Back to Journals » Clinical Ophthalmology » Volume 16
Efficacy and Safety of Temporary in situ Stenting of Ahmed Glaucoma Valve in Eyes with High Risk of Hypotony
Authors Al Houssien AO , Al Owaifeer AM, Ahmad SI, Owaidhah O, Malik R
Received 27 July 2022
Accepted for publication 11 October 2022
Published 9 November 2022 Volume 2022:16 Pages 3689—3700
DOI https://doi.org/10.2147/OPTH.S383489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Abdullah Omar Al Houssien,1 Adi Mohammed Al Owaifeer,2,3 Sameer I Ahmad,4– 6 Ohoud Owaidhah,7 Rizwan Malik8
1Fellowship and Residency Training Program, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 2Research Department, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 3Department of Ophthalmology, College of Medicine, King Faisal University, Al Hasa, Saudi Arabia; 4Department of Ophthalmology, Howard University Hospital, Washington, DC, USA; 5Department of Ophthalmology and Visual Sciences, University of Maryland School of Medicine, Baltimore, MD, USA; 6Glaucoma Consultants of Washington, Herndon, VA, USA; 7Glaucoma Division, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 8Department of Ophthalmology, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates
Correspondence: Abdullah Omar Al Houssien, Fellowship and Residency Training Program, King Khaled Eye Specialist Hospital, 2775 Al Urobah Road, Umm Al Hamam Al Gharbi, Riyadh, 12329-8139, Saudi Arabia, Email [email protected]
Introduction and Objective: To describe a novel technique for providing external ligation of the Ahmed glaucoma valve (AGV) to prevent hypotony in eyes at high risk with a 4/0 nylon stent suture and report outcomes compared to ligation with an absorbable vicryl suture and no ligation in terms of efficacy and safety.
Methods: This was a retrospective cohort study investigating the efficacy and safety of in situ stenting compared to an absorbable ligature and the standard care, in high risk eyes, of hypotony. It included 116 patients; 34 in Group A (ligation + stent), 27 in Group B (ligation – stent), and 55 in Group C (no ligation).
Results: The mean age (in years) of the participants was 53.94± 19.01 in Group A, 44.85± 29.92 in Group B and 52.62± 24.47 in Group C, 59% (n = 20), 63% (n = 17) and 60% (n = 33) were males, respectively. The follow-up period was at least 6 months (Group A: 9.1± 4.2 months, Group B: 9.6± 3.4 months and Group C: 10.2± 6.4 months). The mean baseline Snellen VA (LogMAR) was 1.82± 1.34, 1.30± 0.98 and 1.34± 1.07 and the mean baseline IOP was 32.50± 9.48, 28.22± 7.12 and 28.33± 10.63 mmHg, in Groups A, B and C, respectively. The failure rates, by the Kaplan Meier Survival curve, were higher 27.3% in Group C (no ligation) compared to 20.6% in Group A (ligation + stent) and 18.5% in Group B (ligation – stent) yet not found to be statistically significant (p = 0.4; log rank test). There was lower hypotony 2.9% in Group A and lower complications 25.9% in Group B but no statistical significance was found amongst the groups.
Conclusion: In conclusion, temporary nylon in situ stenting of AGV had lower rates of hypotony. Furthermore, lower failure and complication rates were observed in vicryl only ligated AGV, then nylon in situ stented AGV and lastly in standard AGV controls.
