Back to Journals » Clinical Ophthalmology » Volume 11
Efficacy of 0.05% epinastine and 0.1% olopatadine for allergic conjunctivitis as seasonal and preseasonal treatment
Authors Mizoguchi T, Ozaki M, Ogino N
Received 7 May 2017
Accepted for publication 18 July 2017
Published 27 September 2017 Volume 2017:11 Pages 1747—1753
DOI https://doi.org/10.2147/OPTH.S141279
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Takanori Mizoguchi,1 Mineo Ozaki,2 Nobuchika Ogino3
1Mizoguchi Eye Clinic, Ophthalmology, Sasebo, Japan; 2Ozaki Eye Hospital, Ophthalmology, Miyazaki, Japan; 3Nishigaki Eye Clinic, Ophthalmology, Nagoya, Japan
Purpose: To evaluate the efficacy and safety of 0.05% epinastine and 0.1% olopatadine eye drop preparations as seasonal and preseasonal treatments in patients with seasonal allergic conjunctivitis (SAC).
Subjects and methods: This was a prospective, randomized, case-control study involving two institutions. The subjects were patients diagnosed with SAC at two institutions between February and March in 2014. To examine the clinical effects of seasonal treatment, 0.05% epinastine and 0.1% olopatadine were administered, and their effects were investigated every 2 weeks (Stage 1). To evaluate the clinical effects of preseasonal therapy, in January 2015, the same eye drop preparations as adopted in Stage 1 were administered to patients who had participated in Stage 1 and provided consent to participate in this study, and their effects were investigated every month (Stage 2).
Results: In Stage 1, the 0.05% epinastine group consisted of 43 patients, and the 0.1% olopatadine group consisted of 42 patients. There were significant improvements in the total symptom and objective finding scores at each time point after administration in comparison with those before its baseline, but there were no significant differences between the two groups. In Stage 2, the 0.05% epinastine group consisted of 15 patients, and the 0.1% olopatadine group consisted of 14 patients. The rate of change in the total symptom score in comparison with that at the baseline of preseasonal treatment was significantly higher in the 0.1% olopatadine group 1 month after the start of treatment, suggesting symptom deterioration (P=0.025). There was no significant difference in the rate of change in the total objective finding score between the two groups.
Conclusion: Seasonal treatment with 0.05% epinastine or 0.1% olopatadine was equally effective for patients with allergic conjunctivitis. However, for preseasonal therapy, 0.05% epinastine was more effective than 0.1% olopatadine.
Keywords: 0.05% epinastine, 0.1% olopatadine, seasonal allergic conjunctivitis, preseasonal treatment, seasonal treatment, inverse agonist
Introduction
Ocular allergy occurs in 15%–25% of the general population.1,2 In other countries, antigens include grass,3 differing from those that cause allergic conjunctivitis in Japan.4,5 Japanese cedar pollinosis is a common disease with an age-adjusted estimated prevalence of 19.4% in Japan4 and is considered to be a national affiction.5 Cedar pollen is specific to Japan, differing from antigens in other countries; it is important to examine the therapeutic effects on cedar pollen allergy.
Currently, second-generation antiallergic drugs, which have both anti-histaminic and mast-cell stabilizing activities, are selected as first-choice drugs to treat allergic conjunctivitis. Several studies6,7 have shown that, even in the absence of an agonist, a portion of the G protein-coupled receptor (GPCR) is activated, and that there is a kinetic equilibrium between activated and inactivated receptors. In addition, the entity of an inverse agonist in histamine receptors was proposed,6,8,9 and it has been considered as an important factor influencing the effects of preseasonal therapy. Both 0.05% epinastine and 0.1% olopatadine have inverse agonist activities, but, according to a study, the former exhibits more potent actions.9 As second-generation anti-histamine eye drop preparations, 0.05% epinastine and 0.1% olopatadine are currently used, showing favorable results.10–14 Many studies have reported favorable results for the use of these ophthalmic solutions as preseasonal therapy,15,16 but no study has compared the efficacy of these drugs.
We conducted this study to examine the therapeutic effects of seasonal treatment and preseasonal therapy and compare the efficacy of the drugs.
Subjects and methods
Patients who had a history of seasonal allergic conjunctivitis (SAC) to cedar pollen in both eyes and had itching and signs of ocular allergy every year during the cedar season were recruited for this study between February and March in 2014 and 2015. In Japan, the signs and symptoms of SAC to cedar pollens are characteristically manifested from February to March. The study included 85 patients who consulted Mizoguchi Eye Clinic (Nagasaki, Japan) or Ozaki Eye Clinic (Miyazaki, Japan) after February and March in 2014. Fifty-one patients (9 males, 42 females) visited Mizoguchi Eye Clinic and 34 patients (8 males, 26 females) Ozaki Eye Clinic. The following patients were excluded: 1) patients with anterior ocular disease other than allergic conjunctivitis; 2) those receiving oral steroids, anti-inflammatory drugs, anticholinergic agents, immunosuppressive drugs, or eye drops; 3) those with severe allergic ocular disease and giant papilla formation; and 4) those wearing contact lenses.
