Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Efficacy and Safety of Tranexamic Acid in Cancer Surgery. An Update of Clinical Findings and Ongoing Research
Authors Zec T, Di Napoli R , Fievez L, Ben Aziz M, Ottaiano A, Vittori A , Perri F , Cascella M
Received 1 September 2021
Accepted for publication 22 October 2021
Published 5 July 2022 Volume 2022:15 Pages 1427—1444
DOI https://doi.org/10.2147/JMDH.S337250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tamara Zec,1 Raffaela Di Napoli,1 Lydwine Fievez,1 Mohamed Ben Aziz,1 Alessandro Ottaiano,2 Alessandro Vittori,3 Francesco Perri,4 Marco Cascella5
1Department of Anesthesiology, Institut Jules Bordet, Université Libre de Bruxelles, Bruxelles, 1000, Belgium; 2SSD Innovative Therapies for Abdominal Metastases, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Naples, 80100, Italy; 3Department of Anesthesia and Critical Care, ARCO ROMA, Ospedale Pediatrico Bambino Gesù IRCCS, Piazza S. Onofrio 4, Rome, 00165, Italy; 4Medical and Experimental Head and Neck Oncology Unit, Istituto Nazionale Tumori IRCCS Fondazione G. Pascale, Naples, 80100, Italy; 5Division of Anesthesia and Pain Medicine, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Naples, 80100, Italy
Correspondence: Francesco Perri, Email [email protected]
Abstract: In cancer patients undergoing surgery, tumor biology and anticancer treatments can increase the risk of perioperative bleeding and blood transfusions. Notably, blood transfusions can be potentially associated with an increased risk of life-threatening immune responses, acute lung injury, postoperative infections, and thromboembolism. Moreover, the link between perioperative transfusion and increased risk of cancer recurrence cannot be excluded. On the other hand, cancer patients have an increased risk of thromboembolism due to cancer itself and antineoplastic systemic treatments including chemotherapy and anti-angiogenic drugs. In this complex scenario, effective and safe strategies aimed at the prevention of blood transfusions are warranted. This narrative review addresses the efficacy, and the safety of the synthetic antifibrinolytic agent tranexamic acid (TXA) when used perioperatively in cancer surgery. Although in not oncologic surgery the use of TXA has been extensively studied, in the setting of cancer patients requiring surgery, the evidence is scarce. An overview of the ongoing clinical research is also provided.
Keywords: tranexamic acid, cancer surgery, oncology, anemia, blood transfusion, anti-fibrinolytic
Introduction
In cancer patients, tumor biology features such as tumor angiogenesis, and local tumor invasion, and anticancer treatments included chemotherapy or radiotherapy can increase the risk of perioperative bleeding. In this population, this risk further increases due to the chronic use of medications such as antiangiogenic agents (eg, bevacizumab), nonsteroidal anti-inflammatory drugs (NSAIDs), and anticoagulants and antiplatelet drugs.1 Moreover, during surgery, the extension of the resection area and its vascularization, as well as perioperative conditions such as hemodilution, hypothermia, and metabolic alterations are recognized risk factors for bleeding and coagulopathy.2 Remarkably, changes in the fibrinolytic system usually occur.3 For example, pieces of evidence suggested that hyperfibrinolysis can manifest in up 50% of patients undergoing liver surgery.4
Consequently, cancer patients are often treated with transfusion of blood products to treat perioperative blood loss and anemia.3 Nevertheless, transfusion of blood products is not without risk. In fact, it can lead to an increased risk of potentially life-threatening immune responses, acute lung injury, and postoperative infections.5 In addition, in these patients, blood transfusions can be strongly associated with the development of new or progressive venous thromboembolism (VTE).6,7 Another important issue is the correlation between transfusion and cancer recurrence. Notably, although several studies concluded that perioperative transfusion of red blood cells (RBCs) did not increase the early risk of recurrence after cancer surgery,8,9 other data have shown that it was linked with worse cancer prognosis, in different settings.10–12 These potential risks, in addition to the high cost of transfusions, stimulate the use of hemostatic agents to reduce blood loss and the need for transfusions.13
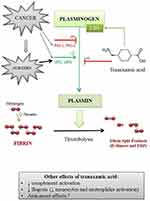
On the other hand, as a result of the hypercoagulable state caused by the production of several procoagulant agents, such as tissue factor, fibrinogen, neutrophil extracellular traps, and platelets activation factors, cancer patients have a higher risk of VTE than non-cancer patients.14,15 Most solid tumors, in particular those originating from the gastrointestinal tract, cause hypercoagulability of the blood and this phenomenon is due to the production by cancer cells of PCA (cancer procoagulant agent), namely a protease able to activate the coagulation cascade through the direct activation of coagulation factor X. Interestingly, cancer cells can interefere with the fibrinolysis by increasing the fibrinolytic activity through the expression on their cell surface of the tissue plasminogen activator (tPA), and the urokinase-type plasminogen activator (uPA). Nevertheless, they can also induce the production of the inhibitor of plasminogen activator type 1 receptor and −2 (PAI-1 and PAI-2) that limit the lysis of the blood clot.16
In this complex scenario, effective and safe potential strategies for the prevention of blood transfusions and their complications are needed. These strategies may include numerous pharmacological and operative approaches (eg, minimally invasive surgery, maintenance of normothermia). Tranexamic acid (TXA) is a potential pharmacological opportunity aimed at counteracting alterations in fibrinolysis. It is a low-cost hemostatic agent with a wide range of applications and minimal adverse effects. Synthesized in the second half of the twentieth century, TXA is widely used for its antifibrinolytics properties during abnormal bleeding.17 A meta-analysis of 129 trials (n=10,488) on its use in surgery concluded that TXA administration reduced the need for a blood transfusion by 38% while the effect on thromboembolic events and mortality was not conclusive.18
This narrative review is aimed to assess the efficacy and the safety of TXA when used in the context of cancer surgery. The authors also provide an overview of ongoing clinical research.
