Back to Journals » Clinical Ophthalmology » Volume 14
Efficacy and Safety of VisuEvo® and Cationorm® for the Treatment of Evaporative and Non-Evaporative Dry Eye Disease: A Multicenter, Double-Blind, Cross-Over, Randomized Clinical Trial
Authors Fogagnolo P , Quisisana C , Caretti A , Marchina D, Dei Cas M, Melardi E, Rossetti L
Received 18 April 2020
Accepted for publication 3 June 2020
Published 18 June 2020 Volume 2020:14 Pages 1651—1663
DOI https://doi.org/10.2147/OPTH.S258081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Paolo Fogagnolo,1 Chiara Quisisana,1 Anna Caretti,2 Daniele Marchina,1 Michele Dei Cas,2 Ettore Melardi,1 Luca Rossetti1
1Eye Clinic ASST Santi Paolo Carlo, Department of Health Sciences, San Paolo Hospital, University of Milan, Milan, Italy; 2Department of Health Sciences, Laboratory of Biochemistry, University of Milan, Milan, Italy
Correspondence: Paolo Fogagnolo
Eye Clinic ASST Santi Paolo Carlo, Department of Health Sciences, San Paolo Hospital, University of Milan, Via Di Rudinì 8, Milan 20142, Italy
Tel +39 02 81844301
Email [email protected]
Purpose: To compare the efficacy of the new lubricating product VisuEvo® (VSE) vs Cationorm® (CTN) in patients with dry eye disease (DED).
Methods: Seventy-two patients with evaporative (n=54) and non-evaporative DED (n=18) were included in a multicenter, double-blind, 12-week cross-over study to receive VSE (6 weeks) and CTN (6 weeks) in randomized sequence. After baseline, two visits were performed during each period (intermediate and final visit, respectively at 2 and 6 weeks from the beginning of each period). Primary (tear break-up time, TBUT) and secondary endpoints (Schirmer I, Ferning, blink rate, osmometry, cytokine and lipid expression, ocular surface staining, patient satisfaction, and OSDI score) were compared.
Results: Sixty-three patients were evaluated for efficacy and 68 patients for safety. The intergroup differences for mean TBUT values were not significant at any study visit (baseline 3.2 ± 1.5 sec; intermediate visits 4.5 ± 1.9 and 4.5 ± 1.8 sec in VSE and CTN groups, respectively, p = 0.10; final visits 5.4 ± 2.4 and 6.0 ± 3.1, respectively, p=0.63). Also, the assessment of secondary endpoints showed no significant difference between the two groups. The two study treatments were equally effective in evaporative and non-evaporative DED. The safety profile was excellent for both ocular treatments; transient blurred vision was observed in 11 patients only during CTN, 10 patients only during VSE, and 16 during both treatments.
Conclusion: VSE was non-inferior to CTN in restoring tear film composition, increasing its stability and reducing ocular surface damage in evaporative and non-evaporative DED patients.
Study Identifier: NCT03833882.
Keywords: evaporative dry eye disease, tear break-up time, Ocular Surface Disease Index questionnaire, meibomian gland disturbance, glaucoma, ocular surface
Introduction
Dry eye disease (DED) is
a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.1
Prevalence of DED ranges from approximately 5% to 50%;2,3 incidence in a Caucasian population aged 48–91 years is about 2%, being more common in women (25%) than in men (17.3%).2
The most relevant risk factors for DED are increasing age, female sex and Asian ethnicity.2 The endocrine system plays a significant role in the regulation of the ocular surface and adnexa. The decrease in serum androgen levels that occurs during menopause, pregnancy, lactation, or the use of estrogen-containing oral contraceptives may trigger tear deficiency. On the other hand, the breakdown of hormonal balance and induction of an androgen-deficient condition is associated with obstructive Meibomian gland disturbance (MGD).4 Obstructive MGD is the most common cause of evaporative dry eye (EDE) and it is believed that MGD-dependent EDE is the most common form of DED overall.5
Other pathogenetic factors are abnormal immune response,6,7 and ocular surface toxicity induced by the chronic use of medications and preservatives.1 In glaucoma, the chronic use of topical medications is frequently associated with impairment of the corneal glycocalyx and of the mucins produced by conjunctival goblet cells,8 which in turn can determine non-evaporative dry eye (NEDE).
