Back to Journals » Drug Design, Development and Therapy » Volume 10
Efficacy and tolerability of vilazodone for major depressive disorder: evidence from phase III/IV randomized controlled trials
Authors Shi LG, Wang JY, Xu SB, Lu YR
Received 11 September 2016
Accepted for publication 27 October 2016
Published 25 November 2016 Volume 2016:10 Pages 3899—3907
DOI https://doi.org/10.2147/DDDT.S122085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Ligen Shi,1,2 Jingyi Wang,1 Shenbin Xu,2 Yunrong Lu1
1Department of Psychiatry, The Fourth Affiliated Hospital of Zhejiang University School of Medicine, Yiwu, 2Department of Neurosurgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China
Abstract: Vilazodone is a new molecule approved for major depressive disorder (MDD). This report focuses on the efficacy and tolerability of vilazodone for MDD. MEDLINE, EMBASE, and Cochrane Library were searched. A total of 1,930 patients from four trials were included. A significant improvement in the Montgomery–Asberg Depression Rating Scale (MADRS) total score was seen as early as week 2 (P<0.01) in vilazodone-treated patients. The results showed a higher rate of MADRS response with vilazodone compared with placebo (P<0.001). There were also greater improvements in the Hamilton Rating Scale for Anxiety as well as the Clinical Global Impressions (severity of illness and improvement of illness) scores from baseline in vilazodone-treated patients compared to placebo patients (P<0.001). Discontinuation rates due to adverse events were higher with vilazodone than placebo (P=0.0002). The most common adverse events of vilazodone were vomiting, nausea, diarrhea, insomnia, somnolence, dizziness, and dry mouth (P<0.05). Treatment-related effects on sexual function were mild compared to placebo in men (P=0.03). In conclusion, 40 mg/day of vilazodone had a rapid onset of response and showed good improvement in anxiety symptoms as well as good tolerability during short-term treatment (8–10 weeks) for MDD. Further studies should focus on the efficacy and tolerability of vilazodone over a longer duration and should utilize active comparators.
Keywords: vilazodone, major depressive disorder, sexual dysfunction, anxiety
Introduction
Major depressive disorder (MDD) is a prevalent and often recurrent debilitating illness associated with severe functional impairment, significant mortality, and high health care costs.1 Although numerous antidepressants have been used in clinical settings for decades, over 30% of patients with MDD do not achieve an adequate response and remission.2 Nonadherence and premature treatment discontinuation are fairly common3 and can be traced back to limited efficacy or delayed onset as well as intolerable adverse events, especially weight gain and sexual dysfunction.4 In addition, it is difficult to achieve treatment response or remission for those patients with depressive and anxiety symptoms.5 Thus, there is a need for novel antidepressants with better efficacy and tolerability than the current therapies.
Vilazodone, a 5-hydroxytryptamine-1A (5-HT1A) receptor partial agonist and selective serotonin reuptake inhibitor (SSRI), was approved by the US Food and Drug Administration (FDA) for treatment of MDD in January 2011.6 SSRI is a well-established first-line antidepressant,7 and its antidepressant efficacy may be enhanced by the 5-HT1A receptor partial agonist working to improve the onset of therapeutic action.8 This unique dual modulation of serotonin neurotransmission by vilazodone offers 30 times greater potency for serotonin reuptake than fluoxetine in functional assays.9 Preclinical studies have demonstrated that vilazodone showed high selectivity for the 5-HT1A receptor as well as a relative lack of affinity for other neuronal receptors, which may lead to a low risk for treatment-induced adverse events, especially sexual dysfunction.10 Moreover, vilazodone has also been proposed to control anxiety symptoms.11 Thus far, two phase III12,13 and two phase IV14,15 double-blind, placebo-controlled trials have explored the efficacy and tolerability of vilazodone for adult patients with MDD. Vilazodone (40 mg) was found to significantly improve depressive symptoms during short-term (8 or 10 weeks) treatment of MDD. However, nausea, diarrhea, insomnia, dizziness, headache, dry mouth, and fatigue were commonly reported in clinical trials.12–15 In addition, several studies reported that vilazodone might have a potential anxiolytic-like effect.12–15
The purpose of the present study was to conduct a meta-analysis of previous clinical trials to evaluate the efficacy and tolerability of vilazodone for adult patients with MDD. Moreover, this study aimed to provide evidence of vilazodone’s effects on managing anxiety symptoms.