Mechanism of Action
The physiological coagulation cascade is a very complex process. The starting point is the activation of the tissue factor, contained mainly in the endothelial cells, by a tissue injury like a surgical act. It is a finely regulated process, which involves the sequential activation of coagulation factors. The result is the formation of a blood clot constituted by a tangle of fibrin and platelets that, finally, can fill the vascular damage. There is a feedback control system that prevents excessive clot formation as the fibrinolysis is activated to limit the extension of the clot and to degrade it. In brief, fibrinolysis works through a cellular mechanism involving the action of white blood cells, and a plasma mechanism. The fundamental step of this latter mechanism is the transformation of plasminogen into plasmin.The plasmin, namely the central molecule of fibrinolysis, is a proteolytic enzyme that can disrupt fibrin producing fibrin split products (eg, D-dimers). Regarding fibrinolysis inhibitors, the hemostasis control system provides several mechanisms which, if necessary, shift the balance in favor of coagulation. These mechanisms include inhibitors of the serine proteases tPA (PAI 1,2,3) and uPA, and several antiproteases such as the glycoprotein alpha2-antiplasmin which binds plasmin and is enhanced by calcium ions and coagulation factor XIII, alpha1-antitrypsin, complement C1 esterase inhibitor, and ATIII (antithrombin).
TXA is a synthetic molecule (trans 4 amino methylcyclohexane carboxylic acid) that derivates from lysine and produces antifibrinolytic effects by blocking lysine-binding sites on plasminogen. This binding prevents the formation of plasmin which is the activated form of plasminogen and the activation of fibrin. As a result, inhibition of plasminogen activation induces the stabilization of the preformed fibrin structure produced by secondary hemostasis.19 TXA exerts its main bleeding limiting mechanism by delaying natural fibrinolysis and, therefore, the degradation of the blood clot.
TXA has other interesting effects. By blocking the formation of plasmin, it can reduce the activation of monocytes and neutrophiles. This mechanism, in turn, can produce an important anti-inflammatory effect. Furthermore, inhibition of complement activation is also described.20 This process is of paramount importance as an increased complement activation correlates with mortality and organ failure.21 Finally, preclinical studies suggested and anticancer effect of TXA22 (Figure 1).