This study was conducted according to the Helsinki Declaration and was approved by the ethics committee of Mizoguchi Eye Clinic. Written informed consent regarding this study was obtained from all the patients. They were registered at http://www.umin.ac.jp/ (Identification No UMIN 000013073).
To evaluate the severity of subjective symptoms including itching, hyperemia, discomfort, lacrimation, and discharge, which was assessed using 5 grades (score 0= none and score 4= most severe), the Japanese allergic conjunctival disease quality-of-life questionnaire version 1 (http://www.joasg.com/article/14534117.html) was adopted.
To evaluate the severity of objective signs including palpebral and bulbar conjunctiva and superficial punctate keratopathy (SPK), which was assessed using 5 grades (score 0= none and score 4= most severe), biomicroscopy was used to grade them.
The objective examinations included examination of palpebral conjunctiva (hyperemia, swelling, follicle, papilla) and bulbar conjunctiva (hyperemia, edema, Trantas spots, swelling); SPK score17 was adopted.
Study design
Stage 1 (seasonal treatment)
This survey was conducted as a seasonal treatment involving patients diagnosed with SAC and consented to participate in this study. These patients were randomly assigned to receive 0.05% epinastine or 0.1% olopatadine using the envelope method. Subsequently, eye drop treatment was performed for 3 months, and they were instructed to consult the clinic every 2 weeks for a questionnaire survey and examinations. They were received in each eye four times a day. During the treatment period, respective eye drops alone were administered, and patients were prohibited from using other antiallergic eye drops (Figure 1).
  | Figure 1 Study design of Stages 1 and 2. |
Stage 2 (preseasonal treatment)
To patients who participated in Stage 1, a request for participation in a study regarding the effects of preseasonal therapy was sent, and Stage 2 study was conducted involving those who consented to this. They were requested to consult the clinic 1 month prior to cedar pollen dispersion. The same eye drop preparation as administered in Stage 1 was administered. They were applied in each eye four times a day. During the treatment period, respective eye drops alone were administered, and they were prohibited from using other antiallergic eye drops (Figure 1). In Stage 2, they were prohibited from receiving steroid eye drops or oral antiallergic drugs.
Statistical analysis
For group comparison in Stage 1, estimation was conducted using a mixed effect model, and the results were compared using the least square mean at each observation point. In Stage 2, findings 1 month prior to cedar-pollen dispersion were regarded as baseline values, and the rate of change from the baseline value was compared at each examination point. To compare the results between the two groups, chi-square test and unpaired t-test were used. A P-value of 0.05 was regarded as significant. The confidence coefficient for interval estimation was 95%. SPSS statistical software version 16.0 for windows (SPSS Inc., Chicago, IL, USA) was used.
Results
The number of cedar pollen grains from January to March in 2014 (Stage 1) and 2015 (Stage 2) is showed in Table 1.
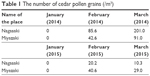  | Table 1 The number of cedar pollen grains (/m3) |
Stage 1 (seasonal treatment)
The patients’ background data at Stage 1 is shown in Table 2. The total subjective symptom scores on initial consultation in the 0.05% epinastine and 0.1% olopatadine groups were 4.86±3.23 and 5.67±3.96, respectively; there was no significant difference between the two groups (P=0.16). The scores at each point are shown in Figure 2. After the start of eye drop treatment, there was a significant improvement in the subjective and objective scores at each point in comparison with those at the baseline period. However, there was no significant difference between the two groups at any point. The total objective finding scores at the start of treatment in the 0.05% epinastine and 0.1% olopatadine groups were 4.64±1.80 and 5.75±2.59, respectively, although the subjects were randomly assigned using the envelope method. The value was significantly higher in the latter (P=0.0048). However, after the start of eye drop treatment, there was no significant difference between the two groups at any point of time (Figure 2).
