Back to Journals » OncoTargets and Therapy » Volume 12
Efficacy and tolerability of stereotactic body radiotherapy for lung metastases in three patients with pediatric malignancies
Authors Deck J, Eastwick G, Sima J, Raymond A , Bogart J , Aridgides P
Received 15 November 2018
Accepted for publication 27 March 2019
Published 15 May 2019 Volume 2019:12 Pages 3723—3727
DOI https://doi.org/10.2147/OTT.S194812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Jared Deck,1 Gary Eastwick,1 Jody Sima,2 Amanda Raymond,1 Jeffrey Bogart,1 Paul Aridgides1
1Department of Radiation Oncology, SUNY Upstate Medical University, Syracuse, NY, USA; 2Department of Pediatrics, SUNY Upstate Medical University, Syracuse, NY, USA
Purpose: To report a case series of 3 pediatric patients treated with Stereotactic Body Radiation Therapy (SBRT) for lung metastases.
Patients and methods: Three patients (ages 9, 11, and 21) received SBRT for rhabdoid tumor, Ewing sarcoma, and Wilms tumor histologies, respectively. SBRT doses were 37.5–50 Gy in 3–5 fractions treating twelve lesions.
Results: Three patients (ages 9, 11, and 21) received photon SBRT for pulmonary metastases. The patients were as follows: 1) 21-year-old male with favorable histology Wilms tumor and 1 lesion treated, 2) 11-year-old female with Ewing sarcoma and 1 lesion treated for relapse after previous whole lung radiation (15 Gy), and 3) 9-year-old female with rhabdoid tumor of the left thigh with 10 lesions treated over a two-year period. Median dose delivered was 40 Gy (range, 37.5–50 Gy), delivered in a median of 4 fractions (range, 4–5) of a median of 10 Gy per fraction (range, 9.4–10 Gy). Within a minimum follow-up of 1.9 years (range 1.9–4 years), local control for all 13 treated metastases is 100% without any observed acute toxicities. One possible late toxicity (grade 2 rib fracture) developed 1.3 years following SBRT for treatment of a peripheral lesion (rhabdoid tumor) in an area of disease progression and was managed conservatively. Two patients are surviving 2.9 years (Wilms tumor) and 1.9 years (Ewing sarcoma) after SBRT, and one (rhabdoid tumor) expired 2 years after her final course (4 years after initial SBRT). Two patients (rhabdoid tumor and Ewing sarcoma) suffered disease progression outside of the treated lesions and one patient (Wilms tumor) is without evidence of disease and has not required whole lung irradiation or further systemic therapy.
Conclusion: SBRT appears effective and well tolerated for pediatric lung metastases, however further studies are warranted.
Keywords: SBRT, lung metastases, pediatric oncology, Ewing’s sarcoma, Wilms tumor, rhabdoid tumor
Introduction
Radiotherapy in combination with chemotherapy is an effective modality for treatment of lung metastases in pediatric tumors.1,2 Stereotactic body radiotherapy (SBRT) delivers conformal and ablative treatment in 1–5 fractions, while sparing the volume of heart and lung irradiated. In adults local control >90% with SBRT for pulmonary metastases has been achieved in a prospective study.3 This treatment relies on high doses per fraction, which have been sparingly adopted in pediatric patients (usually to bony sites) due to unknown long-term toxicity.4–6 We presently report our institutional experience in 3 patients who received SBRT for lung metastases in pediatric tumors.