Pharmacological Properties
TXA is available as intravenous and oral formulations. The recommended posology varies greatly between studies, ranging from 10 mg/kg to 100 mg/kg.16 It is poorly metabolized by the liver and is mainly excreted by the kidney as renal clearance is the primary mechanism of excretion.16 This correlates to an increased incidence of complications in the case of renal dysfunction.23,24 Thus, a dose reduction in both oral and intravenous formulations should be considered depending on serum creatinine values.16 Indeed, for a serum creatinine between 120 and 150 µg/L, the recommended dose is 10 mg/kg twice a day instead of 2 to 4 gr per day;16 for a serum creatinine between 250 and 500 µg/L, the recommended posology is 10 mg/kg/24h, and 10 mg/kg/48h when the serum creatinine is greater than 500 µg/L.16
The therapeutic plasma concentration of TXA varies from 5 mg/kg to 10 mg/kg.25 Plasma concentration after an intravenous dose of 10 mg/kg is maintained for up 3 hours, but it appears that this dosage is insufficient in some types of surgery such as urologic25,26 and orthopedics surgery.27 A dose of 10 mg/kg can maintain a therapeutic level for 8 hours but appears to increase the risk of thromboembolic events.25,27
After oral administration, TXA bioavailability is 30 to 50%. The volume of distribution is 9 to 12 liters and the half-time is of 2 hours.16 When studied in healthy volunteers, intravenous TXA has a half-life of 2 hours.18 Finally, food intake does not affect time to maximum concentration, as detected by the area under the receiver operating characteristic curve (ROC AUC).16–18,28
Compared to the other lysine analog epsilon-aminocaproic acid, TXA is up to ten times more potent in binding affinity to plasminogen. Moreover, TXA provokes inhibition of fibrinolysis, which manifests as reductions in serum D-dimer levels without affecting the results of serum markers of coagulopathy.16 Furthermore, since concomitant heparin use does not influence the activity of TXA, it can be safely administrated in heparinized patients.18
The main contraindications of TXA are arterial or venous thromboembolism diseases, and convulsions.16 Furthermore, precautions should be taken in case of renal dysfunction and/or hematuria (eg, due to potential obstructive urinary complications). The side effects of TXA are rare and they are mainly nausea, vomiting, and diarrhea, as well as visual or ocular adverse effects, dizziness, hypotension, and seizures (particularly during cardiovascular surgery).16 It only exceptionally can cause dangerous allergic reactions. Remarkably, in old studies, due to its inhibitory role on complement, it has even been used to treat anaphylactic shock.29 About drug interactions, concomitant use of chlorpromazine may result in an increased risk of bleeding.17 The drug is contraindicated in patients with subarachnoid hemorrhage, in those with active intravascular clotting, and in the case of a previous severe hypersensitivity reaction after TXA administration. The Food and Drug Administration (FDA) approved TXA in individuals with hemophilia for short-term use (2–8 days) to reduce or prevent hemorrhage especially in the case of tooth extraction, and for heavy menstrual bleeding. Nevertheless, several off-label uses of oral, topical, and intravenous TXA are described in the literature, mostly in surgical settings and in trauma to reduce blood loss.17–24
Clinical Usage and Efficacy of Tranexamic Acid in Oncologic Surgery
The efficacy and safety of TXA in oncological surgery has been investigated in several clinical settings. The main results are summarized according to the surgical context.
Head and Neck Surgery
In patients with head/neck cancer, the use of TXA is poorly investigated (Table 1). In 2016, Kulkarni et al16 published a double-blind, placebo-controlled study, whose data showed that preoperative intravenous TXA, given at the dosage of 10 mg/kg (after induction and repeated every 3 hours), did not reduce blood loss and the need for blood transfusions in patients undergoing major surgery for oral cancers, with or without reconstruction. Moreover, the TXA administration was safe and the incidence of deep venous thrombosis (DVT) did not increase. In another randomized, controlled trial, Chen et al30 demonstrated that, compared to placebo, TXA did not significantly reduce drainage time.
 |
Table 1 Summary of Tranexamic Acid in Head and Neck Surgery |
Urological Surgery
To date, there is only a randomized controlled trial evaluating the TXA during cystectomy for bladder cancer (10 mg/kg intravenously before incision, followed by a 5 mg/kg/h maintenance infusion).26 The study is still ongoing (clinicaltrials.gov identifier: NCT01869413). Results are pending and they will soon be published.
Concerning prostate cancers, Crescenti et al31 demonstrated that, compared to placebo, the intraoperative use of TXA (500 mg 20 minutes before the surgery, then 250 mg/h until skin suture) was able to reduce the transfusion rate. Similar results were also obtained by Pourfakhr et al.32 Several investigations have been conducted on transurethral resection of the prostate (TURP), but the results seem to be inconclusive.33–36 In a systematic review and meta-analyses, Longo et al37 demonstrated that, in patients who undergo prostatectomy and TURP, TXA was able to reduce both intraoperative blood loss and the need for blood transfusion, without increasing in complications, such as DVT and pulmonary embolism. However, presently, there are few and very heterogeneous (both in the dosage and method of administration of TXA) high-quality studies. Therefore, more clinical trials with a large number of patients and with a robust methodology are necessary to confirm these findings (Table 2).
 |
Table 2 Summary of Tranexamic Acid in Urological Surgery |
The advantages of TXA on postoperative outcomes can also be evaluated indirectly. For example, TXA seems to decrease surgical time during TURP.38,39 This advantage is desirable to decrease the potential complications, like thromboembolism events, blood loss, and effects due to irrigating fluid absorption, especially in oncological patients.