Tear supplementation is considered a mainstay of DED therapy, in order to increase or stabilize the natural tear film. Two main categories of lubricating molecules are available: those increasing tear volume (polymers such as hyaluronic acid or cellulose derivates), and those improving tear stability (lipids). Despite eye drops targeted to aqueous supplementation are among the most commonly used, lipid-containing eye drops are growing in both availability and popularity, primarily due to the increased attention paid to hyper-evaporation in the pathogenesis of DED.9
Cationorm® (CTN)(Santen Ltd, Japan) is a clinically well-established lipid treatment for EDE. It is a preservative-free cationic nano-emulsion containing mineral oils, surfactants (cetalkonium chloride, tyloxapol, poloxamer), and glycerin. VisuEvo® (VSE)(Visufarma SpA, Italy) is a new multidose, non-preserved ophthalmic solution with antioxidant activity that uses a liposomal nano-dispersion associated with vegetable oil rich in Omega 3 (docosahexaenoic acid – DHA and eicosapentaenoic acid – EPA), Vitamin D3 and Vitamin A palmitate. Due to their compositions, both treatments are expected to be effective in EDE but also in non-Sjogren NEDE patients. The lipid structures are capable of effectively stabilizing impaired lipid layer (as already shown for CTN).10 The presence of cetalkonium chloride in CTN would modulate inflammatory response,11 as well as the presence of Omega 3 (DHA and EPA) and Vitamin D in VSE is able to modulate the immune and inflammatory responses in DED by means of resolving expression.12,13 VSE also contains Vitamin A which may support goblet cells activity and epithelial integrity.14
In literature, there are numerous clinical trials on lubricating eye drops but, in most cases, they are cohort studies or uncontrolled studies; there is relative lack of prospective randomized head-to-head studies. The present study aimed at comparing the clinical performances of two lipid eye drops (VSE and CTN) by means of a prospective randomized study performed on both EDE and NEDE patients.
Materials and Methods
Patients and Study Design
This was a pre-market, multicenter, double-blind, randomized, prospective cross-over study. It was conducted at the Eye Clinic, ASST Santi Paolo Carlo, San Paolo Hospital, University of Milan, Milan, Italy and at the Department of Biomedical and Clinical Sciences, Fatebenefratelli-Sacco Hospital, Milan, Italy. It was registered at www.clinicaltrial.gov (identifier: NCT03833882) and approved by Comitato Etico Milano Area 1; it followed the Tenets of the Declaration of Helsinki; informed consent was obtained from participants.
72 consecutive patients with DED fulfilling the inclusion and exclusion criteria listed in Box 1 were included. Two groups of patients were studied: EDE (n = 54) and NEDE (n = 18). EDE patients had MGD or abnormal hormonal state or high tear film instability; NEDE were patients treated with BAK-preserved anti-glaucoma eyedrops for at least 2 years, showing mucous layer instability (Box 1).
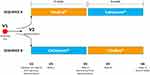
The flow of the study is described in Figure 1. The study lasted 12 weeks for each patient (two 6-week treatment cycles) and included six visits: Screening visit (V1,1 week prior to baseline), baseline visit (V2, randomization, dispensation of the first treatment to be started the following day), week 2 (V3, intermediate visit of the first cycle), week 6 (V4, final visit of first cycle, dispensation of the second treatment to be started the following day), week 8 (V5, intermediate visit of the second cycle), and week 12 (V6, final visit of second cycle and end of study visit). At screening visit patients stopped any eventual lubricating treatment; at baseline untreated DED parameters were measured and patients were then randomized (centralized randomization by means of a list of random numbers) to receive either VSE or CTN with a 1:1 ratio for the following 6 weeks. After this time lapse, patients were switched to the opposite therapy for 6 additional weeks without wash-out period. From the day after baseline to the end of the study, patients self-administered one drop of the study devices into conjunctival sac three times daily.
Study Procedures
At each visit, patients underwent the following tests in the following order: Ocular Surface Disease Index questionnaire (OSDI, Copyright 1995, Allergan Inc, Irvine, California, US),16 measurement of blinking, osmometry, TBUT, fluorescein staining, lissamine staining, Schirmer I test, tear Ferning test, tear sampling and cytokine and lipid expression. Measurement of blinking, osmometry, tear sampling, Ferning test was performed on subgroups of patients. Test procedures are described in detail on Box 2.
