Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy and Safety of TACE Combined with Regorafenib Plus PD-1 Inhibitor in the Treatment of Hepatocellular Carcinoma After Sorafenib Resistance
Authors Zou X, Xu Q, You R, Yin G
Received 1 December 2022
Accepted for publication 7 February 2023
Published 16 February 2023 Volume 2023:10 Pages 267—279
DOI https://doi.org/10.2147/JHC.S399874
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mohamed Shaker
Xinhua Zou, Qingyu Xu, Ran You, Guowen Yin
Department of Tumor Interventional Therapy, Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing City, People’s Republic of China
Correspondence: Guowen Yin, Tel +86-19868589105, Email [email protected]
Purpose: To evaluate the efficacy and safety of TACE combined with regorafenib plus PD-1 inhibitor as a second-line therapy for hepatocellular carcinoma after sorafenib resistance.
Materials and Methods: The clinical data of 76 patients with hepatocellular carcinoma who were drug-resistant to sorafenib from September 2018 to May 2022 in the tumor intervention department were collected. Among them, 35 patients used TACE combined with regorafenib plus PD-1 inhibitor (TACE-R-P) as second-line treatment, and the remaining 41 patients used TACE combined with regorafenib (TACE-R) as second-line treatment. The mRECIST (modified Response Evaluation Criteria in Solid Tumors) standard was used to evaluate the therapeutic effect. The progression-free survival (PFS) and overall survival (OS) of the two groups were compared. Blood samples were collected before and after treatment to detect the changes in biochemical indicators, and the adverse events (AEs) related to treatment were recorded.
Results: A total of 76 patients were included in the study, including 35 patients receiving TACE-R-P treatment and 41 patients receiving TACE-R treatment. Patients in the TACE-R-P group had longer median OS (19.7months vs 15.2months, HR:0.7716, 95% CI:0.4767– 1.2490, P=0.03), longer median PFS (6.3months vs 3.8months, HR:0.6032, 95% CI:0.3727– 0.9763, P=0.0029), higher objective response rate (37.14% vs 19.51%, P=0.001) and higher disease control rate (71.43% vs 48.78%, P=0.001) than those in the TACE-R group. Multivariate analysis showed that Child–Pugh grade (B/A; HR=1.283, 95% CI: 0.623– 1.707, P=0.014), PVTT (Yes/No, HR=1.455, 95% CI: 0.977– 2.038, P=0.018), extrahepatic metastasis (Yes/No, HR=1.766, 95% CI: 1.135– 2.302, P=0.022) and treatment option (TACE-R/TACE-R-P, HR=1.930, 95% CI: 1.461– 2.850, P=0.017) were independent prognostic factors for OS. There was no significant difference in the incidence and severity of AEs between the two groups.
Conclusion: TACE-R-P treatment can be more effective than TACE-R treatment for HCC after sorafenib resistance and can be given priority as a second-line treatment for HCC.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, regorafenib, sorafenib, PD-1 inhibitor
Introduction
Primary liver cancer is the seventh of the newly diagnosed cancer cases, 75–85% of which are hepatocellular carcinoma (HCC).1 More than 70% of the patients are unsuitable for curative therapy, such as hepatectomy, transplantation or liver ablation.2,3 Sorafenib, as a molecular targeted drug of tyrosine kinase, can block the activities of vascular endothelial growth factor receptor (VEGF), platelet-derived growth factor receptor (PDGF) and Raf family kinases to play an anti-tumor role,4,5 which has been proven to prolong the survival of patients with advanced HCC. In the SHARP clinical study published in 2008, sorafenib was shown to have a good clinical effect on advanced HCC, median overall survival (mOS) was nearly 3 months longer for patients treated with sorafenib than for those given placebo (10.7months vs 7.9months, HR:0.69, 95% CI: 0.55–0.87, P<0.001), and the time to radiologic progression was (5.5months vs 2.8months, HR:0.58, 95% CI: 0.45–0.74, P<0.001); thus, sorafenib was considered as the first-line systemic treatment for advanced HCC.6 In many subsequent studies, the efficacy of sorafenib as a first-line systemic treatment for advanced HCC was also confirmed, including TACE combined with sorafenib.2,3,7–10 However, the drug resistance of sorafenib will lead to the progression and recurrence of HCC. Therefore, the second-line treatment is required to prolong the survival of HCC patients after sorafenib resistance. The REFLECT trial study showed that lenvatinib could improve survival as a first-line system treatment, but there is no effective second-line treatment after the drug