Neurosurgery
Patients undergoing craniotomy for tumor excision are at high risk for major bleeding. They usually require allogeneic red blood cells (RBC) transfusions which can increase the risk for infectious disease transmission, transfusion reaction, and immunosuppression. Previous studies have suggested that the leptomeninges are rich in tPA.40 Therefore, it can be supposed that blood loss in intracranial meningiomas might be made worse by local tPA induced by hyperfibrinolysis. So, prolonged intracranial surgery and the following surgical stress may be complicated by a consumption-induced coagulopathy.41
To the best of our knowledge, there are currently two studies evaluating the TXA effectiveness in patients undergoing craniotomy for tumoral excision. Indeed, Vel et al42 and Hooda et al43 showed that TXA at 10 mg/kg and 20 mg/kg, respectively, with a maintenance dose of 1 mg/kg/h until the end of the surgery, can reduce the blood loss but not the need for blood transfusions. Moreover, the duration of surgery was not affected by TXA use.
Other investigations have been conducted in oncological surgery of the spine. Bednar et al44 performed a pilot study including a small number of patients with spinal metastases who required surgery for spinal stabilization. They showed that intravenous TXA did not significantly reduce blood loss and the need for blood transfusion. Damade et al45 in a similar trial, did not show a significant decrease in intraoperative and postoperative blood loss in patients receiving TXA for spinal tumor surgery. However, they demonstrated that blood transfusions are statistically less frequent in the group that received TXA. More recently, in a retrospective analysis, Zhang et al46 proved that, about surgery for spinal tumors, TXA can significantly reduce (p<0.05) the amount of intraoperative blood loss and postoperative drainage without increasing the risk of DVT. The duration of the surgical intervention was not significantly reduced by the administration of the TXA41,42 (Table 3).
 |
Table 3 Summary of Tranexamic Acid in Neurosurgical Surgery |
Despite limitations, further data can be obtained from studies conducted on non-cancer patients. In a large study enrolling patients treated with spinal reconstructive surgery, Wong et al47 demonstrated that the administration of TXA significantly reduced the perioperative blood loss by up to 30%, if compared with those not receiving TXA. However, the amount of perioperative blood products transfusions was not different among groups.
Visceral Surgery
Several studies have been conducted to evaluate the efficacy of TXA in visceral surgery (Table 4).
 |
Table 4 Summary of Tranexamic Acid in Visceral Surgery |
In 2006, Wu and al48 published a prospective randomized study evaluating the rate of blood transfusion in patients who received TXA before hepatectomy for primary or secondary malignancies. They concluded that a perioperative intravenous low dose administration of TXA (500 mg intravenously administered before surgery followed by 250 mg, every 6 hours, for 3 days) was associated with a significantly lower amount of blood loss (p=0.0001) and blood transfusions (p<0.0001). The duration of surgical intervention was significantly lower in the group receiving TXA (p=0.003). In addition, even if the length of hospitalization was similar, the global hospital cost was significantly lower in the experimental group receiving TXA (p=0.036).
More recently, Grass et al49 carried out a retrospective study in patients (n=213) who underwent elective colorectal surgery for cancer and received 1 g of TXA intravenously as induction therapy and before anesthesia emergence. Despite their study failed to prove a significant reduction of post-operative transfusions rate, the authors provided insight into the potential benefits of perioperative TXA administration in this surgical setting. Regarding major oncologic surgery (eg, cytoreductive surgery with heated intraperitoneal chemotherapy, esophagectomy, and pancreatectomy), Wright et al50 conducted a double-blind, placebo-controlled randomized testing the effects of a single dose (1 g) of TXA before surgery on perioperative transfusion rate. They did not find lower perioperative allogeneic blood transfusion rates, but TXA did not increase the incidence of postoperative adverse effects.
Currently, the TAC-DP (Tranexamic Acid during Pancreatic Duodenectomy) study is ongoing.51 It is a multicentre randomised, double-blind, placebo-controlled trial whose objectives are to investigate the effect of TXA on the incidence of perioperative blood transfusions, operation time, anesthesia time, postoperative laboratory elements, and complications. The study also investigates the safety of TXA, especially in terms of incidence of thromboembolic events within 28 days of surgery or requiring readmission. Results are pending.