Methods
PRISMA guidelines were followed to draft the study protocol for this project.16
Eligibility criteria
Only studies that met the following inclusion criteria were included: 1) study type: double-blind randomized controlled trials (RCTs) phase III and IV; 2) language: English; 3) participants: adult patients with MDD diagnosed by the Diagnostic and Statistical Manual of Mental Disorders (IV); 4) intervention: oral vilazodone; and 5) control: oral placebo.
Exclusion criteria included: 1) study types: case reviews, case reports, cohort studies, and retrospective studies; 2) parallel setups: positive control such as fluoxetine; and 3) drug administration: intravenous injection.
Search strategy and information sources
Two authors (LS and JW) independently searched three major databases; MEDLINE, EMBASE, and the Cochrane Library. The combination of the variables “vilazodone (viibryd)” AND “major depressive disorder” was used as the search strategy for MEDLINE. The study only focused on the titles and abstracts of studies written in English. A similar search strategy was used for EMBASE and Cochrane Library. Reference lists from the included studies were also manually screened to ensure all relevant studies were included in this systematic review.
Study selection and data collection
Two authors (LS and JW) independently evaluated all records from the systematic search of the electronic database and reference lists of RCTs as well as the systematic reviews. The following information was extracted from the included RCTs: overview of the included trials, eligibility criteria and study design, patient demographic characteristics, MDD history, and outcome assessments (Table 1).
The mean change in the Montgomery–Asberg Depression Rating Scale (MADRS) total score was the primary outcome. Data on the change in MADRS total score from baseline to weeks 1, 2, 4, 6, and 8 were extracted from the figures using Graph Data Extractor. The secondary outcomes included the MADRS response, mean change in Hamilton Rating Scale for Anxiety (HAM-A), Clinical Global Impressions-Severity of Illness (CGI-S), and Clinical Global Impressions-Improvement of Illness (CGI-I) total scores from baseline to the end of treatment, and adverse events. The definition of a MADRS response was ≥50% decrease in MADRS from baseline to week 8. Tolerability was defined by a discontinued rate due to adverse events.
Risk of bias and quality assessment
The risk of bias in individual studies was independently assessed by two authors (LS and JW) using Review Manager 5.2 software. Uniform criteria were applied from the Cochrane Collaboration to evaluate the risk of bias of RCTs: detection bias, selection bias, reporting bias, performance bias, attrition bias, and other potential biases as previously used in this meta-analysis.17
Statistical analysis
Review Manager 5.2 was used from the Cochrane Collaboration to assess the data. Differences between vilazodone and placebo groups were evaluated according to changes in MADRS, HAM-A, CGI-I, CGI-S, and adverse events. Dichotomous outcomes were analyzed by the risk ratio (relative risk [RR]; 95% confidence interval [CI]). Continuous outcomes were analyzed by the mean difference (MD) and 95% CI. A fixed effect model (Mantel–Haenszel technique) was used for statistics. The study was considered to be homogeneous only when the analysis showed P>0.05. Statistical heterogeneity was estimated by the I2 statistic as follows: I2<30% means “low heterogeneity,” I2=30%–50% denotes “moderate heterogeneity,” and I2>50% represents “substantial heterogeneity.” All tests were two-tailed, and P≤0.05 was considered to be significant for all analyses.
Results
A total of 311 records from MEDLINE, EMBASE, and Cochrane Library were retrieved with the initially established search strategy. Irrelevant records and duplicates were removed and 22 full-text articles were obtained for the next eligibility assessment. Furthermore, 18 articles were excluded for the following reasons: one was an open-label study, seven were post hoc analysis studies, and ten were reviews. Ultimately, four RCTs containing 1,930 patients were included in the meta-analysis (Figure 1). The main characteristics of the included studies are listed in Table 1.
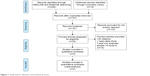  | Figure 1 Study search, selection, and inclusion process. |
Primary outcome analysis
A total of 1,930 patients from four RCTs were available for the analysis of mean change in MADRS total score from baseline to the end of treatment. A significant improvement was seen as early as week 2 (MD −1.40, 95% CI −2.41 to −0.38, P=0.007; Figure 2) and was maintained throughout the therapeutic period in vilazodone-treated patients (week 4: MD −2.30, 95% CI −3.31 to −1.29, P<0.001; week 6: MD −3.38, 95% CI −4.39 to −2.37, P<0.001; week 8: MD −3.73, 95% CI −4.74 to −2.72, P=0.007; Figure 2). Only one trial15 evaluated the efficacy of vilazodone on MADRS total score from baseline to week 10, and this trial also reported sound improvement in vilazodone-treated patients (MD −2.80, 95% CI −4.61 to −0.99, P=0.002; Figure 2). No heterogeneity was shown in any of the three analyses (I2=0%; Figure 2).