resistance of lenvatinib.11 Regorafenib is a multi-kinase inhibitor with similar molecular structure to sorafenib, and mainly acts on VEGFR-1, VEGFR-2, VEGFR-3, TIE-2, PDGFR-β, FGFR, KIT, RET, RAF-1 and BRAF sites.12–14 In 2017, the RESORCE trial found that for advanced HCC after first-line treatment with sorafenib, regorafenib as a second-line treatment can effectively prolong the survival of patients. Regorafenib improved overall survival with a hazard ratio (HR) of 0.63 (95% CI: 0.50–0.79; P<0.0001); mOS was 10.6 months (95% CI: 9.1–12.1) for regorafenib versus 7.8 months (95% CI: 6.3–8.8) for placebo.15 Clinical trials in many countries have also verified the effectiveness of regorafenib as a second-line treatment after sorafenib resistance.16–19 The possible mechanism is that compared with sorafenib, regorafenib targets a wider range of kinases and has stronger inhibitory effects on VEGFR-2, PDGFR-β, FGFR-1, and c-Kit. Besides, regorafenib also inhibits TIE-2, which has a broader anti-angiogenesis effect.5,14,20 In addition, in the CELESTIAL trial, there was an improvement in mOS (10.2months vs 8.0months; HR:0.76, 95% CI: 0.63–0.92, P=0.0049) and median progression-free survival (mPFS) (5.2months vs 1.9months; HR:0.44, 95% CI: 0.36–0.52, P<0.0001) with cabozantinib when compared to placebo.21 Ramucirumab showed an improvement in mOS (8.5months vs 7.3months; HR:0.71, P=0.0199) and PFS (2.8months vs 1.6months; HR:0.45, P<0.0001) in the REACH-2 trial when compared to placebo in patients with HCC who are resistant to sorafenib.22 However, Japanese researchers found that there was no significant survival benefit from using ramucirumab after lenvatinib resistance.23
Recently, it was found that the expression of some immune checkpoint proteins by tumor cells disturbs the anti-tumor immunity of the body, promoting tumor growth and invasion.24 Thus, blockade of the immune checkpoint pathway using immune checkpoint inhibitors (ICIs) is a novel strategy in cancer therapy, including the most studied anti-programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies.25 In the CheckMate-040 study26 and KEYNOTE-224 study,27 it is shown that nivolumab and pembrolizumab have a definite effect on HCC after sorafenib treatment. However, the overall efficacy of immune checkpoint inhibitors has also not achieved very satisfactory results.26–28 Next, in the IMbrave150 trial,29 the combination of atezolizumab and bevacizumab (Atezo/Bev) achieved better outcomes over sorafenib alone, suggesting combined immunotherapy strategy of PD-1/PD-L1 and VEGF inhibitors has gradually become a new treatment option and has also brought new ideas for second-line treatment for advanced HCC. The possible mechanism is that VEGF inhibitors can reverse the immunosuppressive state of tumor microenvironment and improve the immune response rate of PD-1/PD-L1 inhibitors.30 Besides, ORIENT-32 study also show the combination of sintilimab plus bevacizumab achieved better outcomes over sorafenib alone.31 All above-mentioned provide the theoretical principle and excellent practical basis for the combined strategy of regorafenib and PD-1 inhibitors. However, there is still no standard second-line combined therapy for HCC.
TACE is considered an effective way in the treatment guidelines of many countries around the world for patients with unresectable HCC.32 However, repeated TACE treatment increases the up-regulation of VEGF and PDGF, and ultimately cause tumor recurrence.33,34 The combination of TACE and regorafenib can achieve synergistic effects on reducing the expression of VEGFRs after TACE, thereby improving the tumor response and reducing the recurrence rate. Some studies have found that TACE combined with regorafenib (TACE-R) is more effective than regorafenib monotherapy as a second-line treatment for HCC after sorafenib resistance.35,36 At the same time, a multicenter retrospective study showed that regorafenib combined with PD-1 blockade immunotherapy versus regorafenib alone as second-line treatment for advanced HCC, achieved better outcomes.37 Therefore, TACE combined with regorafenib plus PD-1 (TACE-R-P) inhibitor may achieve better survival in the treatment of advanced HCC after sorafenib resistance. However, there are few trials to explore the efficacy and safety of TACE-R-P versus TACE-R in the second-line treatment of advanced HCC after sorafenib resistance. Therefore, we conducted this study to evaluate the efficacy and safety of TACE-R-P as a second-line treatment for advanced HCC after sorafenib resistance.