Patients and methods
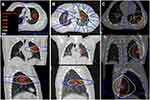
The records of pediatric patients (age ≤21 years) treated with SBRT for lung metastases at our institution between 2012 and 2016 were reviewed. All patients and families were aware and gave written informed consent to publication as a case series. This case series fulfilled institutional criteria for publication without requiring institutional review board approval and necessary documentation was completed. Patients were treated with 4D-CT simulation for respiratory motion assessment, immobilization, selective use of abdominal compression, and image-guided radiation therapy for target verification of each fraction. Treatment planning utilized Eclipse (Varian Medical Systems) with 5–7 mm PTV expansions and was delivered with 6-MV photons on a Vero or Tomotherapy machine. The following critical structures were used for treatment planning: lungs, esophagus, ribs, heart, brachial plexus, spinal cord, proximal trachea and large bronchi. Selected treatment plans are shown in Figure 1. Conformality index ranged from 0.99–1.27. Treatment planning was conducted utilizing established and departmental guidelines for lung SBRT in adult patients. Doses were specified so at least 95% of the PTV received 95–100% of prescribed dose (D95 range 95–100). For intensity modulated radiation therapy (Tomotherapy) doses were homogenous and for 3D conformal radiation therapy (Vero) dose was inhomogenous.
Results
Patients
The patient population is described in Table 1. Patient 1 is a 21-year-old male with Stage IV Wilms tumor who received lung SBRT (50 Gy/5 fractions) to a solitary LUL nodule. His initial treatment consisted of chemotherapy, surgery, and flank RT (10.8 Gy). He declined whole lung radiation, which was reserved in case of pulmonary relapse, and opted for close observation. Patient 2 is an11-year-old female diagnosed with metastatic pelvic Ewing sarcoma and subsequently treated with SBRT (40 Gy/5) for a LUL nodule that was recurrent after previous whole lung radiation (15 Gy), and not amenable to surgical resection due to multiple prior lung metastastectomies. Initial treatment consistent of chemotherapy and hemipelvectomy and SBRT was delivered 2 years following diagnosis. Patient 3 is a 9-year-old female with extrarenal, extracranial rhabdoid tumor of the left thigh treated with SBRT to 8 lesions in bilateral lungs (Course 1, 40 Gy/4), given 0.8 years from initial diagnosis. Her subsequent SBRT treatments for solitary nodules were given 4 months (Course 2, 37.5 Gy/3) and 24 months (Course 3, 40 Gy/4) from initial SBRT.
 | Table 1 Patient and treatment characteristics |
Outcomes
Local control was achieved for all lesions (follow-up 1.9–4 years from SBRT). Disease status following SBRT includes: alive without pulmonary relapse (Wilms tumor), alive with evidence of pulmonary relapse (Ewing sarcoma), and expired with evidence of pulmonary and liver relapse (rhabdoid). SBRT was well-tolerated in all patients with no acute toxicity. There was one case of grade 2 rib fracture (Figure 1C) that was managed conservatively. Rib volume receiving 32 Gy(V32) was 2.1 cm3 with a point dose of 44.6 Gy (111% prescription).
Discussion
SBRT for treatment of pulmonary metastases is an effective treatment for adult patients with limited burden of metastatic disease.7 Caution regarding the use of ablative doses of radiation in children, and therefore a paucity of reported data, have thus far limited its use. Whether the therapeutic ratio of SBRT will hold in the pediatric population is not established, and concerns for toxicity and late effects remain.
Reports of SBRT for pediatric lung metastases are limited to 3 patients with Ewing sarcoma (age 14–19).6,8,9 Herein we described SBRT to younger patients (ages 9 and 11), additional histologies, and feasibility to treat multiple lesions in a single course. Local control was achieved in all treated lesions at 2–4 years, including for Wilms tumor where isolated pulmonary disease was successfully salvaged with SBRT. Importantly, severe acute treatment related toxicity was not observed despite previous treatment including chemotherapy, whole lung radiation, or multiple (10) lesions treated.
The SBRT doses for patients 2 and 3 (37.5–40 Gy in 3–5 fractions) are consistent with the Mayo regimen (median 40 Gy in 5) and regimens for bone metastases on open Children’s Oncology Group Trials ARST1431(NCT02567435) and AEWS1221 (NCT02306161) (35 and 40 Gy in 5). SBRT is not permitted for lung metastases on these studies.6 For patient 1, a regimen comparable to the adult experience was chosen (50 Gy/5) due to his older age and favorable tumor location. Given his favorable histology (Wilms tumor), a lower dose such as 35–40 Gy in 5 fractions would likely also be effective.