Gynecological Surgery
Myomectomy can be associated with a high rate of perioperative blood loss. In a randomized controlled trial, Abdul et al52 combined TXA (10 mg/kg before incision) with the use of the tourniquet. This strategy significantly reduced intraoperative blood loss per 100 g of fibroid removed, when compared to tourniquet alone. In a retrospective study including women with uterine cancer who underwent surgery, Celebi et al53 divided the sample size (n=105) into 4 groups: group I received crystalloid solutions, group II colloid agent, group III received TXA (10 mg/kg), and group IV received (100 mg/kg) epsilon-aminocaproic acid. They found an important decrease (up to 30%) in perioperative blood loss in those who received TXA compared to the crystalloid group (p<0.05). They also proved that, compared to the epsilon-aminocaproic group, women who received TXA had approximately 24% of reduced occurrence of perioperative blood loss (p<0.05). Another placebo-controlled study (n=100) demonstrated that a single dose of TXA (15 mg/kg just before surgery) significantly reduced blood loss and transfusion rates (p=0.03) in patients with advanced ovarian cancer treated with surgery.54 Given these results, it seems that TXA could be recommended as a standard prophylactic strategy in advanced ovarian cancer surgery and other types of gynecological surgery for cancer (Table 5). Nevertheless, to determine if TXA can be used as a standard of care, it is necessary to conduct good quality, randomized and controlled trials to provide solid evidence on the efficacy, and safety profile of the drug. In this regard, a Cochrane analysis concluded that the evidence is not sufficient to recommend the routine use of TXA for reducing blood loss during cytoreductive surgery for advanced ovarian cancer.55
 |
Table 5 Summary of Tranexamic Acid in Gynecological Surgery |
Breast Surgery
In a retrospective monocentric trial (n=868), intravenous TXA (1 g intravenously before incision and 1 g at the end of the surgery) was administrated in women who received implant-based breast reconstruction.56 The authors highlighted a clinical benefit of TXA in plastic reconstruction surgery, with a statistical decrease in postoperative hematoma. Moreover, adverse effects such as DVT and other thromboembolic issues were not observed.
A Norwegian two-center, double-blind randomized trial investigated the effects of wound « moistening » through TXA (25 mg/mL) before closure.57 The authors observed a reduction of postoperative bleeding within 24h in patients undergoing mastectomy57 (Table 6).
 |
Table 6 Summary of Tranexamic Acid in Breast Surgery |
Orthopedic Surgery
In non-cancer orthopedic surgery, TXA has been extensively studied. Recently, the American Association of Hip and Knee Surgeons, the American Society of Regional Anesthesia and Pain Medicine, and the American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society published their combined clinical-practice guidelines regarding the use of TXA in total joint arthroplasty.58 According to them, TXA is profoundly changing the perioperative management of arthroplasty patients. The aforementioned guidelines recommend that in patients without a known history (strong evidence) and in those with a history of a VTE, or myocardial infarction (moderate evidence) TXA does not increase the risk of thromboembolism phenomena.
Nevertheless, there is a lack of evidence about the safety of TXA in cancer patients undergoing orthopedic surgery. Varady et al59 evaluated the safety of TXA in patients with current or former cancer undergoing hip and knee arthroplasty. They found that TXA was not associated with an increased risk of VTE. The same results were found in other studies.60,61 For example, Atalay et al60 demonstrated that preoperative TXA (15 mg/kg as a bolus) significantly reduced postoperative bleeding amount and transfusion needs in wide-margin tumor resection and prosthesis surgery for cancer lesions of the proximal femur. Other studies investigated different modalities of TXA use. Haase et al61 for example, found that topical TXA can reduce perioperative blood loss, transfusion rates, hospitalization, and VTE occurrence.
Differently, Owen et al62 did not show a reduction in blood loss and in blood transfusions when intravenous TXA was administrated before bipolar hemiarthroplasty for metastatic disease and at the time of closure. However, this study was conducted on a small and heterogeneous cohort of patients. Therefore, further investigations via multicenter RCTs are needed (Table 7).
 |
Table 7 Summary of Tranexamic Acid in Orthopedic Surgery |
Ongoing Research
Several clinical investigations addressing multiple important issues are still ongoing (Table 8). Some of them, for example, is establishing the effective and safe dose of TXA to be used.
 |
Table 8 Summary of Ongoing Clinical Research on Tranexamic Acid in Oncological Surgery |
In the NCT04360629 trial, patients with advanced ovarian cancer are randomized to receive three different TXA doses. Group I, receive a low dose of TXA (10 mg/kg bolus followed by an infusion of 1 mg/kg/h); Group II, a high dose of TXA (20 mg/kg bolus followed by an infusion of 5 mg/kg/h); Group III, placebo.
Another hot issue to be elucidated is the occurrence of late-onset postoperative complications. In a randomized controlled trial, researchers are collecting the postoperative thrombotic complications including myocardial infarction, stroke, pulmonary embolism, renal failure, or bowel infarction, 1 month after surgery for colorectal cancer. Patients receive TXA (intravenous bolus of 10 mg/kg) or placebo (NCT03606785). In the NCT03128866 trial on patients undergoing hemipelvectomy, the effect of TXA on postoperative complications, length of intensive care unit (ICU), and hospital stays are under investigation.
Notably, there is also a study in patients with skin cancer (NCT04630886). The hypothesis is that a subcutaneous infiltration of TXA (2% lidocaine with 1:100,000 epinephrine mixed 50/50 with 50 mg/mL TXA) can reduce perioperative complications including bleeding, infections, as well as flap and graft loss. About the topic use of TXA, a study was designed to evaluate the use of topical application of the drug (3 g diluted in 10 mL of normal saline) to the surgical wound to decrease hematoma formation in patients undergoing bilateral breast reduction (NCT04918576).
