Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Efficacy and Safety Profile of Remimazolam for Sedation in Adults Undergoing Short Surgical Procedures
Authors Morimoto Y
Received 9 November 2021
Accepted for publication 16 January 2022
Published 2 February 2022 Volume 2022:18 Pages 95—100
DOI https://doi.org/10.2147/TCRM.S304556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Yasuhiro Morimoto
Department of Anesthesia, Ube Industries Central Hospital, Ube, Yamaguchi, Japan
Correspondence: Yasuhiro Morimoto
Department of Anesthesia, Ube Industries Central Hospital, Ube, Yamaguchi, Japan
, Tel +81-836-51-9221
, Email [email protected]
Abstract: Sedation for short-term procedures is increasingly being used in clinical practice. Selection of appropriate drugs is important for effective and safe sedation; however, an ideal sedative remains unavailable. Remimazolam is a novel, ultrafast-acting benzodiazepine with a shorter duration of action than other agents in this class. It is currently expected to become a popular agent for short-term procedural sedation. Remimazolam shows higher clearance, a smaller volume of distribution, and a shorter half-life than midazolam. It showed dose-dependent sedative action, with onset of sedation within 60s of administration. The results of clinical trials indicate that remimazolam is more useful than midazolam for short procedural sedation such as in patients who undergo colonoscopy and that its safety profile is comparable with that of midazolam. Anesthesia-induced vascular pain is lesser and reduction in blood pressure is lesser with remimazolam than with propofol. Moreover, the availability of flumazenil (a benzodiazepine antagonist) is a specific advantage of remimazolam. These characteristics and the results of clinical trials suggest that remimazolam will be a safer alternative to previous sedative drugs for sedation during the short surgical procedures. Although short-acting agents are useful, they might lead to immediate hyper-sedation. Remimazolam is a promising agent for short-term procedural sedation; however, clinicians should be mindful of the risks of this agent.
Keywords: sedation, propofol, midazolam, remimazolam
Introduction
Sedation for short-term procedures is increasingly being used in clinical practice. Selection of appropriate drugs is important for effective and safe sedation; however, an ideal sedative remains unavailable.
Remimazolam, a novel, ultra-short-acting intravenous anesthetic belongs to the benzodiazepine class of drugs and was first approved in Japan in 2020 as a general anesthetic.1 It was later approved in the United States in 2021 for sedation during endoscopy and has since been approved in other countries. Owing to its short duration of action and the availability of the antagonist flumazenil, it is currently expected to become a popular agent for short-term procedural sedation. Few clinical trials have discussed this subject, and currently limited data are available regarding remimazolam. In this review, I will summarize the role of remimazolam as a potential agent for short procedural sedation.
Characteristics
Remimazolam is a novel, ultrafast-acting benzodiazepine with a shorter duration of action than other agents in this class. Its pharmacological action is similar to that of midazolam. Remimazolam promotes binding of gamma-aminobutyric acid (an inhibitory neurotransmitter) to its receptors and inhibits cellular excitation in the reticular formation of the midbrain. It differs from midazolam with regard to its metabolic pathway; midazolam is metabolized via cytochrome P450 and remimazolam by hepatic tissue esterases. Additionally, alpha-hydroxymidazolam, a metabolite of midazolam, shows 1/8th of the sedative effect of midazolam, whereas CNS7054, a metabolite of remimazolam, shows 1/400th of its sedative effect.2 This difference in metabolic pathways and pharmacokinetic profiles of its metabolites contributes to the rapid onset of action and systemic clearance of remimazolam. Remimazolam-induced sedation is reversed by flumazenil, a benzodiazepine antagonist. In contrast to other intravenous anesthetics, such as propofol, the availability of an antagonist is an advantage associated with remimazolam administration.
Pharmacokinetic Profile
The pharmacokinetics of remimazolam are previously reported. A study that compared a single intravenous infusion of remimazolam and midazolam reported that remimazolam showed higher clearance, a smaller volume of distribution, and a shorter half-life.3 It showed dose-dependent sedative action, with onset of sedation within 60s of administration. The sedation time of remimazolam was 8 min and 40 min at a dose of 0.075 mg/kg and 0.25 mg/kg, respectively. No respiratory depression or hypotension was observed following remimazolam administration.