There are obvious limitations to our report beginning with the potential impact of selection bias given the small cohort of patients. While local tumor control was achieved with SBRT, an optimal dose regimen is not known and likely differs from adult regimens. Moreover, our experience is too limited to define the risk of severe toxicity with lung SBRT in the pediatric population. The impact of SBRT on overall disease progress is also not clear, as new lung metastases were seen in 2 of 3 patients, albeit both had poor prognoses. The place of SBRT in relation to further systemic therapy and/or whole lung radiotherapy needs to be better defined.
We anticipate that if further retrospective reports and hopefully prospective data confirm favorable local control and toxicity, SBRT will be considered as an alternative to surgical resection in appropriately selected patients. In addition, it merits consideration when the disease is unresectable or resection would entail significant morbidity, such as in the setting of multiple prior surgeries or central tumor location.
Conclusion
Excellent local tumor control was observed without severe toxicity following SBRT for lung metastases in select pediatric malignancies. The role of SBRT remains unclear and further studies are warranted to define the role of SBRT for metastatic lung lesions in the pediatric population.
Summary
We report a case series of 3 patients with pediatric malignancies (ages 9, 11, and 21) treated with Stereotactic Body Radiation Therapy (SBRT) for lung metastases for rhabdoid tumor (1 site), Ewing sarcoma (1 site), and Wilms tumor (10 sites) histologies, respectively. SBRT was well-tolerated without acute/long-term toxicity except for one rib fracture (managed conservatively). Local control was 100% for all treated lesions at 2–4 years, however two patients developed new lung metastases (Ewing sarcoma, rhabdoid tumor).
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The abstract of this paper was presented at the 2018 American Radium Society Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the International Journal of Radiation Oncology Biology. Physics:
Disclosure
The authors report no conflicts of interest in this work.
References
1. Meisel JA, Guthrie KA, Breslow NE, Donaldson SS, Green DM. Significance and management of computed tomography detected pulmonary nodules: a report from the National Wilms tumor study group. Int J Radiat Oncol Biol Phys. 1999;44(3):579–585.
2. Mohan AC, Venkatramani R, Okcu MF, et al. Local therapy to distant metastatic sites in stage IV rhabdomyosarcoma. Pediatr Blood Cancer. 2018;65:2. doi:10.1002/pbc.26859
3. Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27(10):1579–1584. doi:10.1200/JCO.2008.19.6386
4. Farnia B, Louis CU, Teh BS, Paulino AC. Stereotactic body radiation therapy (SBRT) for an isolated bone metastasis in an adolescent male with nasopharyngeal carcinoma. Pediatr Blood Cancer. 2014;61(8):1520. doi:10.1002/pbc.v61.8
5. Taunk NK, Kushner B, Ibanez K, Wolden SL. Short-interval retreatment with Stereotactic body radiotherapy (SBRT) for pediatric neuroblastoma resulting in severe myositis. Pediatr Blood Cancer. 2016;63(4):731–733. doi:10.1002/pbc.25863
6. Brown LC, Lester RA, Grams MP, et al. Stereotactic body radiotherapy for metastatic and recurrent ewing sarcoma and osteosarcoma. Sarcoma. 2014;2014:418270. doi:10.1155/2014/418270
7. Singh D, Chen Y, Hare MZ, et al. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis. 2014;6(4):369–374. doi:10.3978/j.issn.2072-1439.2013.12.03
8. Amsbaugh MJ, Bertke M, Cheerva A, Silverman C, Dunlap N. Stereotactic ablative radiotherapy for a lung metastasis in a child with Ewing‘s sarcoma. J Pediatr Hematol Oncol. 2016;38(6):e199–e201. doi:10.1097/MPH.0000000000000617
9. Siddiqui F, Kunos CA, Paulino AC. Stereotactic body radiation therapy in head and neck, gynecologic, and pediatric malignancies. J Radiat Oncol. 2012;1(1):31–42. doi:10.1007/s13566-012-0009-z
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.