Keywords: AGV, hypotony, ligation, stent, ligature
Introduction
Glaucoma drainage devices (GDD) are episcleral implants that are either valved or non-valved that transmit aqueous to the sub-Tenon’s space through a tube inserted into the anterior chamber (AC), ciliary sulcus or vitreous cavity.1,2 The valved devices include various models (FP7, FP8, S2, and S3) of the Ahmed glaucoma valve, AGV, (New World Medical, Inc, Rancho Cucamonga, CA).1
Although GDDs traditionally have been indicated in eyes with prior failed trabeculectomy with antimetabolite, severe conjunctival scarring, and secondary glaucomas like uveitic or neovascular, these devices can be used as primary filtration surgeries.2–5
In addition to corneal edema, complications of both valved and non-valved GDD include plate migration, tube exposure, tube occlusion and overfiltration with subsequent hypotony.1,6–8 The (non-valved) BGI had higher rates of chronic hypotony than the (valved) AGV in both of the Ahmed versus Baerveldt comparison (ABC) studies and the Ahmed versus Baerveldt (AVB) study.9,10 Although the risk of hypotony is lower for AGV,8,9 it still may occur, leading to further potential complications such as AC shallowing, choroidal effusions, and hypotony maculopathy.11 The early post-AGV complication rates were 19% for shallow AC, 15% for choroidal effusion and 3% hypotony maculopathy in the AVB study.12 In eyes at high risk of hypotony, such as aphakic eyes13 or eyes with compromised ciliary body function,14 temporary ligation or stenting of the AGV has been suggested as a way of reducing immediate postoperative hypotony.15–17 Studies, which have compared ligated (by sutures tied adjacent to or outside the tube with vicryl ligation) with non-ligated AGV tubes, have shown lower rates of initial hypotony and complications in the ligated group.15,16
In the previous literature in which AGV tubes were ligated, Kee15 had only 10 patients with glaucoma in their series and used an external 6–0 prolene suture. Another study by Lee16 was descriptive and did not include a comparison group without ligation. They used three external 8–0 nylon sutures for stenting.
In the current study, we described an alternative technique for tube ligation, which minimizes risk of early hypotony, yet allows a stent suture that is tightened to the tube to be removed in the post-operative period if any refractory IOP increase occurs.
Methods
Study Design
This is a retrospective study that was conducted to review patients in the period between January 2017 and December 2020 at the Glaucoma Clinics of King Khaled Eye Specialist Hospital. The study compared uncontrolled glaucomatous eyes with high risk of hypotony treated with AGV ligated with a 6–0 vicryl suture and a temporary external stent of 4–0 nylon suture (Group A), ligation with 6–0 vicryl without a temporary external stent (Group B), and standard AGV without any ligation (Group C).
Inclusion and Exclusion Criteria
All patients with uncontrolled glaucoma regardless of age in need of AGV who were deemed at risk of hypotony (see definition of high-risk eyes below) during the allocated period (mentioned above). Glaucoma patients with less than two postoperative follow up visits within six months of surgery were excluded from the study.
Definitions
High Risks Eyes for Hypotony
Eyes that were aphakic,18 pseudophakic with anterior chamber IOL or secondary sulcus IOL,19 with a history of prior cyclodestruction,20 cyclodialysis or ciliary body cleft,21,22 uveitic,15 vitrectomized,23,24 high myopia (more than −6 diopter or more than 26 millimeters axial length)25,26 or patients equal or older than 80 years of age. These factors were chosen as they have been reported to carry a higher risk of hypotony in the literature. Primary AGV was the surgery of choice for some eyes due to follow up concerns as many of the patients lived far away from our tertiary hospital so post-op management for trabeculectomy would have been difficult.
Failure
IOP that was greater than 21 mmHg or not reduced by 20% below baseline on two subsequent follow-ups after three months, IOP was equal or less than 5 mmHg on two subsequent follow ups after three months, loss of light perception vision or patients that required further glaucoma surgery (apart from tube trimming or stent removal). Conversely, ‘success’ was defined as IOP ≤ 21 mmHg with ≥20% reduction of IOP from baseline and without the need for further glaucoma surgery.
Hypotony
IOP that was equal or less than 5 mmHg at one setting with proven detrimental effect on the visual function and/or ocular structure such as retinal/choroidal folds, macular folds, optic nerve oedema, etc.
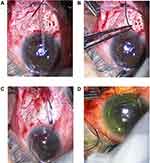
Surgical Technique (Figure 1)
After placing a 7–0 vicryl corneal traction suture to expose the desired quadrant, a fornix-based conjunctival flap dissection was made followed by cautery. Curved Stevens scissors were used to open a subconjunctival pocket in the desired quadrant (usually superotemporally). Then, AGV was primed with a balanced salt solution and the plate was inserted into the exposed quadrant and sutured to sclera with 9–0 nylon with anterior edge 8–9 mm posterior to limbus.
Stenting was achieved by placing a removable external stent (4–0 nylon suture) parallel to the tube. The tube was then tied with the stent suture in situ by a 6–0 vicryl suture. The other end of the stent was extended outside the AGV to be placed under the conjunctiva. (Figure 1D). If ligation was planned with no stent, then AGV is tightened with 6–0 vicryl sutures only.