Materials and Methods
Study Design and Patients
We retrospectively analyzed the clinical data of HCC patients with progression after first-line sorafenib treatment between September 2018 and May 2022. Among these patients, 35 and 41 were included in the TACE-R-P and TACE-R groups, respectively (Figure 1). The inclusion criteria were as follows: (1) patients who had disease progression during sorafenib treatment (including sorafenib monotherapy failure, progression of TACE combined with sorafenib, and progression of other local treatments combined with sorafenib); (2) Pathologically or clinically diagnosed HCC; (3) Age ≥18 years; (4) Child–Pugh A or B; (5) Eastern Cooperative Oncology Group (ECOG) performance status (PS)≤1. The exclusion criteria were as follows: (1) Poor compliance of patients; (2) Discontinued treatment due to serious adverse reactions; (3) Biliary obstruction; (4) Obstruction of main portal vein; (5) Underwent previous other systemic anti-tumor treatments; (6) Incomplete data. This study was approved by the ethics committee of Jiangsu Institute of Cancer Research (NO. 2022LC0322). All patients provided written informed consent and this study was conducted in accordance with the Declaration of Helsinki. The access to personal information for authors can be identified at any time of data capture.
Treatment Protocol
TACE is performed according to the standard process:38,39 the patients received either conventional TACE (C-TACE) or drug-eluting beads TACE (DEB-TACE) according to their own choice. During TACE, superselective catheterization was performed with a microcatheter, and the embolization end point was the tumor-feeding arteries flow stasis. TACE was continued until progression to the unacceptable toxicity, TACE refractory criteria, or withdrawal of consent. Regorafenib (Stivarga, Bayer, Germany, 40–160mg/day), Sorafenib (Nexavar, Bayer, Germany, 800 mg/day), PD-1 inhibitor [Pembrolizumab (Keytruda, Merck & Co., Inc., USA), 200mg every 3 weeks; Sintilimab (Tyvyt®, Innovent Biologics, Suzhou, China), 200mg every 3 weeks] was administered within 7 days before or after the first TACE. Doses were adjusted according to the grade of adverse events (AEs).
Follow-Up
Follow-up of all patients was performed at a 4–6 weeks interval. During the follow-up, laboratory and imaging examinations were carried out. Laboratory examinations included blood routine examination, blood coagulation function, alpha fetoprotein (AFP), albumin (ALB), cholinesterase (CHE) other blood biochemical indicators. Contrast enhanced CT/MRI was checked every 4–6 weeks. Record and timely treat adverse reactions during follow-up.
Assessments
Treatment response was assessed according to the mRECIST.40 Tumor responses were categorized as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) according to mRECIST. Calculate and compare the disease control rate (DCR=CR+PR+SD) and objective response rate (ORR=CR+PR) of the two groups. PFS is defined as the time from the start of second-line treatment to tumor progression, and OS is defined as the time from the start of second-line treatment to death from any cause. Treatment-related AEs were evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE).41
Statistical Analysis
SPSS version 22.0 statistical software (IBM Corp, Armonk, NY, USA) and GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA) were applicated for statistical analysis and drawing, respectively. Pearson’s χ2 test and independent t-test were performed for analyzing clinical data. Survival curves was performed by the Kaplan–Meier method. Cox regression model was used to determine independent prognostic factors for survival. Statistical significance was set at P<0.05.
Results
Patient Characteristics
A total of 104 HCC patients who received TACE-R-P or TACE-R treatment were eligible for screening. Among them, 28 were excluded because they met the exclusion criteria (Figure 1). Finally, 76 patients were recruited in this study (TACE-R-P, 35; TACE-R, 41). The categories of PD-1 inhibitor received by the patients were as follows: sintilimab for 19 (54.29%) and pembrolizumab for 16 (45.71%). The baseline patient characteristics, including sex, age, ECOG score, Child–Pugh grade, AFP level, viral hepatitis, PVTT, number of tumors, hepatic vein invasion and extrahepatic metastasis, were not significantly different between the two groups (Table 1).
 |
Table 1 Baseline Demographic and Clinical Characteristics of Patients Enrolled in This Study |
Efficacy Outcomes
Comparing TACE-R-P and TACE-R groups, the CR was 5.71% vs 0.00%; the PR was 31.43% vs 19.51%; the ORR was 37.14% vs 19.51%; the DCR was 71.43% vs 48.78% (Table 2). The tumor response of the TACE-R-P group was better than that of the TACE-R group, and there was significant statistical difference between the two groups (P=0.001).
