Back to Journals » Clinical Ophthalmology » Volume 13
Efficacy and safety of tafluprost 0.0015% – retrospective analysis of real-world data from the Philippines
Authors Tumbocon JA , Macasaet AM
Received 25 March 2019
Accepted for publication 29 July 2019
Published 27 August 2019 Volume 2019:13 Pages 1627—1634
DOI https://doi.org/10.2147/OPTH.S209942
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Joseph Anthony Tumbocon,1,2 Anne Marie Macasaet1
1Eye Institute, St. Luke’s Medical Center, Quezon City, Philippines; 2Department of Ophthalmology, St. Luke’s College of Medicine, Quezon City, Philippines
Correspondence: Joseph Anthony Tumbocon
Eye Institute, St. Luke’s Medical Center, 279 E. Rodriguez Sr. Blvd., Quezon City 1102, Philippines
Tel +632 789 7700 Ext 7212
Fax +632 789 7700 Ext 7212
Email [email protected]
Purpose: To investigate the IOP lowering effect and safety of tafluprost 0.0015% in a routine clinical setting in the Philippines.
Patients and methods: A retrospective review of glaucoma patients receiving tafluprost 0.0015% (BAK 0.001% preserved) with a minimum follow-up of 3 months was conducted. Main outcome measure was the mean IOP change at month 3. Secondary outcome measures included longitudinal IOP assessments and occurrence of any adverse events.
Results: Three-hundred twenty-nine eyes of 177 patients with mean age 64.8 years were included and followed for mean 8.8 months. Most common diagnosis was primary open-angle glaucoma (POAG) (34.9%), followed by primary angle-closure (PAC) glaucoma post-laser iridotomy (24.0%), PAC post-laser iridotomy (15.5%), ocular hypertension (OHT)(14.6%), secondary glaucoma (6.7%), and normal-tension glaucoma (4.3%). Mean IOP change at month 3 was −6.18 mmHg (SD 4.06), a −26.37% reduction (p<0.001) and IOP reduction was sustained throughout the study period (p<0.001). Sub-group analysis of treatment naïve (n=203); add-on (n=53) and replacement therapy (n=73) showed a 3-month mean IOP reduction of −8.34 mmHg (SD 2.57, p<0.001) or –31.24%, −5.08 mmHg (SD 2.86, p<0.001) or –23.68%, and −1.00 mmHg (SD 3.08; p=0.007) or –6.31%, respectively. There was significant IOP reduction from baseline in both POAG/OHT sub-group and the PAC/PACG post-laser iridotomy with ≥90° open-angle sub-group (p<0.001), sustained up to month 12 post-treatment. However, there was no significant difference in the average absolute (mmHg) or proportional IOP change from baseline between the two sub-groups. Conjunctival hyperemia was the most common adverse reaction occurring in 15% of patients.
Conclusion: Tafluprost was a safe and effective IOP-lowering treatment in this routine clinical setting.
Keywords: tafluprost 0.0015%, primary open-angle glaucoma, IOP reduction
Introduction
Optimal medical management of glaucoma requires an individualized treatment approach; utilizing potent, convenient patient-friendly agents to achieve a target IOP required to preserve the patient’s visual function and quality of life.1
There are several classes of topical antiglaucoma drugs available, including prostaglandin analogs (PGAs), beta blockers, alpha agonists, carbonic anhydrase inhibitors, and rho kinase inhibitors.2 These topical therapies reduce the production of aqueous humor, enhance outflow, or both. For many years, beta blockers were the most commonly used therapy; however, the introduction of newer agents over the past decades has given physicians a wider variety of choices for both initial and combination treatment.