A 23G-gauge needle was used to make a limbal stab incision, aiming for a 2 mm tunnel before entry to ensure a tight tube entry, then the tube was trimmed to an appropriate length and inserted into AC or sulcus as planned. For non-ligated AGVs, viscoelastic was placed in the eye (Provisc ophthalmic viscosurgical device, Alcon, Fort Worth, Texas), but not used for ligated tubes. The tube was sutured to episclera with 9–0 nylon and a patch graft (pericardium, cornea or sclera) is placed over and sutured with 10–0 nylon to cover the tube and entry site. Lastly, conjunctiva was sutured with 9–0 vicryl sutures and concluded with subconjunctival administration of cefazoline and dexamethasone. For unligated AGVs, viscoelastic was left inside the anterior chamber to minimize the risk of hypotony. The eye was treated with Maxitrol (Alcon Inc, Fort Worth, Texas) and 1% cyclopentolate ointment and patched.
Stent Removal
For Group A, the nylon stent was removed after the first two weeks if the IOP was deemed to be too high for the level of optic nerve damage; within the first two weeks, it was removed if the IOP was greater than 30 mmHg. For Group B, vicryl sutures were left to be resorbed (typically at 5–6 weeks post-operatively) any IOP spike during this time was managed medically.
Data Collection
Data were collected retrospectively from the medical records of the patients following the inclusion criteria in the specified timeframe above. Baseline demographic, preoperative clinical details, operative data and postoperative complication rates and interventions of all groups were collected. These data were retrieved from patients’ last pre-operative visit and three post-operative visits at 1 month, 3 months and 6 months (at least two post-operative visits within 6 months of the surgery). All data were entered into customized data collection forms then transferred to Excel sheets (Microsoft Excel 2013, Microsoft Inc, Redmond, WA).
Sample Size
Considering hypotony rate in partially AGV ligated tubes is estimated to be 6.3% and was 15% in the non-ligated tubes of AGVs respectively13 (95% CI, power 0.8) the calculated sample was estimated as 63 patients for each of the ligated and non-ligated groups.
Data Analysis
Baseline demographics were displayed as proportions for categorical data such as gender, mean and standard deviation for continuous Gaussian data. Proportions were compared using a chi-square or Fisher test as appropriate. Continuous data was compared with an unpaired T-test.
Visual acuity was converted to LogMar for the ease of analysis. Repeated VA and IOP measurements and the number of antiglaucoma medications over time were displayed with a boxplot to show the difference between the groups at each time point and to estimate an overall effect between the three groups. Survival analysis was performed using the above definitions of failure to compare success rates of the nylon ligated, vicryl ligated and non-ligated groups using a Kaplan Meier Survival Curve and survival probabilities compared using a Log rank test. Statistical significance was set at p<0.05. Data was analysed using STATA 16.1 (StataCorp LLC, College Station, TX, USA).
Ethical Considerations
The study follows the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of the Research Center of King Khaled Eye Specialist Hospital.
Informed consent documentation was waived by the IRB as the study was retrospective in nature that carried no risk to participants, no adverse effect to the rights and welfare of the participants, and assured the participants confidentiality.
Results
The study included 116 eyes, with 34 eyes in Group A (ligation with stent suture), 27 eyes in Group B (ligation only, no stent) and 55 eyes in Group C (no ligation). The follow periods were at 1 month, 3 months and 6 months (Group A: 9.1±4.2 months, Group B: 9.6±3.4 months and Group C: 10.2±6.4 months).
The baseline characteristics and diagnosis of all groups are shown in Table 1. In the postoperative period (first 3 months), 12 patients (35.3%) had high IOP spikes in Group A (ligation + stent). All the 12 patients had their IOP controlled with 9 patients by stent removal (7 within 1 month and 2 within 3 months) and 3 patients by topical antiglaucoma drops. No case of hypotony was recorded after stent removal. For Group B (ligation without stent), 11 patients (44.4%) had early postoperative high IOP spikes managed medically with 3 patients failed as they needed further glaucoma surgery and 1 patient failed for persistently high IOP. For the early postoperative high IOP spikes in Group C (no ligation), 18 patients (32.7%) were identified with 9 responding to antiglaucoma medications, 7 failed due to persistent uncontrolled IOP and 2 needed further surgeries for tube blockage (manual cutting of iris and vitreous tissue and intracameral TPA for blood clot).