Outcome Assessments
The primary objective was the comparison of both ophthalmic treatments in improving tear film stability (TBUT increase in seconds). The secondary outcomes were improvements of symptoms (OSDI score), and the other ocular signs (Box 2). The safety profile of both medical devices was assessed by monitoring the occurrence of adverse events (AEs) during the study.
Statistical Analysis
Both eyes of each patient, if eligible, were tested and treated. The analyses were then performed only on the worse eye per patient based on TBUT. In case of identical TBUT value the right eye was used.
Sample Size
Sample size estimate was calculated setting the error to 5%, the worth-detecting difference was 1 second, delta of −0.5, standard deviation of 2.3 seconds, with a power of 80%. 64 patients were necessary. Taking into account a 10% drop-out rate, 72 patients were enrolled.
Efficacy Analysis
This analysis was performed using intent-to-treat set. Cross-over data were analyzed by summing up the data of patients treated with the same eye drop during the first and the second 6-week period. A mixed-model analysis of variance was used to compare treatment groups and changes from baseline to each study visit, including the following effects: patients as random effect, drug, visits, drug by visit interaction, treatment sequence and baseline value as fixed effects. This model corrected the estimates for baseline value. The presence of a potential carry-over effect was evaluated using a likelihood ratio test between two mixed models including carryover effect or not. Study groups were also compared at each visit by means of t-test for paired data after Kolmogorov–Smirnov test was used to check data normality.
To test non-inferiority of VSE vs CTN, confidence intervals (CI) were estimated from mixed model and lower boundary of the CI was compared to the non-inferiority margin (equal to −0.5) in order to test the non-inferiority. The null hypothesis was TBUT difference between two treatments of 0.5 sec or less. If it was rejected, non-inferiority of the study treatment was accepted.
Results
Overall, 72 subjects were enrolled in the study; age was 65±17 years; 73% were female. In EDE patients age was 63 ± 17 years; 82% were females. In NEDE patients age was 71 ± 12 years; 43% were females. Nine patients were excluded from efficacy analysis (n=63); four patients were excluded from safety analysis (n=68) because they did not self-administer the eye drop correctly.
Efficacy Data, Whole Population
TBUT increased from baseline to intermediate visits by +1.3 seconds in both treatment groups; afterwards, a further increase was shown: +0.9 for VSE, and +1.5 seconds for CTN (p < 0.001 vs baseline, Table 1). Differences between the two treatment groups were not significant at any visit (p>0.10). The estimated effect of treatment products was −0.57 (CI 95% −1.19 to 0.05), and non-inferiority of VSE over CTN was achieved. The similar effect of the two products is also confirmed by Blandt–Altmann plot between baseline and final visits (Figure 2).
 |
Table 1 Results of Primary and Secondary Endpoints on the Whole Study Population (N=63) |
 |
Figure 2 Blandt–Altmann plot for comparison of baseline and final TBUT values between study treatments. |
OSDI scores significantly decreased from baseline (37 ± 12) to the following visits (23 ± 15 in both groups at intermediate visit, and 19 ± 13 in VSE group and 19 ± 14 in CTN group at final visits; p < 0.001 for both treatments compared with baseline, Table 1, Figure 3), with no inter-treatment differences at any visit (p > 0.43).
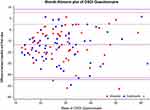 |
Figure 3 Blandt–Altmann plot for comparison of baseline and final OSDI scores between study treatments. |
Osmometry was 314 ± 23 mOsm/L at baseline and 305 ± 16 at intermediate visits in both groups. At final visits, it was 305 ±15 in VSE groups and 307 ± 20 in CTN group (p < 0.05 compared with baseline for all visits and both treatments). No significant differences between treatments were shown (p > 0.46).
Blinking analysis was performed excluding 4 outliers due to side effects (data not shown). The trend of blinks during the study was similar in CTN and VSE: the number of complete blinks was overall stable, whereas a significant, progressive reduction of incomplete blinks was shown throughout the study (p < 0.001); this phenomenon also caused a significant reduction of the number of total blinks (p < 0.001; Table 1).