  | Figure 2 MADRS change from baseline. |
Secondary outcomes analysis
All 1,930 patients from the four RCTs were enrolled in the analysis of MADRS response, HAM-A, and CGI-S but not CGI-I (three trials with 1,365 patients). MADRS response rates showed a significant improvement in the vilazodone group compared with placebo (RR 1.42, 95% CI 1.28–1.56, P<0.001; Figure 3A). There were significantly greater improvements in HAM-A (MD −1.44, 95% CI −2.08 to −0.80, P<0.001; Figure 3B), CGI-I (MD −0.43, 95% CI −0.59 to −0.27, P<0.001; Figure 3C), and CGI-S (MD −0.42, 95% CI −0.56 to −0.29, P<0.001; Figure 3D) scores from baseline in vilazodone-treated patients compared to placebo patients. Low heterogeneity was shown in all four analyses (I2≤15%, P>0.05).
Rates of discontinuation due to adverse events were significantly higher with vilazodone than placebo (RR 2.11, 95% CI 1.42–3.15, P=0.0002; Figure 4A). The most common adverse events of vilazodone were vomiting, nausea, diarrhea, insomnia, somnolence, dizziness, and dry mouth (P<0.05; Figure 4B). Vilazodone resulted in mild sexual dysfunction in men as measured by the Changes in Sexual Functioning Questionnaire (CSFQ) compared with placebo (MD −1.63, 95% CI −3.11 to −0.16, P=0.03; Figure 4C) but had no such effect in women (MD 0.22, 95% CI −1.18 to 1.62, P=0.76; Figure 4C).
Risk of bias of the included studies
Figure 5 shows the risk of bias of the included studies. Two studies showed unclear risk in allocation concealment due to their allocation scheme not being mentioned in the trials.12,13 Three studies showed unclear risk in the blinding of outcomes assessment.12,14,15 No high or unclear risk of bias was observed in the other items, except these two items.
  | Figure 5 Risk of bias: a summary table for each risk of bias item for each study. |
Discussion
Summary of evidence
The present meta-analysis showed that 40 mg/day of vilazodone had a rapid onset of response and showed good tolerability in short-term treatment (8–10 weeks) for MDD. A significant improvement was observed in MADRS total score as early as week 2, and this was maintained throughout the therapeutic period in vilazodone-treated patients. The MADRS response rate, defined as a ≥50% decrease in MADRS score from baseline to the end of treatment, was apparently higher in vilazodone-treated patients than in placebo patients. Interestingly, HAM-A scores showed a significant improvement, which suggested that vilazodone treatment was associated with reduced anxiety symptoms in patients with MDD. Although rates of discontinuation due to adverse events were significantly higher with vilazodone than with placebo, the most common adverse events were mild to moderate in severity. Importantly, vilazodone showed a mild improvement in sexual function in men as measured by the CSFQ.
Efficacy of vilazodone for MDD
An acceptable definition of clinical improvement in depressive symptoms is a 2-point difference in the MADRS score between an active drug and placebo.18 The results of the present meta-analysis showed a 3.3-point difference in the MADRS score between vilazodone and placebo at week 8. This encouraging efficacy of vilazodone was supported by other crucial indicators, such as the MADRS response, CGI-I, and CGI-S. The vilazodone-treated group had a 16% higher result in MADRS response than control patients, which is associated with longer remission and better outcomes.19 Previous studies reported similar differences between other active drugs and placebo in MADRS response, such as a 15% difference for escitalopram18 and an 11% difference for tricyclic antidepressants (TCAs).20 The significant improvement in CGI-S and CGI-I suggested that vilazodone could broadly reduce global disease severity. Moreover, a statistically significant difference in the MADRS score between vilazodone and placebo was presented as early as week 2 (Figure 2), though the 1.40-point difference did not reach the criterion of clinical improvement due to the lack of effective dosage. Early improvement in the course of treatment is regarded as predictive of later remission and better functional recovery; this has been confirmed by previous studies on SSRIs, serotonin–norepinephrine reuptake inhibitors, TCAs, and others.19,21 All of these results suggested that vilazodone might at least be an effective antidepressant with a rapid onset of efficacy in the treatment of MDD. Interestingly, vilazodone showed a statistically significant improvement in anxiety symptoms in patients with MDD. Depression combined with anxiety is considered to be associated with severe depressive symptoms,22 delayed treatment response,23 and high risk of MDD relapse.24 Previous studies have demonstrated that patients with anxious depression were less responsive to conventional antidepressants than those with non-anxious depression.5 The present meta-analysis showed that vilazodone resulted in a significant improvement in the HAM-A score compared with placebo, indicating its potential value in the management of non-anxious depression. However, it should be noted that the persistent efficacy of vilazodone is difficult to demonstrate due to the short duration of antidepressant clinical trials. Although an open-label trial reported that the efficacy of vilazodone over a long-term 52-week duration was similar to the results of an 8-week treatment,25 further multicentric double-blind RCTs must assess the long-term effect of vilazodone on the treatment of MDD.