Pharmacokinetics of continuous intravenous infusion of remimazolam was also investigated in twenty healthy men volunteers who received the following doses: 5 mg/min for 5 min, 3 mg/min for the next 15 min, and 1 mg/min for further 15 min.4 Pharmacokinetics was best described by a three-compartment model. Remimazolam showed high clearance (1.15 ± 0.12 L/min, mean ± SD), a small steady-state volume of distribution (35.4 ± 4.2 L), and a short terminal phase half-life (70 ± 10 min). The context-sensitive half time (CSHT) calculated using the pharmacokinetic parameters obtained in this study was as short as 6.8 ± 2.4 min. The mean blood pressure decreased by 24%, and the heart rate increased by 28% during the study. Spontaneous breathing was maintained throughout the study. The results of this study suggest that remimazolam was characterized with fast onset and fast recovery with mild hemodynamic side effects.
Results of Clinical Trials
Currently, no clinical study has reported short-term sedation. Therefore, the results of initial clinical trials are introduced.
A phase Ib study was performed in multiple doses of remimazolam in volunteers undergoing colonoscopy. The study also designed to assess reversing sedative effects with flumazenil. Healthy volunteers received intravenously administered 50 μg of fentanyl and remimazolam; the initial dose of remimazolam was divided as 0.04 mg/kg, 0.075 mg/kg, or 0.1 mg/kg.5 An additional dose of 0.04 mg/kg was added at the physician’s discretion during the 30-min evaluation, and >70% of the patients were well sedated and awake within 10 min after administration. Patients who received flumazenil were awake within 1 min and were not re-sedated. No serious adverse events were reported. This study showed that remimazolam is useful for sedation during colonoscopy and that its sedative effect is easily antagonized by flumazenil.
In a phase IIa study, three doses of remimazolam were compared with those of midazolam.6 Patients scheduled to undergo a diagnostic upper gastrointestinal endoscopy were randomized to receive 1 of 3 doses of remimazolam or midazolam, 825 per group. The success rates of the colonoscopy were 32%, 56%, and 64% for the low (0.1 mg/kg), medium (0.15 mg/kg), and high (0.2 mg/kg) doses of remimazolam, respectively, and 44% for midazolam (0.075 mg/kg). The time to onset of sedation was 1.5–2.5 min for remimazolam vs 5 min for midazolam. There were no obvious differences in the safety profiles of remimazolam and midazolam. The study showed that a single administration of remimazolam (0.1–0.2 mg/kg) was capable of inducing rapid sedation with a quick recovery in patients undergoing upper gastrointestinal endoscopy.
A Phase III study compared an initial 5 mg dose of remimazolam followed by an additional 2.5 mg dose of remimazolam with a placebo for outpatient colonoscopy.7 A single midazolam dose of 1.75 mg group was also added for comparison. There were 461 randomized patients. Endoscopy was completed in 91.3%, 1.7%, and 25.2% of patients who received remimazolam, placebo, and midazolam, respectively. Compared with midazolam, remimazolam resulted in more rapid recovery of neuropsychiatric function and earlier discharge from the hospital. The incidence of hypoxia was 1% in both the remimazolam and midazolam groups. The results of these clinical trials indicate that remimazolam is more useful than midazolam for short procedural sedation such as in patients who undergo colonoscopy and that its safety profile is comparable with that of midazolam. These clinical trials showed that remimazolam can be used safely as a sedative during endoscopy.
Advantages of Remimazolam vs Conventional Sedatives
Compared to midazolam, remimazolam has a shorter duration of action and therefore it better regulates sedation levels. However, owing to its short duration of action, administration of additional doses may be required based on the time of the procedure. Continuous intravenous infusion is required for prolonged sedation. Surgeons who are accustomed to midazolam administration should be mindful when using remimazolam. However, the CSHT of midazolam is significantly increased with prolonged administration. Continuous intravenous infusion of remimazolam maintains prolonged sedation and thereby scores over midazolam.
Anesthesia-induced vascular pain is lesser and reduction in blood pressure is lesser with remimazolam than with propofol. The intraoperative hypotension rate was lower in the remimazolam (22%) than in the propofol (49%) group in a clinical trial that investigated patients who received general anesthetics.8 Both propofol and remimazolam show nearly the same CSHT; therefore, arousal time after anesthesia is nearly the same. However, the availability of flumazenil (a benzodiazepine antagonist) is a specific advantage of remimazolam. In view of the aforementioned factors, it is reasonable to conclude that remimazolam scores over propofol.