 |
Table 2 Tumor Response in Patients to TACE-R-P Group and TACE-R Group |
Compared with the patients in the TACE-R group, the patients in the TACE-R-P group had significantly better survival outcomes (Figure 2). In the TACE-R-P and TACE-R groups, the mOS was (19.7months vs 15.2months, HR:0.7716, 95% CI:0.4767 to 1.2490, P=0.03), the median PFS (mPFS) was (6.3months vs 3.8months, HR:0.6032, 95% CI:0.3727 to 0.9763, P=0.0029), respectively.
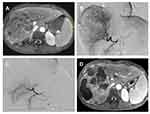
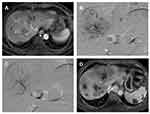
In the TACE-R-P group, as shown in Figure 3, the combination treatment could effectively inhibit tumor progression after sorafenib resistance, and the tumor was well controlled with necrosis in most lesions and no distant metastasis. However, in the TACE-R group, there were still many lesions and tumor progression occurred after TACE combined with regorafenib (Figure 4).
Prognostic Factors Analysis for OS
Additional Cox regression analyses of OS were performed (Table 3). Univariate analysis showed that OS was significantly associated with Child–Pugh grade (B/A, HR=1.523, 95% CI: 0.756–1.822, P=0.002), AFP (>400/≤400µg/L, HR=1.149, 95% CI: 0.590–1.603, P=0.009), tumor size (>5/≤5cm, HR=1.446, 95% CI: 0.801–1.885, P=0.006), PVTT classification (Yes/No, HR=1.759, 95% CI: 1.206–2.115, P=0.001), extrahepatic metastasis (Yes/No, HR=1.815, 95% CI: 1.106–3.058, P=0.003) and treatment option (TACE-R/TACE-R-P, HR=1.634, 95% CI: 1.203–2.627, P=0.001). On the multivariate analysis, Child–Pugh grade (B/A, HR=1.283, 95% CI: 0.623–1.707, P=0.014), PVTT (Yes/No, HR=1.455, 95% CI: 0.977–2.038, P=0.018), extrahepatic metastasis (Yes/No, HR=1.766, 95% CI: 1.135–2.302, P=0.022) and treatment option (TACE-R/TACE-R-P, HR=1.930, 95% CI: 1.461–2.850, P=0.017) were identified as the independent prognostic factors for OS.
 |
Table 3 Univariate and Multivariate Analyses of Risk Factors for OS in HCC Cases After Sorafenib Resistance |
Safety
Treatment-related AEs are summarized in Table 4. In the TACE-R-P group, the most common AEs were thrombocytopenia (grade 1–2, 25.71%; grade 3–4, 14.29%), anorexia and nausea (grade 1–2, 25.71%; grade 3–4, 8.57%), fatigue (grade 1–2, 25.71%; grade 3–4, 5.71%), hand-foot syndrome (grade 1–2, 22.86%; grade 3–4, 14.29%), abdominal pain (grade 1–2, 28.57%; grade 3–4, 5.71%) and hypertension (grade 1–2, 17.14%; grade 3–4, 11.43%). In the TACE-R group, the most common AEs were thrombocytopenia (grade 1–2, 26.83%; grade 3–4, 14.63%), anorexia and nausea (grade 1–2, 21.95%; grade 3–4, 9.76%), fatigue (grade 1–2, 21.95%; grade 3–4, 7.32%), hand-foot syndrome (grade 1–2, 24.39%; grade 3–4, 9.76%), abdominal pain (grade 1–2, 29.27%; grade 3–4, 9.76%) and hypertension (grade 1–2, 17.07%; grade 3–4, 12.20%). There was no significant difference in AEs between the two groups (P>0.05).