Tolerability of vilazodone for MDD
Intolerable adverse events of antidepressants are a common reason for premature discontinuation that results in relapse and recurrence with poor long-term outcomes.4 Previous studies have indicated that antidepressants have similar efficacy and varying tolerability,26 suggesting that adverse events might be a major limitation in the treatment of MDD. The present meta-analysis showed that vilazodone was safe and generally well tolerated. Although the discontinuation rate due to adverse events in vilazodone-treated patients was significantly higher than that in placebo-treated patients, these frequently reported adverse events, including vomiting, nausea, diarrhea, insomnia, somnolence, dizziness, and dry mouth (Figure 3B), were transient in nature and mild to moderate in severity. Additionally, the 7% discontinuation rate in the present vilazodone treatment is superior to the 10% rate with other serotonergic antidepressants and is less than the 20% rate with TCAs.27 Moreover, a 1-year open-label study indicated that 40 mg/day of vilazodone was as safe as the 8-week duration.25 Importantly, vilazodone resulted in a mild improvement in sexual function as measured by the CSFQ in men; this is superior to conventional antidepressants, especially SSRIs. Treatment-induced sexual dysfunction occurred in at least 30%–40% of patients, and the most common symptom was a decrease in libido.3 The exact rate of sexual dysfunction might be higher than reported due to low spontaneous reporting. A previous study indicated that only 14% of patients spontaneously reported sexual problems while taking SSRIs, but 58% reported problems when asked specifically.28 The results of the present meta-analysis showed that vilazodone did not increase the incidence of sexual dysfunction, either in men or in women, compared with placebo. Additionally, a 1-year open-label study showed a similar incidence of sexual dysfunction with 8-week duration trials.25 Due to its high selectivity of the 5-HT1A receptor, vilazodone has no effect or a slight effect on the 5-HT2 receptors involved in regulating sexual behavior.29 Weight gain is also considered to be a leading cause of discontinued treatment.3 However, detecting weight gain in short duration clinical trials is unlikely. A previous long-term open-label study reported a mean 1.7 kg increase after 52 weeks of vilazodone. Additionally, no notable differences were observed in clinical laboratory tests, vital signs, and electrocardiogram (data not shown). Based on the above data, it can be concluded that 40 mg/day of vilazodone has good tolerability in short-term treatment of MDD.
Limitations of this meta-analysis
There were several limitations in the present analysis. First, the present meta-analysis included only four trials with 1,930 patients. Moreover, none of the treatment arms could separate from placebo in the primary outcome of these phase II trials, making it impossible to extract data. Second, the short duration of vilazodone was restrictive in allowing detection of long-term efficacy and tolerability, such as remission rates, weight gain, and sexual dysfunction. Finally, only one trial15 contained an active comparator, which made it impossible to evaluate the difference in effect between vilazodone and the active comparator.
Conclusion
The present meta-analysis indicated that 40 mg/day of vilazodone had a rapid onset of response, good improvement in anxiety symptoms, and good tolerability in short-term treatment (8–10 weeks) of MDD. Further studies should focus on the efficacy and tolerability of vilazodone over a longer duration and should include active comparators.
Acknowledgments
This study was supported by a grant from the Education Department Foundation of Zhejiang Province (Y201432662) and the Medical Science and Technology Project of Zhejiang Province (2015KYB232) to YR Lu.
Disclosure
The authors report no conflicts of interest in this work.