Zhang et al compared the effects of remimazolam vs propofol administration in patients who underwent hysteroscopy.9 Remimazolam was administered at a dose of 0.2 mg/kg and maintained at a dose of 1 mg/kg/hour. Propofol was administered at 1.5–2 mg/kg and maintained at 3–6 mg/kg/hour. The authors aimed to achieve a modified observer’s assessment of alertness/sedation score (MOAA/S) of ≤2, and remifentanil was subsequently added to the drug regimen. Vascular pain during injection occurred in 80.5% and 2.4% of patients in the propofol and remimazolam groups, respectively. Hypoxia, bradycardia, and hypotension were less frequent in the remimazolam group, which suggests that remimazolam is a safer alternative to propofol for sedation during hysteroscopy.
Effects on Electroencephalography
Electroencephalographic (EEG) monitoring, including the bispectral index (BIS) (Medtronic, Minneapolis, MN, USA) is widely used to control sedation levels in recent times and is important for evaluation of sedation levels for decision-making regarding the use of sedatives administered in clinical practice.
In a study that included volunteers, the authors monitored changes in the blood levels of remimazolam, the MOAA/S score, and BIS and observed that an increase in blood remimazolam concentrations was associated with a decrease in the MOAA/S score and BIS, which suggests that the BIS is a useful indicator to determine the sedative effects of remimazolam.10
EEG changes during remimazolam-induced sedation are reported to be an increase in power in the beta wave region.11 This change can be evaluated using the β-ratio utilized for the calculation of BIS; therefore, the BIS is a useful indicator of the degree of remimazolam-induced sedation.
However, studies have reported that midazolam administration which is expected to cause EEG changes similar to those caused by remimazolam does not result in a reduction in the BIS value to <60 despite a decrease in blood midazolam concentrations.12 Further studies are warranted to determine whether BIS can be used to confirm deeper levels of sedation. The effects of concomitant opioid administration should also be considered in clinical settings. Therefore, further accumulation of clinical data is necessary.
Practical Applications
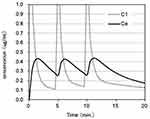
A phase III colonoscopy study in the United States investigated the effectiveness of remimazolam for short-term sedation.7 In this study, an initial dose of remimazolam (5 mg) was followed by an additional dose of 2.5 mg. The dose/body weight was perhaps based on the 0.075 mg/kg dose used in the Phase I study. Therefore, an initial dose of 0.075 mg/kg or 5 mg should be administered and 50% of the dose should be added if necessary. Figure 1 shows the simulation of the concentration after the administration of an initial dose of 0.075 mg/kg followed by an additional dose of 0.0325 mg/kg at 5 and 10 min. Simulation of intravenous anesthetics involves the blood concentration and effect-site concentration; the concentration in the central nervous system or the effect-site concentration correlates with the degree of drug-induced sedation. Pharmacokinetic simulation of remimazolam was performed using Microsoft Excel for Mac by the pharmacokinetic parameters by initial clinical trial13 (Appendix).
A rapid decrease in the concentration is observed after a single dose of remimazolam; therefore, additional doses need to be administered every 5 min. Reportedly, the concentration of remimazolam required to reduce the MOAA/S to <1 is 0.7 μg/mL,4 which is slightly lower than the maximum concentration of 0.45 μg/mL. This protocol may be sufficient for sedation during short procedures, such as colonoscopy and upper gastrointestinal endoscopy. Analgesics such as fentanyl may be combined with the anesthetic, if needed.
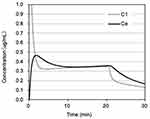
Continuous intravenous infusion should be used for prolonged sedation. Zhang et al administered 0.2 mg/kg of remimazolam for uterine evaluation, followed by a maintenance dose of 1 mg/kg/hour, which is equivalent to the dose used during general anesthesia.9 It is reasonable to conclude that a lower dose can be used for sedation. Figure 2 shows the change in concentration following administration of 0.075 mg/kg as a single dose, followed by continuous intravenous infusion at the rate of 0.5 mg/kg/hour (50% of the dose used by Zhang et al). Nearly the same concentration was maintained after a single dose. An additional dose of 0.0325 mg/kg may be useful in patients in whom sedation appears to be insufficient.
