Back to Journals » Drug Design, Development and Therapy » Volume 12
Efficacy and safety of Tongfu powder in acute pancreatitis patients with gastrointestinal dysfunction: a clinical trial
Authors Miao B, Li FW, Zhang SW, Wang H, Qi WJ, Wang C
Received 24 January 2018
Accepted for publication 30 May 2018
Published 31 October 2018 Volume 2018:12 Pages 3665—3673
DOI https://doi.org/10.2147/DDDT.S163645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Bin Miao, Feng-Wu Li, Shu-Wen Zhang, Hong Wang, Wen-Jie Qi, Chao Wang
Department of Infectious Disease, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
Objective: To evaluate the efficacy and safety of Tongfu powder for external application on Shénquè (the umbilicus, hereafter, Tongfu powder) versus mosapride in acute pancreatitis (AP) patients with gastrointestinal dysfunction.
Methods: A total of 102 AP patients were diagnosed using the latest Atlanta Criterion and recruited at the Department of Infectious Disease, Beijing Friendship Hospital (Beijing, People’s Republic of China) from August 2014 to December 2016. Patients were randomized into the Tongfu powder group and mosapride group using the random table. Information on scores (eg, the gastrointestinal function score) on days 1 and 7 of hospitalization, biochemical indicators (eg, interleukin [IL]-2 and IL-6), indicators for curative effects (eg, first defecation time, bowel sound recovery time, hospitalization costs, and duration) were collected and compared between the 2 groups.
Results: The gastrointestinal function score decreased significantly after treatment, and the changes were significantly different between the Tongfu powder group and the mosapride group (P<0.05). Significantly shorter time to first defecation and bowel sound recovery was observed in the Tongfu powder group versus the mosapride group (P<0.05). The improvements of IL-2, IL-4, intestinal fatty acid binding protein, motilin, and vasoactive intestinal peptide in the Tongfu powder group were higher than those in the mosapride group (P<0.05). There were no significant differences in hospital cost and length of hospital stay between the 2 groups.
Conclusion: This study suggested that Tongfu powder for external application may improve gastrointestinal function for AP patients compared with mosapride.
Keywords: Tongfu powder, mosapride, gastrointestinal dysfunction, acute pancreatitis
Introduction
Acute pancreatitis (AP), which occurs as a result of activation of pancreatic enzymes, has a wide variety of causes. The disease is characterized by local pancreatic inflammation with or without dysfunction of other organs. As one of the most common acute abdominal emergencies, most cases of AP are slight and self-limiting. However, nearly 10%–20% of AP patients have the severe form of AP that is characterized by speedy onset, rapid deterioration, and high mortality (about 10%–20%).1
According to recent Chinese studies, the incidence of AP with gastrointestinal dysfunction ranged from 50% to 74.7%.2,3 Additionally, the incidence of AP with dysfunction of the intestinal barrier was reported as 59%.4 AP is often highly related to gastrointestinal dysfunction, leading to bacterial and endotoxin translocation, and ultimately, a multiple organ dysfunction syndrome (MODS). The mortality rate of AP is closely related to MODS. The mortality rate for AP patients with 2 organ failures was as high as 50%–91%.5
The dysfunction of the intestinal barrier in the early stage of AP and its association with bacterial translocation, necrosis, and infection of the pancreas and organ failure are commonly acknowledged.6 In some studies, pancreatic infection and organ failure were reported as indicators of severity of pancreatitis.7,8 Additional reports have shown that the origin of microbes that induced pancreatic infection was mostly from the intestine,9 which further indicates that the injury of the intestinal barrier might cause systemic inflammatory response syndrome (SIRS), MODS, and peripancreatic infection. The degree of gastrointestinal dysfunction was closely linked with the development and prognosis of severe pancreatitis patients, and could be used as an important prognostic predictor of AP.10,11 Therefore, early detection of gastrointestinal insufficiency is crucial in order to improve the function of the gastrointestinal tract.