In both treatment groups, fluorescein staining showed an initial improvement by 15% between baseline and the intermediate visit, then remaining constant until the end of the study (Figure 4), with negligible inter-treatment differences (p > 0.60).
 |
Figure 4 Changes of proportion (%) of patients with staining grade with fluorescein during the study visits with both ophthalmic solutions (VisuEvo® and Cationorm®). |
The expression of cytokine IL-6, IL-8, and IFN and lipids is reported in Table 2. All these parameters reduced during the study; the difference was statistically significant for IFN, sphingosine and sphingosine 1 phosphate both in the whole population (0.06 < p < 0.0014); no inter-treatment differences were shown.
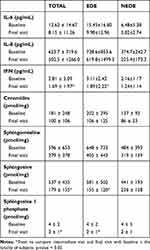 |
Table 2 Tear Expression of Cytokines and Lipids |
Efficacy Data, EDE and NEDE Patients
The main findings for these patients are shown in Table 3. The significant improvements of TBUT shown in the global data with both treatments were confirmed in both EDE and NEDE patients, even though NEDE had worse TBUT at baseline and a minor response throughout the study. The effects of treatments on OSDI improvements were similar for EDE and NEDE patients. Of note, NEDE were less symptomatic at baseline (39 ± 13 EDE vs 32 ± 8 NEDE). Relevant differences were shown at osmometry. EDE had similar findings with both treatments (p=0.26), whereas VSE showed better performances than CTN in NEDE patients (299 ± 18 vs 318 ± 26 mOsm/L) although this difference was not statistically significant (p = 0.10).
 |
Table 3 Results of Primary and Secondary Endpoints for Evaporative (EDE) and Non-Evaporative Dry Eye (NEDE) Groups |
Blinking patterns were also very different in the two groups. At baseline, EDE patients had a slightly higher number of total blinks (23 ± 12 EDE, 20 ± 13 NEDE), a significantly higher number of complete blinks (16 ± 13 EDE, 9 ± 5 NEDE, p = 0.02), and a significantly lower number of incomplete blinks (7 ± 6 EDE, 11 ± 9 NEDE, p = 0.04). At the end of the study, the scenario between EDE and NEDE was completely changed. In EDE patients, no statistically significant changes were found: total blinks had slightly increased to 25 ± 17 and complete blinks to 22 ± 18; incomplete blinks halved to 3 ± 4. In NEDE patients, total blinks nearly halved (11 ± 6, p < 0.001), complete blinks remained stable, and an important decrease of incomplete blinks was shown (3 ± 4, p < 0.001).
Relevant differences in the two groups were also shown at fluorescein staining. EDE patients had no staining in 58% of cases at baseline, and this percentage progressively increased at 66% at final visits. On the other hand, NEDE patients had no staining in just 35% of cases at baseline. After a temporary improvement at intermediate visits, this percentage returned stable at final visits. The effects of the two treatments were similar in the two DED populations.
On EDE patients, biochemical analysis reflected the data on the whole population, with a statistically significant reduction of IFN, sphingosine and sphingosine 1 phosphate in the course of the study. No differences were shown for NEDE patients.
Ferning test was assessed in 17 NEDE patients who had type 3–4 at screening visit. The two treatments were effective in progressively improving the proportion of normal (type 1–2) Ferning tests: at intermediate visit, they were 21% in both groups; at the final visit, 57% with VSE and 50% with CTN (p = 0.70, Fisher exact test) (Figure 5).
 |
Figure 5 Distribution of proportion (%) of patients during study visits, according to Ferning test grading scale, between the two treatment groups. |
Safety Data
The two treatments had high safety profiles, with mild or unrelated to treatment AEs. VSE caused less blurred vision than CTN, both for number of days with blurring (median of 6 days for CTN and 3 days for VSE, p = 0.37) and the minutes with blurred vision after instillation (13 vs 11 min, respectively, p = 0.96).
Discussion
This randomized prospective study compared the efficacy of a new lipid eye drop (VSE) with one of the most commonly prescribed lipid eye drops (CTN) and it showed that the two treatments are equally effective in reducing signs and symptoms on a population with DED.
VSE was as effective as CTN in providing a significant and progressive TBUT amelioration throughout the study, reaching +2.5 sec. This beneficial effect was confirmed by a reduction of symptoms (mean OSDI was 39 at baseline, ie severe symptoms, and 19 at the end of the study, ie mild symptoms). A 15% amelioration of ocular surface staining was also found with both treatments. The agreement of TBUT, OSDI and ocular surface staining has been shown in other studies,21,22 and it is probably, together with ease of use, the main reason of the diffusion of these tests. Schirmer test did not change over the course of the study, and this is an expected finding in a group of non-Sjogren patients with normal function of the main lacrimal gland.