With regard to continuous intravenous infusion, it is important to remember that the CSHT of remimazolam is approximately 7 min. Additionally, the CSHT for the effect-site concentrations is 10 min.4 If the maintenance concentration is within twice the waking concentration, the patient will be aroused 10 min after completion of drug administration.
Other anesthetics, such as propofol and remifentanil use target-controlled infusion (TCI), in which a pump controls the dose to maintain the desired blood concentration using pharmacokinetic parameters. TCI may likely be used for remimazolam in the near future.
Complications
A case of remimazolam-induced anaphylactic shock was reported.14 In the case, the patient may have been sensitized by previously administered midazolam. Remimazolam should not be used in patients with suspected allergy to midazolam.
The administration of flumazenil (a benzodiazepine antagonist) should be considered in patients with inadequate or delayed arousal. However, a re-sedation case in a patient who was aroused following flumazenil administration was reported.15 Re-sedation is likely to occur following the administration of a large volume of remimazolam during general anesthesia. However, even brief sedation can result in re-sedation. The patient should be monitored for some time after arousal by flumazenil. Further studies are warranted to confirm the optimal timing and dosage of flumazenil.
I have used remimazolam in my patients for approximately a year and have observed that its effect significantly varies between individuals. Elderly patients seem more vulnerable to the effects of remimazolam; therefore, this drug should be used cautiously in this patient population. The patient’s consciousness level and respiratory status require close monitoring, and prompt respiratory support should be available if needed. Moreover, immediate administration of flumazenil should be performed to antagonize the effects of remimazolam in cases of a suspected overdose.
Although short-acting agents are useful, they might lead to immediate hyper-sedation, which may precipitate respiratory arrest and hypotension. Remimazolam is a promising agent for short-term procedural sedation; however, clinicians should be mindful of the risks of this agent and be familiar with the remedial measures in cases of a suspected overdose.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Masui K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J Anesth. 2020;34:479–482. doi:10.1007/s00540-020-02755-1
2. Kilpatrik GJ, Mclntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107:60–66. doi:10.1097/01.anes.0000267503.85085.c0
3. Antonik LJ, Goldwater DR, Kilpatrick GJ, et al. A placebo-and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam 8CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274–283. doi:10.1213/ANE.0b013e31823f0c28
4. Schuttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi:10.1097/ALN.0000000000003103
5. Worthington MT, Antonik LJ, Goldwater DR, et al. A phase Ib. dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117:1093–1100. doi:10.1213/ANE.0b013e3182a705ae
6. Borkett KM, Riff DS, Schwartz HI, et al. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120:771–780. doi:10.1213/ANE.0000000000000548
7. Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427–437. doi:10.1016/j.gie.2018.04.2351
8. Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:545–553.
9. Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. 2021;21:156. doi:10.1186/s12871-021-01373-y
10. Eisenried A, Schuttler J, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II. Pharmacokinetics of electroencephalogram effects. Anesthesiology. 2020;132(4):652–666. doi:10.1097/ALN.0000000000003102
11. Upton R, Martinez A, Grant C. A dose escalation study in sheep of the effects of the benzodiazepine and the respiratory and cardiovascular systems. Br J Pharmacol. 2008;155:52–61. doi:10.1038/bjp.2008.228
12. Miyake W, Oda Y, Ikeda Y, et al. Electroencephalographic response following midazolam-induced general anesthesia: relationship to plasma and effect-site midazolam concentrations. J Anesth. 2010;24(3):386–393. doi:10.1007/s00540-010-0907-4
13. Doi M. Remimazolam. J Jpn Soc Clin Anesth. 2014;34(7):860–866. Article in Japanese. doi:10.2199/jjsca.34.860
14. Tsurumi K, Takahashi S, Hiramoto Y, et al. Remimazolam anaphylaxis during anesthesia induction. J Anesth. 2021;35:571–575. doi:10.1007/s00540-021-02934-8
15. Yamamoto T, Kurabe M, Kamiya Y. Re-sleeping after reversal of remimazolam by flumazenil. J Anesth. 2021;35:322. doi:10.1007/s00540-021-02915-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.