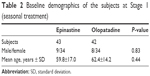  | Table 2 Baseline demographics of the subjects at Stage 1 (seasonal treatment) |
Stage 2 (preseasonal treatment)
The background data of patients in Stage 2 who consented to initial therapy, among those who participated in Stage 1, are shown in Table 3. The total subjective and objective baseline scores in Stages 1 and 2 in the 0.05% epinastine and 0.1% olopatadine groups are presented in Tables 4 and 5, respectively. The total baseline subjective scores in the 0.05% epinastine and 0.1% olopatadine groups in Stage 2 were 2.73±3.88 and 1.57±1.55, respectively; there was no significant difference (P=0.25) between the groups. The comparison of each subjective and objective score at the start of treatment is shown in Table 6. Before the start of treatment, there was a significant difference in itching score between the two groups (P=0.03). However, there were no significant differences in any other symptom score. To evaluate the effects of treatment, we examined the rate of change in the symptom score from before the start of treatment to the end of treatment (Figure 3). The total symptom scores at 0 and 8 weeks were significantly lower in the 0.05% epinastine group (P=0.024 and 0.026, respectively) (Figure 3A). In addition, the rate of change in the total symptom score was low, as the symptoms were stable. When investigating each symptom, there were no significant differences in the itching or foreign body sensation scores between the two groups, but the eye discharge score at 8 weeks was significantly lower in the 0.05% epinastine group (P=0.028) (Figure 3A).
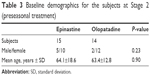  | Table 3 Baseline demographics for the subjects at Stage 2 (preseasonal treatment) |
  | Table 6 Comparison of baseline demographics with preseasonal treatment of epinastine and olopatadine (Stage 2) |
The total objective finding scores at the start of treatment in the 0.05% epinastine and 0.1% olopatadine groups were 2.33±1.52 and 3.07±1.18, respectively, showing no significant difference (P=0.30). However, there was a significant difference in the conjunctival follicle score between the two groups (Table 6). To evaluate the effects of treatment, we examined the rate of change in the objective finding score from before the start of treatment to the end of treatment (Figures 3B). The hyperemic score at 0 weeks was significantly lower in the 0.05% epinastine group, but there was no significant difference at any other point of time. There were no significant differences in the tear volume or follicular/corneal findings between the two groups.
Discussion
Stage 1 (seasonal treatment)
Currently, drugs with two pharmacological actions, histamine H1 receptor antagonism and mediator-release inhibition, are selected as first-choice drugs. These two actions are showed by 0.05% epinastine and 0.1% olopatadine and are commonly used as antiallergic eye drop preparations in Japan. A conjunctival allergen challenge (CAC) test with cedar pollen was conducted to compare the effects of 0.05% epinastine with those of 0.1% olopatadine. It was reported that the latter more markedly reduced pruritus and congestion compared with the former.12 However, in clinical practice, there is no opportunity for single exposure to a massive amount of antigen, as performed in the CAC test; the results of this test reflect the therapeutic effects under a specific environment. In this study, we reviewed symptoms related to cedar pollen dispersion under a natural environment; it is clinically important to evaluate the effects under this circumstance. Based on the total symptom score, both 0.05% epinastine and 0.1% olopatadine significantly reduced the symptoms after administration, showing an improvement. However, there was no significant difference in objective and subjective symptoms between the two groups at each time point. Based on these results, it can be concluded that the efficacy of 0.05% epinastine, as seasonal treatment, may be similar to that of 0.1% olopatadine.
Stage 2 (preseasonal treatment)
Histamine H1 receptors are a type of GPCR.6 There are two H1 receptor isoforms: an active form and an inactive form. In the absence of agonist stimulation, inactive histamine H1 receptors are predominant, showing an equilibrium.7–9 Inverse agonists inhibit active histamine H1 receptors before agonist dispersion.6 Ophthalmic solutions of 0.05% epinastine and 0.1% olopatadine exhibit inverse agonistic action for histamine receptor. Preseasonal therapy with these eye drop preparations is reportedly effective for allergic conjunctivitis. However, there was a difference in inverse agonist activity between the two drugs. Mizuguchi et al7 reported that the inverse agonistic activity of 0.05% epinastine was more potent than that of 0.1% olopatadine. If eye drop treatment before histamine dispersion increases the number of inactive histamine H1 receptors, this inhibits the receptor expression and decreases the stimulation response threshold on allergic reactions, resulting in a possible reduction of subjective symptoms after dispersion; in particular, this may be important for preseasonal therapy.7,15 In our patients, eye drop treatment was started 4 weeks prior to cedar pollen dispersion, and the rate of change in the total symptom score in the 0.05% epinastine group was significantly lower than in the 0.1% olopatadine group. In addition, stable effects were obtained for the subsequent 3 months. This suggests that 0.05% epinastine, which has a potent inverse agonist activity, is useful for preventing symptoms and maintaining the effects as preseasonal therapy.
