(4):437–448. | ||
Arslan N, Yılmaz Ö, Demiray-Gürbüz E. Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol. 2017;23(16):2854–2869. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