 |
Table 1 Baseline Characteristics of 116 Patients Underwent AGV |
The changes of VA, IOP and number of antiglaucoma drops over time in each group are presented in box-plot charts shown in Figures 2–4respectively. The means ± SD of VA (LogMAR) for Groups A, B and C were 1.82 ± 1.33, 1.30 ± 0.98 and 1.34 ± 1.07 at baseline and 2.13 ± 2.47, 1.49 ± 1.49 and 1.44 ± 1.38 at 6 months, respectively. IOP levels (mmHg) for Groups A, B and C were at baseline 32.50 ± 9.48 mmHg, 28.22 ± 7.12 mmHg and 28.34 ± 10.63 mmHg (means ± SD) and 14.83 ± 4.35 mmHg, 16.57 ± 6.19 mmHg and 17.20 ± 8.02 mmHg at 6 months (mean ± SD), respectively. Also, numbers of medications for Groups A, B and C were at baseline 3.41 ± 0.70, 3.44 ± 0.97 and 3.96 ± 0.77 (mean ± SD) and 1.70 ± 1.29, 1.89 ± 1.22 and 1.48 ± 1.22 at 6 months (mean ± SD), respectively. When comparing IOP levels in the first postoperative visit using One-way ANOVA, no statistically significant difference was found with respect to all groups. Group C (no ligation) had a significantly lower number of anti-glaucoma medications in the early postoperative period compared to other groups using Fisher test (p = 0.0005).
 |
Figure 2 (Box Plot Chart). Visual acuity at baseline and follow-ups in the three groups. |
 |
Figure 3 (Box Plot Chart). Intraocular pressure at baseline and follow-ups in the three groups. |
 |
Figure 4 (Box Plot Chart). Number of medications at baseline and follow-ups in the three groups. |
The cumulative survival is illustrated in the Kaplan Meier Survival curve shown in Figure 5. The success rates were 79.4% for Group A, 81.5% for Group B and 72.7% for Group C. The failure was higher in Group C than the other two groups. The log rank test was performed to compare the difference in survival (Group A vs Group C, Group B vs Group C and Group A+B vs Group C) which gave p-values of p = 0.4, p = 0.4 and p = 1.0 respectively indicating that the groups did not differ significantly in survival.
 |
Figure 5 (Kaplan Meier Survival Curve). Survival rate amongst all groups in the follow-ups. |
Causes of failure (as defined above) are presented in frequencies and proportions amongst all groups. Inadequate IOP control was the main cause of failure in Group A 14.7% (n = 5) and Group C 23.6% (n = 13) whereas reoperation was the cause in Group B 11.1% (n = 3). Early postoperative and late postoperative complications are shown in Table 2 (Fisher test was used to compare early, late, and overall complications amongst the groups). Early postoperative complications were 26.5%, 25.9% and 21.8% and late postoperative complications were 20.6%, 0% and 18.2% in Group A, Group B and Group C, respectively. Early hypotony rates were 2.9%, 7.4% and 7.3% and late hypotony rates were 0%, 0% and 3.6% in Group A, Group B and Group C, respectively. No statistical significance was found comparing early, late and overall hypotony rates amongst the groups, although there was a tendency for early hypotony to be lower in Group A.
 |
Table 2 Complications in the Three Groups |
In Group A, 3 patients needed revision (tube trimming, tube ligation and anterior vitrectomy) and 1 patient needed additional surgery (PPV). No patients needed revision and 3 patients needed additional surgery (PPV, AC washout and CPC) in Group B. Only one patient needed intervention, which was a TPA injection, in Group C.
Discussion
Early post-AGV hypotony can occur despite the implant being coupled with a valve-like mechanism.8–10 This complication is more common in certain eyes that are at a higher risk of hypotony and these patients need careful operative and postoperative considerations to avoid this risk and its subsequent complications. Tube ligation is an intraoperative measure that may reduce the risk of early postoperative hypotony but at the expense of increased postoperative IOP. The use of an in situ nylon suture with external vicryl ligation is a further surgical modification that allows for more control in the postoperative period since it allows for immediate filtration as the external suture can be removed at the slit lamp at any time without the use of a laser or return to the operating room.