Among symptoms of allergic conjunctivitis, pruritus, discomfort, and eye discharge reduce the quality of life most markedly. It is important to clarify the preventive effects of preseasonal therapy on these symptoms. In this study, eye discharge was significantly reduced in the 0.05% epinastine group 3 months after the start of administration. There were no significant differences in the preventive effects on pruritus or discomfort between the two groups; the effects may have been similar. However, symptom changes were less marked in the 0.05% epinastine group; the symptoms may have been more markedly reduced, and stable symptom-reducing effects may have been maintained.
Limitations
There are several limitations in this study. In Stage 1 (seasonal treatment), there was a difference in the total objective finding score at baseline between the two groups despite random drug assignment using the envelope method. However, there were no significant differences at any subsequent point of time; therefore, there may have been no difference in the efficacy of eye drop treatment. In the future, a larger number of patients should be reviewed. In Stage 2 (preseasonal treatment), the number of patients was small. Further examination may be necessary in the future.
In conclusion, the efficacy of 0.05% epinastine may be similar to that of 0.1% olopatadine as seasonal treatment. However, as preseasonal therapy, 0.05% epinastine may be more effective than 0.1% olopatadine.
Disclosure
TM received a partial research funds from Santen Pharmaceutical Co.. The authors report no other conflicts of interest in this work.
References
Bielory BP, Shah SP, O’Brien TP, Perez VL, Bielory L. Emerging therapeutics for ocular surface disease. Curr Opin Allergy Clin Immunol. 2016;16(5):477–486. | ||
La Rosa M, Lionetti E, Reibaldi M, et al. Allergic conjunctivitis: a comprehensive review of the literature. Ital J Pediatr. 2013;14:18. | ||
Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1999. J Allergy Clin Immunol. 2010;126(4):778–783. | ||
Okuda M. Epidemiology of Japanese cedar pollinosis throughout Japan. Ann Allergy Asthma Immunol. 2003;91:288–296. | ||
Yamada T, Saito H, Fujieda S. Present state of Japanese cedar pollinosis: the national affiction. J Allergy Clin Immunol. 2014;133(3):632.e5–639.e5. | ||
Leurs R, Church MK, Taglialatela M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002;32(4):489–498. | ||
Mizuguchi H, Ono S, Hattori M, et al. Usefulness of HeLa cells evaluate inverse agonistic activity of antihistamines. Int Immunopharmacol. 2003;15:539–543. | ||
Bakker RA, Wieland K, Timmerman H, Leurs R. Consecutive activity of the histamine H(1) receptor reveals inverse agonism of histamine H(1) receptor antagonist. Eur J Pharmacol. 2000;387(1):R5–R7. | ||
Das AK, Yoshimura S, Mishima R, et al. Stimulation of histamine H1 receptor up-regulates histamine H1 receptor itself through activation of receptor gene transcription. J Pharmacol Sci. 2007;103:374–382. | ||
Trattler WB, Luchs J, Majmudar P. Elestat (epinastin HCl ophthalmic solution 0.05%) as a therapeutic for allergic conjunctivitis. Int Ophthalmol Clin. 2006;46(46):87–99. | ||
Fujishima H, Ohashi Y, Takamura E. Efficacy of epinastine hydrochloride ophthalmic solution in allergic conjunctivitis by conjunctival cedar pollen allergen challenge. Ann Allergy Asthem Immunol. 2014; 113(4):476–481. | ||
Fukushima A, Ebihara N. Efficacy of olopatadine versus epinastine for treating allergic conjunctivitis caused by Japanese cedar pollen: a double-blind randomized controlled trial. Adv Ther. 2014;31:1045–1058. | ||
Nakagawa Y, Oohashi Y, Takamura E, Fujishima H. Safety and efficacy of long-term treatment with 0.05% epinastine ophthalmic solution for allergic conjunctivitis. Atarashii Ganka. 2014;31(1):97–104. | ||
Ogiso T, Takano Y, Kawashima S, Fujishima H. Clinical efficacy of olopatadine hydrochloride ophthalmic solution for allergic conjunctivitis: comparison with combination therapy using anti-histamic and mast cell stabilizer ophthalmic solutions. Atarashii Ganka. 2008;25(11):1553–1556. | ||
Ebihara N. Preventive effect of olopatadine ophthalmic solution in seasonal allergic conjunctivitis. Atarashii Ganka. 2007;24(11):1523–1525. | ||
Shimura M, Yasuda K, Miyazawa T. Pre-seasonal treatment with topical olopatadine suppresses the clinical symptoms of seasonal allergic conjunctivitis. Am J Ophthalmol. 2011;151(4):697–702. | ||
Miyata K, Amano S, Sawada M, Nishida T. A novel grading method for superficial punctate keratopathy magnitude and its correlation with corneal permeability. Arch Ophthalmol. 2003;121(11):1537–1539. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.