The advent of PGAs heralded a significant landmark in research and development of anti-glaucoma therapy, providing a treatment that slows the progression of glaucomatous optic nerve injury and visual field loss in these patients.3 PGAs act by increasing uveoscleral and trabecular meshwork outflow and often, IOP reduction is observed within hours after first administration.2 The combination of effectiveness and tolerability has made topical PGAs the first-line medical therapy for primary open-angle glaucomas (POAG) and ocular hypertension (OHT) owing to their potent IOP-lowering capacity, convenient once-a-day dosing and negligible systemic side effects.4
Tafluprost, the fourth PGA to be developed, following latanoprost, travoprost, and bimatoprost, is a prostaglandin F2α analog, with a prostanoid FP-receptor affinity 12 times greater than that of latanoprost.5 Tafluprost has also demonstrated minimal potential for binding to other receptors.5 Its rapid IOP-reducing effect (2–4 hrs after first instillation)6 is mediated by enhanced uveoscleral outflow.5 In patients with glaucoma or OHT receiving once-daily tafluprost ophthalmic solution 0.0015% (preserved with benzalkonium chloride (BAK) 0.001%), maximum IOP reduction was achieved on day 7 of treatment.7 Empirical evidence of the efficacy and safety of tafluprost 0.0015% in patients with glaucoma and OHT has been shown in prospective, randomized clinical trials.8,9 However, there is a paucity of data related to outcomes in routine clinical settings. Additionally, studies have shown that Filipinos appear to have more convex and thicker irises, smaller lens vault and narrower angles compared to Caucasians and that POAG and normal-tension glaucoma (NTG) may be more common in Filipinos than Caucasians, with the propensity for NTG being particularly higher in the former relative to the latter group.10,11
We note a paucity in the literature about glaucoma treatment in Filipino eyes, more specifically real-world clinical data and outcomes in this population; therefore, the objective of the current study was to investigate the IOP-lowering effect and safety of tafluprost 0.0015% in a routine clinical setting in the Philippines.
Materials and methods
This retrospective chart review was conducted at a single-center, outpatient clinic within a hospital (St. Luke’s Medical Center, Quezon City, Philippines) and included patients attending regular outpatient visits. Retrospective data were collected on management and follow-up of patients who received tafluprost 0.0015% between January 2014 and May 2016. Data were collected and de-identified as required by the International Conference on Harmonization Guidelines for Good Clinical Practice. Patients were included in the review if they were on tafluprost 0.0015% (BAK 0.001% preserved) eye drops and had a minimum follow-up of 3 months after starting the medication. Patients were excluded if they had incomplete clinical data in their medical records. Patients were informed and have provided their consent, while maintaining patient confidentiality, and following the tenets of the Declaration of Helsinki. Being a retrospective chart review only, the institution declared this investigation exempted from IRB approval.
Data collection
Data were collected from the patient chart beginning at the visit immediately prior to initiation of tafluprost treatment (baseline) and all subsequent visits, to a minimum of 3 months and extending to 24 months. Data extracted from patient charts included the following: patient demographics; diagnosis details, medical history, current/prior medical treatment, ocular surgery, IOP (by Goldmann applanation tonometry) at baseline, 1, 3, 6, 12, 18, and 24 months after initiation of treatment; therapeutic pattern (treatment naïve monotherapy, add-on, or switch), concomitant medications; and adverse events. Patient groups were defined as follows based on previous and concomitant drugs: “naïve,” no previous treatment or concomitant drugs; “switched,” switched to tafluprost monotherapy after treatment with at least one previous drug; “add-on,” addition of or switching to tafluprost combined with other anti-glaucoma medications.
The main outcome measure of this study was the mean change in IOP, 3 months from baseline. Sub-group analysis was planned, based on therapeutic pattern and diagnosis. Other secondary outcome measures included longitudinal IOP measurement, and occurrence of any adverse events or reactions.
Statistical analysis
Standard one-way ANOVA (Bartlett’s test) was used to determine the statistical significance of mean IOP change from baseline. Two-way ANOVA test was used to determine the level of statistical significance of IOP changes between particular sub-groups.
Eyes of patients in which both eyes qualified for inclusion were treated independently for all outcomes.
Results
Three-hundred twenty-nine eyes of 177 patients were included and had data collected during the chart review. The mean age was 64.8 years (range 14–94 years) and these patients were followed for a mean of 8.8 months (range 3–24 months).