Finally, other studies are evaluating the use of TXA in different settings of major oncological surgery. Among the various studies the NCT01980355 from the United States (TXA 1 g prior to surgery), the multi-centre Phase IV TRACTION Trial (NCT04803747) from Canada and investigations in pediatric oncological surgery (NCT05024253, NCT04410042) will surely provide interesting data.
Conclusions
Despite the harmful effects of blood transfusions, administration of TXA is not standard clinical practice for patients undergoing cancer surgery. Based on the results of current studies, the use of TXA could be associated with an important reduction in blood loss and transfusions need, and importantly, it seems to positively impact the incidence of some feared side effects, such as thromboembolic phenomena. Nevertheless, most of the published studies on the subject are small and often questionable in terms of statistical power. Consequently, further randomized clinical trials are warranted to corroborate the abovementioned findings, and more importantly, randomized investigations are needed to determine efficacy, safety, adequate therapeutic doses, and the appropriate treatment regimen of TXA. Finally, it is necessary to verify in which surgical areas the use of TXA is most appropriate. For example, pelvic surgery for cancer is associated with an increased risk of VTE and, in this setting, extended prophylaxis is recommended with low-molecular-weight heparin or, more recently, direct oral anticoagulants. The potential interaction of these strategies with TXA requires proper investigation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Johnstone C, Rich SE. Bleeding in cancer patients and its treatment: a review. Ann Palliat Med. 2018;7(2):265–273. doi:10.21037/apm.2017.11.01
2. Cata JP, Gottumukkala V. Blood transfusion practices in cancer surgery. Indian J Anaesth. 2014;58(5):637–642. doi:10.4103/0019-5049.144675
3. Kim HJ, Moon SH, Cho SH, Lee JD, Kim HS. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97(7):776–781. doi:10.2340/00015555-2668
4. Leebeek FW, Rijken DC. The fibrinolytic status in liver diseases. Semin Thromb Hemost. 2015;41(5):474–480. doi:10.1055/s-0035-1550437
5. Abeysiri S, Chau M, Richards T. Perioperative anemia management. Semin Thromb Hemost. 2020;46(1):
6. Ramsey G, Lindholm PF. Thrombosis risk in cancer patients receiving red blood cell transfusions. Semin Thromb Hemost. 2019;45(6):
7. Goel R, Patel EU, Cushing MM, et al. Association of perioperative red blood cell transfusions with venous thromboembolism in a North American registry. JAMA Surg. 2018;153(9):
8. Oliveira GSD, Schink JC, Buoy C, Ahmad S, Fitzgerald PC, McCarthy RJ. The association between allogeneic perioperative blood transfusion on tumour recurrence and survival in patients with advanced ovarian cancer. Transfus Med. 2012;22(2):
9. Schiergens TS, Rentsch M, Kasparek MS, Frenes K, Jauch K-W, Thasler WE. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum. 2015;58(1):
10. Chang KY, Tai Y-H, Lin S-P, Chan M-Y, Chen -H-H, Chang K-Y. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4030 patients. Sci Rep. 2018;8(1):13345. doi:10.1038/s41598-018-31662-5
11. Hallet J, Tsang M, Cheng ESW, et al. The impact of perioperative red blood cell transfusions on long-term outcomes after hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2015;22(12):
12. Pushan Z, Manbiao C, Sulai L, Jun L, Ruidong Z, Hanshen Y. The impact of perioperative blood transfusion on survival and recurrence after radical prostatectomy for prostate cancer: a systematic review and meta-analysis. J Cancer Res Ther. 2018;14(Supplement):
13. Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost. 2017;117(2):
14. Laridan E, Martinod K, Meyer SFD. Neutrophil extracellular traps in arterial and venous thrombosis. Semin Thromb Hemost. 2019;45(1):
15. Belghasem M, Roth D, Richards S, et al. Metabolites in a mouse cancer model enhance venous thrombogenicity through the aryl hydrocarbon receptor–tissue factor axis. Blood. 2019;134(26):
16. Kulkarni AP, Chaukar DA, Patil VP, Metgudmath RB, Hawaldar RW, Divatia JV. Does tranexamic acid reduce blood loss during head and neck cancer surgery? Indian J Anaesth. 2016;60(1):
17. Ng W, Jerath A, Wąsowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339–350. doi:10.5603/AIT.a2015.0011
18. Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi:10.1136/bmj.e3054
19. Cai J, Ribkoff J, Olson S, et al. The many roles of tranexamic acid: an overview of the clinical indications for TXA in medical and surgical patients. Eur J Haematol. 2020;104(2):
20. Barrett CD, Vigneshwar N, Moore HB, et al. Tranexamic acid is associated with reduced complement activation in trauma patients with hemorrhagic shock and hyperfibrinolysis on thromboelastography. Blood Coagul Fibrinolysis. 2020;31(8):578–582. doi:10.1097/MBC.0000000000000938
21. Shakur H, Roberts I, Bautista R, et al.; CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi:10.1016/S0140-6736(10)60835-5
22. Suojanen J, Sorsa T, Salo T. Tranexamic acid can inhibit tongue squamous cell carcinoma invasion in vitro. Oral Dis. 2009;15(2):170–175. doi:10.1111/j.1601-0825.2008.01507.x
23. Shakur H, Beaumont D, Pavord S, Gayet‐Ageron A, Ker K, Mousa HA. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2018(2):CD012964.