Numerous clinical and experimental studies have focused on the effects of traditional Chinese medicines (TCMs) in many diseases.12–14 TCM external therapy is a well-established treatment method with a long history, which is characterized by ease of use and wide application with safe and reliable effects. TCM external therapy shows a unique treatment for those who refused or were unable to take oral drugs. Because oral treatment is common, it is plausible and necessary to compare the efficacy between TCM external therapy and oral treatment.
Here, we performed a study to evaluate the effect of Tongfu powder for external application on Shénquè (the umbilicus, hereafter, Tongfu powder) and compared it with mosapride that is taken orally, by assessing the gastrointestinal function in AP patients.
Methods
Patient inclusion and exclusion criteria
Patients
A total of 102 AP patients were recruited at the Department of Infectious Disease, Beijing Friendship Hospital affiliated with Capital Medical University (Beijing, People’s Republic of China) between August 2014 and December 2016. The study protocol was approved by the Ethics Committee of Beijing Friendship Hospital. All patients provided written informed consent before participation in the study. This trial was registered with at ClinicalTrials.gov (NCT02204189).
Diagnosis criteria
AP was diagnosed using the 2012 Atlanta Criteria.15 Those who met 2 or 3 of the following criteria were diagnosed as AP: 1) abdominal pain consistent with AP (acute, sudden, persistent upper abdominal pain, often radiating to the back); 2) the activity of serum amylase being at least 3 times higher than the normal upper limit; 3) enhanced computed tomography, magnetic resonance imaging, or abdominal ultrasound showing evidence of AP.
Organ failure diagnosis
Patients who had a Marshall score of >2 were defined as having organ failure, including lung, heart, and kidney failure.16 Failure of other organs (the liver, the gastrointestinal tract, the coagulation system, and the brain) was diagnosed according to the China MODS scoring criteria.17
Severity rating
AP was categorized into 3 types using the 2012 Atlanta Standard.15 Mild AP (MAP) was characterized by no organ failure, and no local or systemic complications; moderately severe AP (MSAP) was the one with organ failure, but had persisted for <48 hours with local or systemic complications; severe AP (SAP) was characterized by at least 1 organ failure (organ failure persists more than 48 hours).
Inclusion criteria
Patients who met the diagnostic criteria for AP, with a gastrointestinal function score of 1 were admitted to hospital within 72 hours.
Exclusion criteria
AP patients who had the following conditions were excluded: gastrointestinal cancer, chronic gastrointestinal diseases, acute gastrointestinal disease with positive fecal occult blood; or confusion; <18 years of age; pregnant or lactating women.
Patient removal criteria
Patients who died in the hospital within 24 hours of admission were removed from the study as they received no drugs due to the rapid progress of the disease. Patients who quit the study or discharged on their own were also removed.
Grouping
Patients who met the inclusion criteria were randomized into either the Tongfu powder group or the mosapride group using the random table. The study participants were unblinded because the 2 groups had different delivery methods.
Treatment and assessment
Treatment
General treatment
All patients were treated in accordance with the guidelines for AP.18
The Tongfu powder group
Patients in this group were treated with Tongfu powder via umbilical compress therapy. The Tongfu powder was made of raw rhubarb (Polygonaceae), Magnolia officinalis (Magnoliaceae Juss), and aurantii (Citrus L.). Every 1 g of Tongfu powder was mixed with 1 mL Huoxiang Zhengqi Liquid (Beijing Tongrentang Pharmaceutical Co., Ltd., Beijing, People’s Republic of China); then, the mixture was applied in patients’ navel covered with a Shexiang Zhuanggu plaster (Henan Lingrui Pharmaceutical Co., Ltd., Xinyang, People’s Republic of China).
The powder and plaster were changed every 24 hours and the treatment lasted for 7 days.
The mosapride group
Patients in this group were given 5 mg mosapride (Sumitomo Pharmaceutical Co., Osaka, Japan; packaged by Suzhou Ltd. Co., Suzhou, People’s Republic of China). The China State Food and Drug Administration approval number was J20110022. It was administered orally or via gastric tube 3 times a day for 7 days.