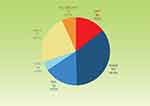
Distribution of baseline diagnoses is shown in Figure 1. The most common diagnosis was POAG (34.9%), followed by primary angle-closure glaucoma (PACG) post-laser iridotomy (24.0%), primary angle-closure post-laser iridotomy (15.5%), OHT (14.6%), secondary glaucoma (6.7%), and normal-tension glaucoma (4.3%). Patients medical history or pre-existing medical conditions did not appear to be relevant to or potentially influential to study outcomes.
The majority of eyes , 203 (62%), were treatment naïve at baseline, while 73 (22%) received tafluprost as replacement therapy (switch) and 53 (16%) received tafluprost as an add-on to current therapy. The most common treatment prior to switch to tafluprost was latanoprost (Table 1). All add-on therapy was dosed according to recommended dosing schedules per product label.
 |
Table 1 Pre-study medication of patients switched to tafluprost (n=73) |
The mean IOP change from baseline to month 3 for the entire cohort was −6.18 mmHg (SD 4.06) or −26.37% decrease from a baseline IOP of 23.44 mmHg (p<0.001). This reduction in IOP was sustained throughout the study period (p<0.001) to month 12 (Figure 2).
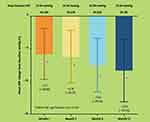
Sub-group analysis demonstrated significant mean IOP reduction from baseline to month 3 of −8.34 mmHg (SD 2.57) or −31.24% decrease from a baseline IOP of 26.70 mmHg (p<0.001) for the treatment naïve group (n=203); −5.08 mmHg (SD 2.86) or −23.68% decrease from a baseline IOP of 21.45 mmHg (p<0.001) for the add-on sub-group (n=53); and −1.00 mmHg (SD 3.08) or −6.31% decrease from a baseline IOP of 15.84 mmHg (p=0.007) for the replacement therapy sub-group (n=73) (Figure 3).
There was a significant mean IOP reduction from baseline in both the POAG/OHT sub-group and the PAC/PACG post-laser iridotomy with ≥90° open-angle sub-group (p<0.001), which was sustained up to month 12 post-treatment initiation.
However, there was no significant difference in the average absolute (mmHg) or proportional IOP change from baseline between the two sub-groups (Figure 4).
Safety
The majority of patients did not experience any adverse signs or symptoms (≥85%). Eye redness (10%) was the most common adverse symptom reported and conjunctival hyperemia was the adverse advent noted most frequently in the patient chart (15%) (Table 2). No patients discontinued treatment due to adverse symptoms or signs during the reviewed period.
 |
Table 2 Adverse reactions recorded |
Discussion
In this study, we evaluated the safety and IOP-lowering effects of tafluprost 0.0015% within a real-world clinical practice setting. We demonstrated significant IOP reduction at an early post-treatment initiation timepoint of month 3, which was sustained throughout the patient follow-up period. Importantly, the IOP-lowering effect was significant in all patient groups, naïve patients, those switched to tafluprost, as well as those receiving tafluprost as add-on therapy.