Also “second-level” tests showed consistent results. Osmometry showed a progressive, similar reduction in the two populations, from a mean of 314 mOsm/L at baseline (corresponding to mild DED) to 305–307 mOsm/L at the end of the study (corresponding to normality according to Sullivan et al4). Another sign of enhanced tear film stability was the progressive reduction of incomplete blinks found with both treatments.23,24 Also, proinflammatory cytokine and lipids reduced in the course of study in both treatment arms.
The effects of the two drops used in this study are close to those observed for CTN using TBUT on mild to moderate DED,10 and OSDI and ocular surface staining on moderate to severe DED.25 The limited number of clinical studies comparing polymers and lipids suggests a superiority of lipids,10,25–27 particularly on evaporative DED.24 In the absence of a gold standard for DED treatment, our data may have been modified using a different comparator, as well as a population with different types and severity of DED.
The analysis performed on EDE and NEDE patients clearly shows how DED may be a very different condition between different types of patients. Yet, lipids were highly effective in both subgroups. At baseline NEDE patients had more severe TBUT and ocular surface staining than EDE, though symptoms at OSDI were lower. During the study, NEDE patients had a lower TBUT increase than EDE; nevertheless, the clinical amelioration was sufficient to reduce corneal staining by about 10% and increase the mucous characteristics of tear (Ferning test), with VSE patients performing slightly better than CTN (57% vs 50%). NEDE also showed peculiar blinking patterns: during study period, the number of complete blinks remained stable (though largely less than EDE: 9 ± 5 vs 16 ± 13 at baseline, 8 ± 5 vs 22 ± 18 at the end of the study), whereas incomplete blink rate reduces from 11 ± 9 at baseline to 3 ± 4 at final visits; as a consequence total blinks nearly halved in the course of the study (20 ± 13 vs 11 ± 6). Also, in these subgroups of patients VSE proved at least non-inferior to CTN, with better final osmometry in NEDE patients (299 ± 18 vs 318 ± 26 mOsm/L, p = 0.10).
Lipids have been introduced in clinical practice as ideal treatments in EDE, but in this paper, we showed a clinically usefulness also on glaucoma patients. This could be due to several factors: the advantage of tear film stabilization also in NEDE, the anti-inflammatory activity11,12 of lipids and, for VSE, the role by Vitamin D14 and the trophic effect on tear mucous layer13 by vitamin A. In NEDE patients, VSE showed better Ferning tests and osmometry than CTN. This may be due to a more favorable composition of VSE over CTN on patients with associated mucous deficits, a fact which require further investigation. Also, future studies may explore the efficacy of VSE and, more generally of lipids, on patients with combined DED (hyposecretive and hyperevaporative).
The strength of the study is randomization and crossover design, which allowed all patients to receive both treatments and therefore provided a true head-to-head comparison. Yet it also has some limitations, the most important being the drop-out rate which may limit our results; luckily the small inferiority to the desired number apparently did not affect study power, which – using a post hoc analysis – was 0.93. When present, carry-over effect in this study likely reflects the progressive amelioration of the ocular surface thanks to chronic treatments rather than a study bias. Finally, the results on secondary results and NEDE group should be considered with caution because of the low number of patients.
Conclusion
In conclusion, we were able to prove that the new ophthalmic solution (VSE) was as effective and safe as the reference cationic emulsion (CTN) in restoring proper tear film composition, increasing tear film stability and reducing ocular surface damage and symptoms in patients with different subclasses of DED.
 |
Box 1 Inclusion Criteria and Exclusion Criteria |
 |
Box 2 Diagnostic Procedures of the Study |
Data Sharing Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on request.
Acknowledgments
1MED, a Contract Research Organization (CRO), contributed to data monitoring, acquisition, and statistical analysis.
Disclosure
Paolo Fogagnolo and Luca Rossetti received honoraria for medical meetings and advisory board from Visufarma S.p.A, Rome, Italy. The authors report no other conflicts of interest in this work.
References
1. Craig JP, Nichols KK, Akpek E, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276e83.
2. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. doi:10.1016/j.jtos.2017.05.003.
3. No authors listed. The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye workShop. Ocul Surf. 2007;5(2):93–107. doi:10.1016/S1542-0124(12)70082-4.