Observational indicators: symptoms, signs, and scores
The endpoint of the study was death at 3 months. Data on days 1, 3, and 7 during hospitalization were recorded, including symptoms (abdominal pain, distention, hematemesis, hematochezia, the number of independent defecations, and digestive function), signs (tenderness, rebound tenderness, muscle tension, number of bowel sounds), and scores (Acute Physiology and Chronic Health Evaluation II score and the gastrointestinal function score used for MODS diagnostic criteria). The Chinese syndrome score, Ranson score, and computed tomography grade were obtained on days 1 and 7 of hospitalization.
Biochemical indicators
Biochemical indicators were measured with relative test kits, including interleukin (IL)-2, IL-6, intestinal fatty acid binding protein (IFABP), diamine oxidase (DAO), D-lactic acid (DLA), endotoxin, vasoactive intestinal peptide (VIP), IL-4, motilin, and cholecystokinin (CCK).
Curative effects
Indicators for curative effects were recorded, including the time to first defecation, bowel sound recovery time, adverse reactions, number and type of organ failure, complications, and hospitalization costs and duration.
Statistical analyses
All statistical analyses were performed using SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA). Continuous variables and categorical variables were summarized as mean ± standard deviation or frequencies (and percentages). Comparisons were performed using the independent sample t-test for normally distributed variables and nonparametric test (ie, signed rank test) for nonnormally distributed variables. A P<0.05 was considered statistically significant.
Results
General information
A total of 102 AP patients were enrolled. Six patients were excluded due to nonparticipation; 96 cases were chosen for further analysis in this study, with 50 cases in the Tongfu powder group and 46 cases in the mosapride group. There were no significant differences in sex, age, etiology, and Acute Physiology and Chronic Health Evaluation II score, MODS score, and gastrointestinal function score on day 1 between the 2 groups (P>0.05, Table 1). AP patients underwent stratified analysis according to the classification of AP, including 40 MAP cases, 56 MSAP cases, and no SAP case. There were also no significant differences in sex, age, etiology, and APACHE II score, MODS score, and gastrointestinal function score on day 1 between the Tongfu powder and the mosapride group regarding MAP and MSAP classification (all P>0.05, Tables S1 and S2).
The incidence of organ failure in AP patients
Among 96 AP patients, 19 patients developed organ failure when admitted, including liver failure (11 cases), gastrointestinal failure (15 cases), and respiratory failure (4 cases). No patient developed circulation, kidney, brain, and coagulation function and metabolism failure. Additionally, 3 patients developed 2-organ failure and 1 patient developed 3-organ failure.
Changes of gastrointestinal score in AP patients before and after treatment
The gastrointestinal score gradually decreased after treatment in both the mosapride group and the Tongfu powder group (Table 2). The score on day 7 was significantly lower than that on day 1 (all P<0.05, Table 2).
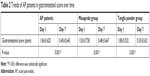  | Table 2 Trends of AP patients in gastrointestinal score over time |
The comparison of gastrointestinal dysfunction treatment effect in the 2 groups
As is shown in Table 3, all AP patients in the Tongfu powder group showed shorter time to first defecation and bowel sound recovery than that in the mosapride group, and the difference was statistically significant (all P<0.05). MAP patients in the Tongfu powder group also had a shorter time to first defecation than that in the mosapride group, and the difference was statistically significant (P<0.05). MSAP patients in the Tongfu powder group had a shorter time to bowel sound recovery than the mosapride group, and the difference was statistically significant (P<0.05). There was no significant difference among AP, MAP, and MSAP patients in improved gastrointestinal function (gastrointestinal score at day 1 subtracting score at day 7, Table 3).
The comparison of biochemical indicators in the 2 groups
The changes of biochemical indicators were evaluated using value on day 1 subtracted from value on day 7 (including IL-2, IL-6, IFABP, DAO, DLA, endotoxin, and VIP) or value on day 7 subtracted from that on day 1 (including IL-4, motilin, and CCK). Among these indicators, the improvements of IL-2, IL-4, IFABP, motilin, and VIP in the Tongfu powder group were significantly higher than those in the mosapride group (P<0.05, Table 4).