Our results are similar to short-term12 and long-term follow-up results13 from a large-scale post-marketing study in Japanese patients with glaucoma or OHT who initiated tafluprost treatment. The study by Kuwayama et al prospectively observed 4502 patients over a 2-year period in the real-world setting, collecting IOP and safety data. They reported a mean baseline IOP of 18.6±5.9 mmHg, lowered to ≤15 mmHg over the 2-year observation period. They reported a 3.2–3.6 mmHg (15.3–16.7%) reduction in IOP for all patients over 2 years, whereas we noted a mean reduction of 26.37% for all eyes. Their baseline IOP was lower than that observed in our study (23.4±6.1 mmHg). This suggests that the degree of IOP reduction may be directly proportional to the baseline untreated IOP. Overall, authors concluded that tafluprost showed significant long-term IOP-lowering effects regardless of treatment patterns or diagnosis, with minimum safety concerns in the actual clinical practice.13
Similar to our study, Kuwayama et al also described statistically significant responses to treatment when patients were categorized into treatment naïve, add-on/concomitant, and replacement/switch therapy.12 They also found that eyes naïve to previous therapy showed the highest reduction in IOP (19.2–20.9%) which again is lower than the 31.2% reduction we observed for this group. Notably, we found a reduction of 23.68% and 6.31% for replacement and add-on therapy, respectively, compared to their 8.7–10.7% and 13.8–15.4% reported reduction for the same groups. The mean baseline IOP observed by Kuwayama et al was 18.6 (SD 6.0) versus 23.4 (SD 6.0) observed in our study. The findings are consistent with the suggestion that eyes that are naïve to treatment are more highly reactive to these agents, therefore demonstrating a greater reduction in IOP. Significant reductions were also noted when tafluprost was included as an add-on therapy, delivering further reductions than patients had experienced on monotherapy, which is an important finding for patients not achieving optimal response to monotherapy. Our cohort demonstrated a lower, yet still significant reduction in IOP for those eyes that were switched to tafluprost, although the reason for treatment switch was not known, whether patients were non-responders or other factors may have contributed. Previous studies have shown non-responder rates up to 15% for other PGAs in normal-tension glaucoma.14
About half (49.5%) of the patients in our study had either POAG or OHT. In a comparative study, Uusitalo et al compared the long-term efficacy and safety of tafluprost 0.0015% with latanoprost 0.005% preserved with BAK in patients with POAG or OHT.9 In this double-blinded, multi-center Phase III study, 533 patients were randomized to once-daily treatment with either tafluprost or latanoprost for up to 24 months. The authors found that the IOP-lowering effects of both treatments were similar throughout follow-up and concluded that tafluprost is an effective and well-tolerated treatment for glaucoma and OHT.9
A study in Japanese patients with normal-tension glaucoma compared IOP reduction efficacy and safety of tafluprost 0.0015% and latanoprost 0.005%.15 The study included 30 patients who had used latanoprost monotherapy for more than 4 weeks and were then randomized to either continue latanoprost or switch to tafluprost. Following 12 weeks of treatment, there was a cross over to the opposite group. The authors evaluated patients’ IOP and adverse events at specified timepoints and concluded that latanoprost and tafluprost have equivalent efficacy and safety in Japanese patients with normal-tension glaucoma.15 In our study, patients with normal-tension glaucoma treated with tafluprost showed sustained IOP reduction to 22% and 24% of baseline at months 3 and 6, respectively.
Furthermore, our study also suggests that tafluprost 0.00015% has a similar degree of IOP reduction in eyes with PAC/PACG post-laser iridotomy, compared to eyes with POAG/OHT
The adverse events profile described in our study are similar to findings from previous randomized trials and large prospective post-marketing studies.9,15 Conjunctival hyperemia was the most frequently reported adverse reaction, observed in 15% of patients in this study. Although according to the tafluprost label, additional adverse reactions may be expected, only eye redness and hyperemia were documented within the patient charts of the current cohort.
We acknowledge that our study has several limitations. The retrospective design of data collection and relatively short follow-up duration may not have captured the complete clinical course and all events for each of these patients. In addition, our small sample size impacts the statistical power. The variations in outcomes observed can be ascribed to the variety of clinical conditions in patients enrolled. In addition, dropouts and drug compliance were issues in the study.
Our study has several strengths. We provide the first report of clinical data on IOP-lowering efficacy of tafluprost 0.0015% in real-world settings in Filipino patients with POAG and OHT. In addition, safety parameters reported in both randomized trials and post-marketing studies in other populations were validated in our study.
We conclude that tafluprost 0.015% is a safe and effective IOP-lowering treatment in a routine clinical setting in the Philippines. Large, multi-site studies with longer follow-up periods in Filipino patients are warranted.