 |
Table 4 Treatment-Related Adverse Events in Two Groups |
Discussion
Our study showed that TACE-R-P was more effective than TACE-R for HCC resistant to sorafenib. In the TACE-R-P and TACE-R groups, the mOS was (19.7months vs 15.2months, HR:0.7716, 95% CI:0.4767–1.2490, P=0.03), the mPFS was (6.3months vs 3.8months, HR:0.6032, 95% CI:0.3727–0.9763, P=0.0029), the DCR was (71.43% vs 48.78%, P=0.001), respectively. Patients in the TACE-R-P group had higher ORR (37.14% vs 19.51%) and DCR (71.43% vs 48.78%) than those in the TACE-R group, and the difference was statistically significant (P=0.001). However, there was no statistical difference in AEs between the two groups (P>0.05). The RESORCE study evaluated the second-line treatment of advanced HCC after sorafenib resistance and compared the efficacy of regorafenib and placebo. The results showed that regorafenib significantly prolonged mOS (10.6 months vs 7.8 months, HR:0.63, 95% CI:0.50–0.79, P<0.0001) and mPFS (3.1 months vs 1.5 months, HR:0.46, 95% CI:0.37–0.56, P<0.0001) compared with placebo.15 In our study, the mOS and mPFS of TACE-R-P group and TACE-R group were higher than those of the RESORCE study. This shows that TACE-R-P treatment and TACE-R treatment can achieve better efficacy than regorafenib monotherapy. The study of Han et al36 showed that the mOS of TACE combined with regorafenib was 14.3 months, which was similar to the mOS of TACE-R group in this study. The study of Liu K et al42 showed that regorafenib combined with PD-1 inhibitor achieved longer mOS (12.9months vs 10.3months, P=0.010) and mPFS (5.9months vs 3.0months, P<0.001) than regorafenib monotherapy, indicating that regorafenib can cooperate with PD-1 inhibitor to treat advanced HCC. Multivariate analysis in this study also showed that the addition of PD-1 inhibitors to TACE-R treatment was an independent predictor of prolonged OS and PFS. TACE, as an interventional therapy, has been proved to be effective and safe for advanced HCC. Therefore, in order to improve the second-line therapeutic effect of advanced HCC after sorafenib resistance, we conducted this clinical study comparing TACE-R-P with TACE-R. Although we found that TACE combined with regorafenib extended survival, the TACE combined with regorafenib plus PD-1 inhibitor had a better survival benefit.
The clinical benefit found in the study may be attributed to the synergistic antitumor effect of PD-1 inhibitors, regorafenib and TACE. First, TACE can enhance the cytotoxicity of regorafenib to local tumor cells, thus prolonging the disease control time. Liver function is an independent risk factor for poor prognosis of TACE, so regorafenib combined with TACE can reduce the damage to liver function by reducing the number of TACE.43 Second, TACE can cause tumor hypoxia and increase serum HIF-1α and VEGF levels.44 HIF-1α can bind to the VEGF promoter region and induces VEGF transcription and expression, leading to neovascularization and increased oxygen supply in tumor tissues.45,46 The high expression of VEGF can induce tumor recurrence and metastasis. Regorafenib is a small molecular multi-target tyrosine kinase inhibitor (TKI) that inhibits CSF1R, RAF-1, B-RAF, VEGFR1-3, KIT, RET, TIE-2, PDGFR-β, FGFR, etc,14 so it has a good therapeutic effect on malignant tumors.47 Regorafenib may inhibit the hypoxia-induced angiogenesis after TACE to enhance the anti-tumor activity of TACE and reduce the tumor recurrence rate. The above-mentioned may be why regorafenib combined with TACE is effective in patients with advanced HCC after the failure of first-line targeted therapy. Third, in some previous studies, immune checkpoint inhibitors did not achieve particularly satisfactory results.27,48 Some studies have shown that regorafenib can reverse the immunosuppressive state of the tumor microenvironment, normalize the vascular permeability and immune environment, and thus enhancing the therapeutic effect of PD-1 inhibitors.37,49,50 The possible mechanism is that regorafenib have been found to suppress the generation of Tregs, TAMs, and MDSCs, enhance the release of immune-suppressive cytokines including IL-5, IL-8, IL-10 and TGF-β,30,51 and inhibiting the release of immune-activating cytokines including IL-1, TNFα/β, and IFN-γ.52 Moreover, regorafenib can inhibit CSF1R to damage tumor immunity, and regulate macrophages to increase the proliferation and activation of CD8+T cells by targeting VEGFR2/3, which can improve the efficacy of PD-1 inhibitors.49,53 Therefore, the combination of TACE, regorafenib and PD-1 inhibitor can achieve better therapeutic effect.