The comparison of TCM syndromes scores in the 2 groups
Patients were evaluated by TCM syndrome scores after treatment; the results in both groups were decreased compared to pretreatment (all P<0.05, Table S3), but there was no significant difference in changes in scores between the 2 groups (P>0.05, Table S4).
The comparison of hospital cost in the 2 groups
As is shown in Table 5, there were no significant differences in hospital cost and duration between the 2 groups. However, MAP patients in the Tongfu powder group spent significantly less time and cost compared to those in the mosapride group. There were no significant differences for MASP patients in hospital cost and duration (Table 5).
The comparison of clinical outcomes in the 2 groups
There was no significant difference in the incidence of organ failure, operation rate, and mortality rate after treatment between the 2 groups (Table S5).
The comparison of adverse reactions
Two cases of 50 AP patients in the Tongfu powder group developed umbilical red rash, which disappeared when patients stopped using Tongfu powder. The incidence of adverse reactions was 4% in the Tongfu powder group. One case out of the 46 AP patients in the mosapride group developed diarrhea, which disappeared when medication was discontinued. The incidence of adverse reactions was 2.8% in the mosapride group. The difference in the incidence of adverse reactions was not statistically significant between the 2 groups.
Discussion
To the best of our knowledge, our clinical trial represents the first effort to investigate the effect of TCM external therapy on gastrointestinal dysfunction in AP patients. We found that the Tongfu power group showed shorter time to first defecation and bowel sound recovery than the mosapride group. The improvements of IL-2, IL-4, IFABP, motilin, and VIP in the Tongfu powder group were significantly higher than those in the mosapride group. However, there were no significant differences in changes in the gastrointestinal function scores and TCM syndrome, hospital cost and duration, and rates of adverse reaction between the 2 groups. Although the findings require validations in multicenter studies with a larger sample size, they provide important empirical evidence for AP therapy.
With the 2 groups showing some differences in time to first defecation and bowel sound recovery, they did not, however, differ in changes in gastrointestinal function scores, indicating that Tongfu powder and mosapride perform similarly in improving gastrointestinal function. A comprehensive and accurate assessment of gastrointestinal function would help early detection of the occurrence of SIRS MODS. However, because gastrointestinal function involves many aspects, including food delivery function, digestion and absorption function, and immune function,19,20 evaluation of gastrointestinal function becomes complicated and, to date, no consistent standard methods are available. Methods in clinical setting mainly include scores of the clinical manifestations of gastrointestinal function, gastrointestinal permeability detection, intestinal mucosal tissue, morphological changes of Cajal cells, and flora shift detection. Considering the limitations in ethics and experimental techniques, we used the time to bowel sound recovery and first defecation and the gastrointestinal function score used for MODS diagnostic criteria as indicators of gastrointestinal function, and they are predictive for improvements and prognosis for AP patients.21,22
Currently, treatments of gastrointestinal dysfunction with Western medicine are mainly through increasing gastrointestinal motility using gastrointestinal motility drugs, regulating intestinal flora by microecological agents, and repairing intestinal mucosa by intestinal nutrition, but the effects are limited.23–25 A variety of TCM combined with Western medicine treatments can improve gastrointestinal function, including the use of Tongfu powder developed by Zhao et al.26 Tongfu powder was made using raw rhubarb and Citrus aurantium, which are ground into a powder. Clinical observations suggest that Tongfu powder could improve gastrointestinal function in AP patients, but no experimental studies have been conducted to evaluate its clinical efficacy. In this clinical trial, the control group was treated with the third-generation gastrointestinal motility drug, mosapride. Mosapride indirectly increases the release of acetylcholine in nerve endings by stimulating the 5-HT4 receptor of the motor ganglion neurons in the intestinal nervous system,27 promotes gastric emptying, and has a certain effect on increasing peristalsis and contraction of gastrointestinal smooth muscle. In this study, we found that the gastrointestinal function score after treatment in either the Tongfu powder group or the mosapride group showed a decreasing trend, and the score on the seventh day was significantly reduced compared to that on the first day, suggesting the 2 treatment methods can reduce gastrointestinal score.