In our study, the early hypotony rates (<3 months) in Groups A, B, and C were 2.9%, 7.4%, and 7.3% respectively. The rate of hypotony in Group A is lower than that reported in previous studies of patients undergoing AGV surgery with either a nylon (8.4%) or a prolene stent (6.3%).15,16 In unligated AGV, viscoelastic is routinely left in the anterior chamber to minimize post-operative hypotony, but hypotony can still develop in a third of eyes.27 Tube ligation can be achieved by using a stent suture as in the present study, with vicryl ligation only or with prolene suture ligation in the anterior chamber.17 In general, both the subconjunctival nylon stent (direct removal with forceps) and intracameral prolene suture (suture lysis using argon laser) can be manipulated at any time during the postoperative period when an undesirable level of IOP is encountered despite medical therapy. In our opinion the external nylon suture has some advantages over prolene suture occlusion in the anterior chamber. Firstly, the nylon stent suture technique can be used in cases where the cornea is opaque where an adequate view for suture lysis may not be possible. Secondly, the stent suture can be easier to remove in younger children or those with nystagmus where laser suture lysis may be difficult. Lastly, not all facilities may have convenient access to an argon or diode laser for postoperative clinic patients. No cases of late hypotony (>3 months) were encountered in the ligated cases of our study (both groups A and B). This contrasts with a reported rate of 2% in previous studies.16,26 Late hypotony can be caused by ciliary body shutdown, overfiltration with lack of adequate encapsulation, wound leak or implant exposure and are not likely related to the surgery itself.
The benefit of using the external stent occlusion technique is to provide more surgeon control over IOP and to allow for stent removal at any time during the post-operative period, especially in patients with advanced glaucoma that may not be able to tolerate prolonged increases in IOP. We did not find a significant difference in the mean IOP between the groups (A, B and C) during the first postoperative visit indicating that ligation did not present an added risk of immediate postoperative IOP elevation in our patient population (17.53±8.48 vs 19.92±10.60 vs 17.74±8.40 mmHg, respectively). These results may be expected due to ligation being performed in patients at higher risk of ciliary body shut down. Through the early postoperative period, 35.3% had high IOP spikes in Group A and all of them had their IOP controlled with 9 patients by stent removal and 3 patients by topical antiglaucoma drops. In contrast, 44.4% had early postoperative high IOP spikes and 4 of them failed to be controlled in Group B and 32.7% had IOP spikes with 9 not responding to treatment in Group C. Having 32.7% of Group C with IOP spikes suggests that other mechanisms were involved in the high postoperative IOP as the “hypertensive phase” which is defined as IOP ≥22 mmHg within 3 months of the procedure (not caused by tube obstruction, retraction or dysfunction) after initial reduction to <22 mmHg in the first postoperative week. A hypertensive phase is known to occur in around half of eyes with unligated AGVs, possibly due to inflammation and fibrosis over the plate bleb and may lead to less favorable outcomes.28,29 In addition, the mean number of glaucoma medications was similar among all groups (A, B and C) at first visits (1.53±1.26 vs 2.2±1.58 vs 0.51±1.1, respectively). Al Owaifeer et al17 found the difference in mean IOP between ligated (either a polypropylene 9–0 or a polyglactin 7–0 suture) and nonligated groups was comparable throughout the study except for the first month (at 1–2 months: 17.3 ± 5.6 vs 17.9 ± 8.1 mmHg) with a greater number of glaucoma medications needed for the ligated group throughout the study which was similar to our findings. Also, Lee et al16 in their one-armed study had higher mean IOP and comparable number of glaucoma medications at 1 month (19.9±8 mmHg and 1.5±1.2 medications) in their 3 external nylon stenting technique compared to the current study.
In the final visits, the mean IOP amongst the groups (A, B and C) were 14.83±4.35 mmHg, 16.29±6.19 and 17.20±8.02 mmHg and the number of glaucoma medications were 1.7±1.29 vs 2±1.19 vs 1.48±1.22, respectively. Lee et al16 had comparable but higher mean IOP (15.8±5.8 mmHg) and lower number of medications (1.5±1.2) in the non-comparable nylon cases as compared to the nylon group in the current study. Kee et al15 had similar mean IOP between the ligated and nonligated groups which differs from our study. The mean IOP at 6 months was 19.6±5.5 mmHg for the partial prolene external stenting and 19.2±6.3 mmHg for the nonligated group.
Long-term IOP control was comparable among all patients in our study irrespective of surgical technique. Group A (the nylon group) had a higher reduction in mean IOP, however, it is important to note that patients in this group had the highest baseline mean IOP too (32.50±9.48 vs 27.52±6.93 vs 28.34±10.63). Though the difference in mean IOP between the groups was comparable throughout the follow-up period, patients in Group A had the lowest mean IOP at the last postoperative visit (14.83±4.35, 16.29±6.19 and 17.20±8.02 mmHg at last visit).