Disclosure
This study was partially funded by Santen Pharmaceutical. The abstract of this paper was presented as a poster presentation in the 32nd Asia-Pacific Academy of Ophthalmology Congress (APAO 2017), Suntec International Convention Center, Singapore, on March 2, 2017. Dr Joseph Anthony Tumbocon reports non-financial support from Santen Pharmaceutical, during the conduct of the study; personal fees, non-financial support from Santen Pharmaceuticals, personal fees, non-financial support from Allergan, personal fees from Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Prum BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern® guidelines. Ophthalmology. 2016;123(1):P41–P111. doi:10.1016/j.ophtha.2015.10.053
2. Harasymowycz P, Birt C, Gooi P, et al. Medical management of glaucoma in the 21st century from a Canadian perspective. J Ophthalmol. 2016;2016:6509809.
3. Teus MA, Arranz-Marquez E. Prostaglandin F2α analogues in glaucoma management. Expert Review of Ophthalmology. 2008;3(2):203–209. doi:10.1586/17469899.3.2.203
4. Li T, Lindsley K, Rouse B, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123(1):129–140. doi:10.1016/j.ophtha.2015.09.005
5. Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Experimental Eye Research. 2004;78(4):767–776. doi:10.1016/j.exer.2003.12.007
6. SANTEN. SAFLUTAN 15 micrograms/mL eye drops, solution, in single-dose container - Summary of Product Characteristics (SmPC) - (eMC): Santen UK Limited. Available from: https://www.medicines.org.uk/emc/product/5115/smpc.
7. Traverso CE, Ropo A, Papadia M, Uusitalo H. A phase II study on the duration and stability of the intraocular pressure-lowering effect and tolerability of Tafluprost compared with latanoprost. J Ocul Pharmacol Ther. 2010;26(1):97–104. doi:10.1089/jop.2009.0066
8. Ge J, Li X, Sun X, He X, Zhang H. [Randomized parallel group study of 0.0015% tafluprost ophthalmic solution in patients with primary open-angle glaucoma or ocular hypertension (comparison with 0.005% latanoprost ophthalmic solution)]. Zhonghua Yan Ke Za Zhi. 2015;51(2):95–102. [Article in Chinese]
9. Uusitalo H, Pillunat LE, Ropo A. Efficacy and safety of tafluprost 0.0015% versus latanoprost 0.005% eye drops in open-angle glaucoma and ocular hypertension: 24-month results of a randomized, double-masked phase III study. Acta Ophthalmologica. 2010;88(1):12–19. doi:10.1111/j.1755-3768.2010.01862.x
10. Siguan-Bell CS, Chansangpetch S, Perez CI, et al. Anterior segment parameters of Filipino-Americans compared to Chinese-Americans and Caucasian Americans using anterior segment optical coherence tomography. Transl Vis Sci Technol. 2019;8(2):11. doi:10.1167/tvst.8.2.11
11. Sáles CS, Lee RY, Agadzi AK, Hee MR, Singh K, Lin SC. Open-angle glaucoma in Filipino and white Americans: a comparative study. J Glaucoma. 2014;23(4):246–253. doi:10.1097/IJG.0b013e318279b3e2
12. Kuwayama Y, Nomura A. Prospective observational post-marketing study of tafluprost for glaucoma and ocular hypertension: short-term efficacy and safety. Adv Ther. 2014;31(4):461–471. doi:10.1007/s12325-014-0109-9
13. Kuwayama Y, Hashimoto M, Kakegawa R, Nomura A, Shimada F. Prospective observational post-marketing study of tafluprost for glaucoma and ocular hypertension: effectiveness and treatment persistence. Adv Ther. 2017;34(6):1411–1425. doi:10.1007/s12325-017-0549-0
14. Inoue K, Setogawa A, Tomita G. Nonresponders to prostaglandin analogs among normal-tension glaucoma patients. J Ocul Pharmacol Ther. 2016;32:90–96. doi:10.1089/jop.2015.0086
15. Ikeda Y, Mori K, Tada K, Ueno M, Kinoshita S, Sotozono C. Comparison study of intraocular pressure reduction efficacy and safety between latanoprost and tafluprost in Japanese with normal-tension glaucoma. Clin Ophthalmol. 2016;10:1633–1637. doi:10.2147/OPTH.S108213
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.