In the study, the biochemical indicators measured included IFABP, DAO, DLA, endotoxin, inflammatory factors (IL-2, IL-4, and IL-6), and gastrointestinal hormones (motilin, CCK, and VIP). The improvements of IL-2, IL-4, IFABP, motilin, and VIP in the Tongfu powder group were significantly higher than those in the mosapride group. This finding suggests the following: 1) Tongfu powder can reduce the level of proinflammatory cytokines (IL-2) and increase the level of anti-inflammatory factors (IL-4) to block the development of SIRS, thereby reducing the occurrence of MODS. 2) IFABP, located in the intestinal mucosa, is released into the circulation when ischemia occurs, resulting in increased level of blood IFABP. Therefore, IFABP can reflect intestinal mucosal permeability, and also be considered as a critical indicator of early gastrointestinal failure in critically ill patients.28,29 In this study, the improvement of IFABP in the Tongfu powder group was significantly higher than that in the mosapride group, indicating that Tongfu powder could improve intestinal mucosal permeability of AP patients. 3) Motilin and VIP are gastrointestinal hormones, and motilin is a stimulatory hormone that can accelerate gastric emptying, and VIP is an inhibitory hormone that can relax the digestive tract smooth muscle and slow down gastric emptying. The Tongfu powder group had significant improvements for motilin and VIP compared with the mosapride group, which suggests that Tongfu powder may improve gastrointestinal motility through the regulation of gastrointestinal hormone levels. No significant changes were observed in IL-6, DAO, DLA, endotoxin, and CCK, which could be explained by the following reasons: 1) mosapride in the control group in this study may have a certain effect on the improvement of gastrointestinal function, leading to the nonsignificant difference; 2) the mechanism underlying the effect of Tongfu powder is complex, with other ways available to improve gastrointestinal function.
The treatment method of Tongfu powder is easy to perform in a clinical setting and it only targets the umbilicus, which is easy for patients to accept with good compliance. For MAP patients who received Tongfu powder, the hospital cost and duration decreased, compared to those who received mosapride, resulting in a lower burden for the patients and their families. Furthermore, only 2 patients presented localized red rash in umbilical region, and both recovered after stopping medications. The incidence of adverse reaction was low and easy to deal with.
Admittedly, there were several limitations in our study. First, our sample size was limited due to resources. A larger population is needed to validate our findings in multicenter studies. We suggest that SAP might be included for comprehensive evaluation of the effect of Tongfu powder on gastrointestinal dysfunction. Second, longer follow-ups are needed to observe outcomes. Third, a comparison between oral treatment and topical therapy might be required when interpreting the findings.
Conclusion
In summary, the present study is the first to demonstrate that Tongfu powder may improve gastrointestinal function for AP patients compared with mosapride. Validations in multicenter studies with a larger sample size are warranted for further evaluation. Nonetheless, our study provides important additional evidence for the therapy of AP.
Acknowledgment
This study was supported by the National Science Foundation of China (No 81774045).
Disclosure
The authors report no conflicts of interest in this work.