The visual outcomes were found to be comparable between the nylon, vicryl and no ligation groups. The mean changes between LogMAR from baseline to last exam were −0.31±1.13, −0.19±0.51 and −0.1±0.3, respectively. The nylon group had higher mean change and this might be due to this group had the worst vision upon presentation (1.82±1.34 vs 1.30±0.98 vs 1.34±1.07).
Early postoperative complications of Group A were comparable to the literature. We found that IOP spike rate was 35.3%, hypotony was 2.9%, hyphema was 5.9% and tube blockage was 5.9% in Group A. Osman et al found that IOP spike rate was 21.4%, hypotony was 1.7%, hyphema was 6% and tube blockage was 1.7%.30
The limitations of our study include its retrospective nature and possible selection bias (eyes at higher risk are more likely to undergo ligation). However, we attempted to overcome this by only including eyes at high risk for hypotony.
In conclusion, failure and hypotony rates were similar amongst the groups though a tendency was observed in the nylon in situ stented AGV group to have a lower early postoperative hypotony rate. So, our recommendation is to use the nylon stent technique to minimize the risk of early hypotony yet allow the stent suture to be removed post-operatively if any refractory IOP increase occurs. Prospective studies are needed to confirm the findings of the current study and investigate the effect of ligation on long-term IOP.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Glaucoma. Basic and Clinical Science Course. San Francisco: American Academy of Ophthalmology; 2016.
2. Gedde SJ, Chen PP, Heuer DK, et al.; Primary Tube Versus Trabeculectomy Study Group. The Primary Tube Versus Trabeculectomy Study: methodology of a Multicenter Randomized Clinical Trial Comparing Tube Shunt Surgery and Trabeculectomy with Mitomycin C. Ophthalmology. 2018;125(5):774–781. doi:10.1016/j.ophtha.2017.10.037
3. Gedde SJ, Feuer WJ, Shi W, et al.; Primary Tube Versus Trabeculectomy Study Group. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 1 Year of Follow-up. Ophthalmology. 2018;125(5):650–663. doi:10.1016/j.ophtha.2018.02.003
4. Gedde SJ, Feuer WJ, Lim KS, et al.; Primary Tube Versus Trabeculectomy Study Group. Treatment Outcomes in the Primary Tube Versus Trabeculectomy Study after 3 Years of Follow-up. Ophthalmology. 2020;127(3):333–345. doi:10.1016/j.ophtha.2019.10.002
5. Gedde SJ, Feuer WJ, Chen PP, Heuer DK, Singh K, Wright MM. Tube Versus Trabeculectomy and Primary Tube Versus Trabeculectomy Study Groups. Comparing Treatment Outcomes from the Tube Versus Trabeculectomy and Primary Tube Versus Trabeculectomy Studies. Ophthalmology. 2021;128(2):324–326. doi:10.1016/j.ophtha.2020.06.059
6. Kanski J, Bowling B. Kanski’s Clinical Ophthalmology. Edinburgh: Elsevier; 2016.
7. Allingham RR, Damji K, Freedman S, et al. Shields’ Textbook of Glaucoma. Philadelphia: Lippincott Williams & Willkins; 2005.