4. Sullivan DA, Rocha EM, Aragona P, et al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15(3):284–333. doi:10.1016/j.jtos.2017.04.001.
5. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;5(3):438–510. doi:10.1016/j.jtos.2017.05.011.
6. Pflugfelder SC, da Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11S):S4–S13. doi:10.1016/j.ophtha.2017.07.010.
7. Semba CP, Gadek TR. Development of lifitegrast: a novel T-cell inhibitor for the treatment of dry eye disease. Clin Ophthalmol. 2016; 10:1083–1094. doi:10.2147/OPTH.S110557.
8. Kahook MY, Noecker R. Quantitative analysis of conjunctival goblet cells after chronic application of topical drops. Adv Ther. 2008;25(8):743–751. doi:10.1007/s12325-008-0078-y.
9. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006.
10. Amrane M, Creuzot-Garcher C, Robert PY, et al. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease - a randomised comparative study. J Fr Ophtalmol. 2014;37(8):589–598. doi:10.1016/j.jfo.2014.05.001.
11. Filion MC, Phillips NC. Anti-inflammatory activity of cationic lipids. Br J Pharmacol. 1997;122(3):551–557. doi:10.1038/sj.bjp.0701396.
12. Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–S215. doi:10.1038/sj.bjp.0707489.
13. Zhang J, Dai Y, Wu D, Xu J. Calcitriol, the active metabolite of vitamin D3, inhibits dry eye related corneal inflammation in vivo and in vitro. Ocul Immunol Inflamm. 2019;27(2):257–265. doi:10.1080/09273948.2017.1372486
14. Hatchell DL, Sommer A. Detection of ocular surface abnormalities in experimental vitamin A deficiency. Arch Ophthalmol. 1984;102(9):1389–1393. doi:10.1001/archopht.1984.01040031131040.
15. Rolando M. Tear mucus ferning test in normal and keratoconjunctivitis sicca eyes. Chibret Int J Ophthalmol. 1984;2:32–41.
16. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615.
17. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–574. doi:10.1016/j.jtos.2017.05.001.
18. Nolfi J, Caffery B. Randomized comparison of in vivo performance of two point-of-care tear film osmometers. Clin Ophthalmol. 2017;11:945–950. doi:10.2147/OPTH.S135068.
19. Masmali AM, Purslow C, Murphy PJ. The tear ferning test: a simple clinical technique to evaluate the ocular tear film. Clin Exp Optom. 2014;97(5):399–406. doi:10.1111/cxo.12160.
20. Fong PY, Shih KC, Lam PY, Chan TCY, Jhanji V, Tong L. Role of tear film biomarkers in the diagnosis and management of dry eye disease. Taiwan J Ophthalmol. 2019;9(3):150–159. doi:10.4103/tjo.tjo_56_19.
21. Lam SM, Tong L, Yong SS, et al. Meibum lipid composition in Asians with dry eye disease. PLoS One. 2011;6(10):e24339. doi:10.1371/journal.pone.0024339.
22. Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S, Wedrich A. Incidence and pattern of dry eye after cataract surgery. PLoS One. 2013;8(11):e78657. doi:10.1371/journal.pone.0078657.
23. Goto E, Shimazaki J, Monden Y, et al. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology. 2002;109(11):2030Y5. doi:10.1016/S0161-6420(02)01262-9.
24. McCann LC, Tomlinson A, Pearce EI, Papa V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea. 2012;31(1):1–5. doi:10.1097/ICO.0b013e31821b71e6.
25. Robert PY, Cochener B, Amrane M, et al. Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol. 2016;26(6):546–555. doi:10.5301/ejo.5000830.
26. Khanal S, Tomlinson A, Pearce EI, Simmons PA. Effect of an oil- in-water emulsion on the tear physiology of patients with mild to moderate dry eye. Cornea. 2007;26(2):175Y81. doi:10.1097/ICO.0b013e31802b492d.
27. Wang TJ, Wang IJ, Ho JD, Chou HC, Lin SY, Huang MC. Comparison of the clinical effects of carbomer-based lipid- containing gel and hydroxypropyl-guar gel artificial tear formulations in patients with dry eye syndrome: a 4-week, prospective, open-label, randomized, parallel-group, noninferiority study. Clin Ther. 2010;32(1):44Y52. doi:10.1016/j.clinthera.2010.01.024.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.