References
Otsuki M, Takeda K, Matsuno S, et al. Criteria for the diagnosis and severity stratification of acute pancreatitis. World J Gastroenterol. 2013;19(35):5798–5805. | ||
Qiang Z. [The clinical observation of acute pancreatitis with gastroenterology dysfunction (83 cases)]. Jiiang Xi Med Pharmacol. 2008;43(2):140–141. Chinese. | ||
Bing T, Ling L, Junchao W. [Severe acute pancreatitis with multiple organ dysfunction (32 cases)]. Huaxi Med. 2006;21(3):560–561. Chinese. | ||
Wu L, Sankaran S, Plank L, Windsor J, Petrov M. Meta–analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101(13):1644–1656. | ||
Vege SS, Gardner TB, Chari ST, et al. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis.” Am J Gastroenterol. 2009;104(3):710–715. | ||
Besselink MG, van Santvoort HC, Renooij W, et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann Surg. 2009;250(5):712–719. | ||
Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139(3):813–820. | ||
Guo Q, Li A, Xia Q, et al. The role of organ failure and infection in necrotizing pancreatitis: a prospective study. Ann Surg. 2014;259(6):1201–1207. | ||
Capurso G, Zerboni G, Signoretti M, et al. Role of the gut barrier in acute pancreatitis. J Clin Gastroenterol. 2012;46:S46–S51. | ||
Al Mofleh IA. Severe acute pancreatitis: pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14(5):675–684. | ||
Chen H, Li F, Jia JG, Diao YP, Li ZX, Sun JB. Effects of traditional Chinese medicine on intestinal mucosal permeability in early phase of severe acute pancreatitis. Chin Med J. 2010;123(12):1537–1542. | ||
Cheng Y, Wang M, Cheng X. [Effect of dachaihu decoction in treating acute mild pancreatitis of Gan-qi stagnant type]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28(9):793–796. Chinese. | ||
Xue P, Deng L, Zhang Z, et al. [Chaiqin Chengqi Decoction decreases pancreatic acinar cell calcium overload in rats with acute pancreatitis]. Zhong Xi Yi Jie He Xue Bao. 2008;6(10):1054–1058. Chinese. | ||
Zhang MJ, Zhang GL, Yuan WB, Ni J, Huang LF. Treatment of abdominal compartment syndrome in severe acute pancreatitis patients with traditional Chinese medicine. World J Gastroenterol. 2008;14(22):3574–3578. | ||
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis – 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. | ||
Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. | ||
Wang C, Su Q, Zhang SW, Yin CH, Wang H, Wang BE. [Scoring system to measure the severity of multiple organ dysfunction syndrome]. Acta Acad Med Sin. 2007;29(4):497–500. Chinese. | ||
Xingpeng W, Guoming X, Yaozong Y, Zhaoshen L. [Diagnosis and treatment guidelines for acute pancreatitis in China]. Med J Chin People’s Liberation Army. 2004;29(7):646–648. Chinese. | ||
Schneeman B. Food factors and gastrointestinal function: a critical interface. Biofactors. 2004;21(1–4):85–88. | ||
Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol. 2008;22(3):391–409. | ||
Massey RL. Return of bowel sounds indicating an end of postoperative ileus: is it time to cease this long-standing nursing tradition? Medsurg Nurs. 2012;21(3):146–150. | ||
Wang C, Su Q, Zhang SW, Yin CH, Wang H, Wang BE. [A clinical study on the diagnostic criteria of multiple organ dysfunction syndrome]. Zhonghua Wai Ke Za Zhi. 2009;47(1):40–43. Chinese. | ||
Greiff JM, Rowbotham D. Pharmacokinetic drug interactions with gastrointestinal motility modifying agents. Clin Pharmacokinet. 1994;27(6):447–461. | ||
Bischoff SC. “Gut health”: a new objective in medicine? BMC Med. 2011;9:24. | ||
Guzman JR, Conlin VS, Jobin C. Diet, microbiome, and the intestinal epithelium: an essential triumvirate? Biomed Res Int. 2013;2013:425146. | ||
Zhao S, Zhang S, Wang B. Clinical application of Tongfu method in acute infectious diseases in internal medicine. Chin J of Integr Tradit Western Med. 1982;2:90–91. | ||
Makimoto N, Sakurai-Yamashita Y, Furuichi A, et al. In vivo assessment of acceleration of motor activity associated with acetylcholine release via 5-hydroxytryptamine4 receptor in dog intestine. Jpn J Pharmacol. 2002;90(1):28–35. | ||
Ludewig S, Jarbouh R, Ardelt M, et al. Bowel ischemia in ICU patients: diagnostic value of I-FABP depends on the interval to the triggering event. Gastroenterol Res Pract. 2017;2017:2795176. | ||
Li H, Chen Y, Huo F, Wang Y, Zhang D. Association between acute gastrointestinal injury and biomarkers of intestinal barrier function in critically ill patients. BMC Gastroenterol. 2017;17(1):45. |
Supplementary materials
  | Table S5 Clinical outcomes of AP patients by randomizations |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.