24. Sentilhes L, Winer N, Azria E, et al.; Groupe de Recherche en Obstétrique et Gynécologie. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med. 2018;379(8):731–742. doi:10.1056/NEJMoa1800942
25. Balík M, Košina J, Hušek P, Broďák M, Čečka F. Safety and efficacy of using tranexamic acid at the beginning of robotic-assisted radical prostatectomy in a double-blind prospective randomized pilot study. Acta Medica. 2020;63(4):
26. Breau RH, Lavallée LT, Cnossen S, et al. Tranexamic acid versus placebo to prevent blood transfusion during radical cystectomy for bladder cancer (TACT): study protocol for a randomized controlled trial. Trials. 2018;19(1):261. doi:10.1186/s13063-018-2626-3
27. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):
28. Alanwar A, Abbas AM, Hussain SH, et al. Oral micronised flavonoids versus tranexamic acid for treatment of heavy menstrual bleeding secondary to copper IUD use: a randomised double-blind clinical trial. Eur J Contracept Reprod Health Care. 2018;23(5):365–370. doi:10.1080/13625187.2018.1515349
29. Hoste S, Van Aken H, Stevens E. Tranexamic acid in the treatment of anaphylactic shock. Acta Anaesthesiol Belg. 1991;42(2):113–116.
30. Chen CC, Wang CC, Wang CP, Lin TH, Lin WD, Liu SA. Prospective, randomized, controlled trial of tranexamic acid in patients who undergo head and neck procedures. Otolaryngol Head Neck Surg. 2008;138(6):762–767. doi:10.1016/j.otohns.2008.02.022
31. Crescenti A, Borghi G, Bignami E, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. BMJ. 2011;343:d5701. doi:10.1136/bmj.d5701
32. Pourfakhr P, Gatavi E, Gooran S, et al. Local administration of tranexamic acid during prostatectomy surgery: effects on reducing the amount of bleeding. Nephro Urol Mon. 2016;8(6):e40409. doi:10.5812/numonthly.40409
33. Jendoubi A, Malouch A, Bouzouita A, et al. Intérêt de l’acide tranexamique dans les résections endoscopiques urologiques: étude prospective randomisée [Safety and efficacy of intravenous tranexamic acid in endoscopic transurethral resections in urology: prospective randomized trial]. Prog Urol. 2017;27(16):1036–1042. French. doi:10.1016/j.purol.2017.09.008
34. Meng -Q-Q, Pan N, Xiong J-Y, Liu N. Tranexamic acid is beneficial for reducing perioperative blood loss in transurethral resection of the prostate. Exp Ther Med. 2019;17(1):
35. Mirmansouri A, Farzi F, Imantalab V, et al. A survey on the effects of intravenous tranexamic acid on the amount of transfusion in patients undergoing T.U.R-P. J Guilan Univ Med Sci. 2016;25(98):
36. Miller RA, May MW, Hendry WF, Whitfield HN, Wickham JE. The prevention of secondary haemorrhage after prostatectomy: the value of antifibrinolytic therapy. Br J Urol. 1980;52(1):
37. Longo MA, Cavalheiro BT, de Oliveira Filho GR. Systematic review and meta-analyses of tranexamic acid use for bleeding reduction in prostate surgery. J Clin Anesth. 2018;48:32–38. doi:10.1016/j.jclinane.2018.04.014
38. Rannikko A, Pétas A, Taari K. Tranexamic acid in control of primary hemorrhage during transurethral prostatectomy. Urology. 2004;64(5):
39. Samir M, Saafan AM, Afifi RM, Tawfick A. Can high-dose tranexamic acid have a role during transurethral resection of the prostate in large prostates? A randomised controlled trial. Arab J Urol. 2021;20:24–29. doi:10.1080/2090598X.2021.1932125
40. Oka K, Tsuda H, Kamikaseda K, et al. Meningiomas and hemorrhagic diathesis. J Neurosurg. 1988;69(3):356–360. doi:10.3171/jns.1988.69.3.0356
41. Goh KYC, Poon WS, Chan DTM, Ip CP. Tissue plasminogen activator expression in meningiomas and glioblastomas. Clin Neurol Neurosurg. 2005;107(4):
42. Vel R, Udupi BP, Satya Prakash MVS, Adinarayanan S, Mishra S, Babu L. Effect of low dose tranexamic acid on intra-operative blood loss in neurosurgical patients. Saudi J Anaesth. 2015;9(1):
43. Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. 2017;41:
44. Bednar DA, Bednar VA, Chaudhary A, Farroukhyar F. Tranexamic acid for hemostasis in the surgical treatment of metastatic tumors of the spine. Spine. 2006;31(8):
45. Damade C, Tesson G, Gilard V, et al. Blood loss and perioperative transfusions related to surgery for spinal tumors. Relevance of tranexamic acid. Neurochirurgie. 2019;65(6):
46. Zhang HZ, Dong L, Wang HM, et al. Safety and efficacy of tranexamic acid in spinal canal tumors: a retrospective cohort study. Br J Neurosurg. 2020;34(3):
47. Wong J, El Beheiry H, Rampersaud YR, et al. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107(5):
48. Wu -C-C, Ho W-M, Cheng S-B, et al. Perioperative parenteral tranexamic acid in liver tumor resection. Ann Surg. 2006;243(2):
49. Grass F, Braafladt S, Alabbad J, et al. The effects of tranexamic acid on blood loss and transfusion rate in colorectal surgery. Am J Surg. 2019;218(5):
50. Wright GP, Wolf AM, Waldherr TL, et al. Preoperative tranexamic acid does not reduce transfusion rates in major oncologic surgery: results of a randomized, double-blind, and placebo-controlled trial. J Surg Oncol. 2020;122(6):
51. Ishii K, Yokoyama Y, Yonekawa Y, Ebata T. Tranexamic ACid during PancereaticoDuodenectomy (TAC-PD): study protocol for a multicentre randomised, blind, placebo-controlled trial. BMJ Open. 2020;10(11):e040914. doi:10.1136/bmjopen-2020-040914
52. Abdul IF, Amadu MB, Adesina KT, Olarinoye AO, Omokanye LO. Adjunctive use of tranexamic acid to tourniquet in reducing haemorrhage during abdominal myomectomy - a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2019;242:150–158. doi:10.1016/j.ejogrb.2019.09.010
53. Celebi N, Celebioglu B, Selcuk M, Canbay O, Karagoz AH, Aypar U. The role of antifibrinolytic agents in gynecologic cancer surgery. Saudi Med J. 2006;27(5):
54. Lundin ES, Johansson T, Zachrisson H, et al. Single-dose tranexamic acid in advanced ovarian cancer surgery reduces blood loss and transfusions: double-blind placebo-controlled randomized multicenter study. Acta Obstet Gynecol Scand. 2014;93(4):335–344. doi:10.1111/aogs.12333
55. Kietpeerakool C, Supoken A, Laopaiboon M, Lumbiganon P. Effectiveness of tranexamic acid in reducing blood loss during cytoreductive surgery for advanced ovarian cancer. Cochrane Database Syst Rev. 2016;2016(1):CD011732. doi:10.1002/14651858.CD011732.pub2
56. Weissler JM, Banuelos J, Jacobson SR, et al. Intravenous tranexamic acid in implant-based breast reconstruction safely reduces hematoma without thromboembolic events. Plast Reconstr Surg. 2020;146(2):
57. Ausen K, Hagen AI, Østbyhaug HS, et al. Topical moistening of mastectomy wounds with diluted tranexamic acid to reduce bleeding: randomized clinical trial. BMJ Open. 2019;4(2):
58. Fillingham YA, Ramkumar DB, Jevsevar DS, et al. Tranexamic acid in total joint arthroplasty: the endorsed clinical practice guides of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. Reg Anesth Pain Med. 2019;44(1):
59. Varady NH, Chen AF, Drayer NJ, Ready J, Lozano-Calderon SA, Hayden BL. Tranexamic acid in patients with current or former cancer undergoing Hip and knee arthroplasty. J Surg Oncol. 2021;123(8):
60. Atalay İB, Yapar A, Ulucakoy C, et al. The effectiveness of tranexamic acid in patients with proximal femoral tumor resection prosthesis. Cureus. 2020;12(8):e10105. doi:10.7759/cureus.10105
61. Haase DR, Templeton KJ, Rosenthal HG, Sweeney KR. Tranexamic acid in patients with cancer undergoing endoprosthetic reconstruction: a retrospective review. JAAOS. 2020;28(6):
62. Owen A, Wellings EP, Wyles CC, Yuan BJ, Rose PS, Houdek MT. Use of tranexamic acid is not associated with complications following bipolar hemiarthroplasty for metastatic disease. J Surg Oncol. 2021;123(4):
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.