8. Wang J, Barton K. Aqueous shunt implantation in glaucoma. Taiwan J Ophthalmol. 2017;7(3):130–137. doi:10.4103/tjo.tjo_35_17
9. Barton K, Gedde SJ, Budenz DL, Feuer WJ, Schiffman J. The Ahmed Baerveldt comparison study: methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011;118(3):435–442. doi:10.1016/j.ophtha.2010.07.015
10. Christakis PG, Tsai JC, Zurakowski D, Kalenak JW, Cantor LB, Ahmed II. The Ahmed versus Baerveldt study: design, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011;118(11):2172–2179. doi:10.1016/j.ophtha.2011.05.003
11. Riva I, Roberti G, Oddone F, Konstas A, Quaranta L. Ahmed glaucoma valve implant: surgical technique and complications. Clin Ophthalmol. 2017;11:357–367. doi:10.2147/OPTH.S104220
12. Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt comparison study after one year of follow-up. Ophthalmology. 2011;118(3):443–452. doi:10.1016/j.ophtha.2010.07.016
13. Jeganathan VS, Ghosh S, Ruddle JB, Gupta V, Coote MA, Crowston JG. Risk factors for delayed suprachoroidal haemorrhage following glaucoma surgery. Br J Ophthalmol. 2008;92(10):1393–1396. doi:10.1136/bjo.2008.141689
14. Rosentreter A, Gaki S, Lappas A, Cursiefen C, Dietlein TS. Previous cyclodestruction is a risk factor for late-onset hypotony and suprachoroidal haemorrhage after glaucoma drainage device surgery. Br J Ophthalmol. 2013;97(6):715–719. doi:10.1136/bjophthalmol-2012-302351
15. Kee C. Prevention of early postoperative hypotony by partial ligation of silicone tube in Ahmed Glaucoma Valve implantation. J Glaucoma. 2001;10(6):466–469. doi:10.1097/00061198-200112000-00005
16. Lee JJ, Park KH, Kim DM, Kim TW. Clinical outcomes of Ahmed Glaucoma Valve implantation using tube ligation and removable external stents. Korean J Ophthalmol. 2009;23(2):86–92. doi:10.3341/kjo.2009.23.2.86
17. Al Owaifeer AM, Alobaida I, Alrefaie S, Malik R, Aljadaan I. The Effect of Tube Ligature on the Safety and Efficacy of Ahmed Glaucoma Valve Surgery. J Glaucoma. 2020;29(12):1173–1178. doi:10.1097/IJG.0000000000001661
18. Thanapaisal S, Wu J, Han Y. Risk of Hypotony in Uveitis Patients after Ahmed Tube Implantation. Invest Ophthalmol Vis Sci. 2020;61(7):969.
19. Vounotrypidis E, Schuster I, Mackert MJ, et al. Secondary intraocular lens implantation: a large retrospective analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257:125–134. doi:10.1007/s00417-018-4178-3
20. Rosentreter A, Gaki S, Lappas A, Cursiefen C, Dietlein TS. Previous cyclodestruction is a risk factor for late-onset hypotony and suprachoroidal haemorrhage after glaucoma drainage device surgery. Br J Ophthalmol. 2013;97(6):715. doi:10.1136/bjophthalmol-2012-302351
21. Fine HF, Biscette O, Chang S, Schiff WM. Ocular hypotony: a review. Compr Ophthalmol Update. 2007;8(1):29–37.
22. Janson BJ, Kam JP, Alward WLM Hypotony: late hypotony from trabeculectomy and Ahmed seton with resulting hypotony maculopathy; 2017; Available from: http://EyeRounds.org/cases/250-Hypotony.htm.
23. Yamane S, Inoue M, Arakawa A, Kadonosono K. Early postoperative hypotony and ciliochoroidal detachment after microincision vitrectomy surgery. Am J Ophthalmol. 2012;153(6):1099–103.e1. doi:10.1016/j.ajo.2011.11.001
24. Bamonte G, Mura M, Stevie Tan H. Hypotony after 25-gauge vitrectomy. Am J Ophthalmol. 2011;151(1):156–160. doi:10.1016/j.ajo.2010.06.042
25. Park HY, Lee NY, Park CK. Risk factors of shallow anterior chamber other than hypotony after Ahmed glaucoma valve implant. J Glaucoma. 2009;18(1):44–48. doi:10.1097/IJG.0b013e31816b2fe7
26. Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003;110(6):1185–1191. doi:10.1016/S0161-6420(03)00227-6
27. Kaderli A, Demirok G, Üney G, Yakın M, Günal B, Ekşioğlu Ü. Assessing risk factors for postoperative hypotony in Ahmed glaucoma valve implantation surgery. Int Ophthalmol. 2021;41(10):3381–3386. doi:10.1007/s10792-021-01900-3
28. Gonçalves ASL, Roque J, Vendrell C, Lisboa M, Vaz FT, Prieto I. Evaluation and management of the hypertensive phase following Ahmed glaucoma valve implantation. OftalSPO. 2019;43(3). doi:10.48560/rspo.17234
29. Nouri-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed Glaucoma Valve. Am J Ophthalmol. 2003;136(6):1001–1008. doi:10.1016/s0002-9394(03)00630-5
30. Osman EA, Alkheraiji NF, Abouammoh MA, Mousa A, Al-Obeidan S. Safety and efficacy of ahmed valve on intractable glaucoma in Saudi population. Middle East Afr J Ophthalmol. 2020;27:40–46. doi:10.4103/meajo.MEAJO_249_19
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.