References
Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. | ||
Kulkarni SK, Dhir A. Current investigational drugs for major depression. Expert Opin Investig Drugs. 2009;18(6):767–788. | ||
Ashton AK, Jamerson BD, Weinstein LW, Wagoner C. Antidepressant-related adverse effects impacting treatment compliance: results of a patient survey. Curr Ther Res Clin Exp. 2005;66(2):96–106. | ||
Geddes JR, Carney SM, Davies C, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361(9358):653–661. | ||
Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–351. | ||
Dawson LA, Watson JM. Vilazodone: a 5-HT1A receptor agonist/serotonin transporter inhibitor for the treatment of affective disorders. CNS Neurosci Ther. 2009;15(2):107–117. | ||
Coplan JD, Gopinath S, Abdallah CG, Berry BR. A neurobiological hypothesis of treatment-resistant depression – mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci. 2014;8:189. | ||
Portella MJ, de Diego-Adelino J, Ballesteros J, et al. Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? A randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. J Clin Psychiatry. 2011;72(7):962–969. | ||
Kehne JH, Bartoszyk GD, Greiner HE. In vitro characterization of vilazodone as a dual-acting serotonin reuptake receptor and 5-HT1A receptor partial agonist. Poster presented at: 65th Annual Meeting of the Society of Biological Psychiatry Meeting; 2010. | ||
Rickels K, Athanasiou M, Reed C. Vilazodone, a novel, dual-acting antidepressant: current status, future promise and potential for individualized treatment of depression. Personali Med. 2009;6:216–224. | ||
Thase ME, Chen D, Edwards J, Ruth A. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351–356. | ||
Rickels K, Athanasiou M, Robinson DS, Gibertini M, Whalen H, Reed CR. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(3):326–333. | ||
Khan A, Cutler AJ, Kajdasz DK, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72(4):441–447. | ||
Croft HA, Pomara N, Gommoll C, Chen D, Nunez R, Mathews M. Efficacy and safety of vilazodone in major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2014;75(11):e1291–e1298. | ||
Mathews M, Gommoll C, Chen D, Nunez R, Khan A. Efficacy and safety of vilazodone 20 and 40 mg in major depressive disorder: a randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 2015;30(2):67–74. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. | ||
Shi L, Pu J, Xu L, Malaguit J, Zhang J, Chen S. The efficacy and safety of cilostazol for the secondary prevention of ischemic stroke in acute and chronic phases in Asian population – an updated meta-analysis. BMC Neurol. 2014;14(1):251. | ||
Kennedy SH, Andersen HF, Lam RW. Efficacy of escitalopram in the treatment of major depressive disorder compared with conventional selective serotonin reuptake inhibitors and venlafaxine XR: a meta-analysis. J Psychiatry Neurosci. 2006;31(2):122–131. | ||
Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry. 2009;70(3):344–353. | ||
Storosum JG, Elferink AJ, van Zwieten BJ, van den Brink W, Huyser J. Natural course and placebo response in short-term, placebo-controlled studies in major depression: a meta-analysis of published and non-published studies. Pharmacopsychiatry. 2004;37(1):32–36. | ||
Papakostas GI, Perlis RH, Scalia MJ, Petersen TJ, Fava M. A meta-analysis of early sustained response rates between antidepressants and placebo for the treatment of major depressive disorder. J Clin Psychopharmacol. 2006;26(1):56–60. | ||
Rao S, Zisook S. Anxious depression: clinical features and treatment. Curr Psychiatry Rep. 2009;11(6):429–436. | ||
Clayton PJ, Grove WM, Coryell W, Keller M, Hirschfeld R, Fawcett J. Follow-up and family study of anxious depression. Am J Psychiatry. 1991;148(11):1512–1517. | ||
Flint AJ, Rifat SL. Two-year outcome of elderly patients with anxious depression. Psychiatry Res. 1997;66(1):23–31. | ||
Robinson DS, Kajdasz DK, Gallipoli S, Whalen H, Wamil A, Reed CR. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31(5):643–646. | ||
Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007;9(6):449–459. | ||
Machado M, Iskedjian M, Ruiz I, Einarson TR. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. Curr Med Res Opin. 2006;22(9):1825–1837. | ||
Montejo-Gonzalez AL, Llorca G, Izquierdo JA, et al. SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther. 1997;23(3):176–194. | ||
Alcantara AG. A possible dopaminergic mechanism in the serotonergic antidepressant-induced sexual dysfunctions. J Sex Marital Ther. 1999;25(2):125–129. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