However, in our study, although we found that TACE combined with regorafenib extended survival time for the failure of sorafenib, not all patients can benefit from TACE combined with regorafenib, which may be related to the mechanism of escaping T cell recognition, that is, the immune escape ability of cancer cells.30,54 Studies have shown that IFN-γ binds to IFN-γ receptors on the surface of cancer cells and upregulates PD-L1 expression through the Janus kinase (JAK)-signal transduction and transcriptional activator (STAT) signaling pathway, which leads to immune escape.55 According to the existence of tumor infiltrating CD8+T cells and the expression of PD-L1,56 cancer can be divided into four types including Type I/II/III/IV tumors. Among, Type I tumors contain tumor-infiltrating lymphocytes and express PD-L1, as well as sufficient response to PD-1/PD-L1 inhibitors. In contrast, type IV tumors lack the expression of PD-L1, although they contain tumor-infiltrating lymphocytes and have no response to PD-1/PD-L1 inhibitor monotherapy because of the existence of tumor immunosuppressive microenvironment to inhibit T cell activity. That is to say, in the case of the high frequency of circulating and tumor-infiltrating PD-1+ CD8+T cells and high expression of PD-L1, PD-1/PD-L1 antibodies could inhibit immune escape through the PD-1/PD-L1 axis.57,58 In type IV tumors, the combination of TACE, regorafenib and PD-1 inhibitor can lead to significantly inhibited tumor proliferation. However, in type II and III tumors without tumor infiltrating lymphocytes, another strategy to improve immunogenicity may be needed.30 Moreover, previous studies showed that under the combination of Atezo/Bev, inflammatory cytokine IL-6 was a biomarker reflecting the response to PD-1 inhibitor.52 Whether the cytokine signaling pathway can benefit from TACE combined with regorafenib or the combination of TACE, regorafenib and PD-1 inhibitor, which is also one of the reasons for the difference between the two treatments.
More importantly, tumor heterogeneity and tumor immune microenvironment restrict the response ability of PD-1/PD-L1 inhibitors.59,60 Tumor heterogeneity is an essential part of tumor development and progression, including gene alterations (eg, EGFR, ROS, ALK and TP53), chromosome stability (proliferative rate), epigenetic modification and so on,60 which are associated with the treatment efficacy of anti-PD-1/anti-PD-L1 therapy. For tumor immune microenvironment, Galon’s group proposed a classification of different immune stages of tumors based on the quantity and quality of immune infiltrates, ranging from “hot” to “immune-suppressed/excluded” or even “deserted” (cold).61 In other words, PD-1/PD-L1 inhibitors exhibit the best overall tumor response rate when immune effector cells such as tumor-infiltrating lymphocytes are spatially close and not inhibited by other humoral or cellular regulatory mechanisms. Additionally, Sia et al62 investigated the response of tumor cells to immune cells in HCC and divided tumors with high expression of PD-L1 and PD-1 into two subgroups, immune-active or exhausted groups. Exhausted groups had activated stroma features with immunosuppressive components, such as TGF-β signaling activation, and up-regulation of the immunosuppressive factors LGALS1, CXCL12 and CCL2. Shimada and Mogushi et al63 further analyzed the molecular, clinicopathological and immunological characteristics and identified three molecular subgroups (MS1/MS2/MS3). Among, MS2 was enriched in activated Wnt/β-catenin pathway tumors associated with CTNNB1 mutations, and their immunoreactivity was low, immunologically “cold”. Therefore, tumors with rich immune cells (“hot tumors”) usually respond strongly to immune modulators, whereas other immune-depleted or immune-excluded tumor areas are less responsive and require alternative treatment regimens such as modified immune effectors cells or immune-stimulating agents. Briefly, the methods of immunotherapy64 include “unleashing the immune system” and “turning a cold tumor into a hot tumor”. The first is that it can relieve any immunosuppression or allow T cells to infiltrate the tumor such as oncolytic viruses to immunize the tumor environment alone or combined with primed immune cells. The second is VEGF inhibitor, TGF-β inhibitor and so on can reprogram the immunosuppressive microenvironment into an immunostimulatory microenvironment, and then enhance the antitumor activity of the PD-1/PD-L1 antibodies.
The main risk factor for HCC in China is HBV, although some patients are combined with hepatitis C virus (HCV) infection, while HCV infection is more common in Western countries. In our study, the TACE combined with regorafenib plus PD-1 inhibitor had a significant survival benefit for the treatment of HCC with HBV infection. The DAAs are more effective for HCV clearance than interferon (IFN)-based therapy, exhibiting better tolerability and cure rates >95%.65,66 It is noted that hepatitis B and hepatitis C dual infection increases the risk of HCC development and promotes disease progression more than mono-infection.67 Hepatitis B and hepatitis C dual infection has a negative impact on the prognosis of liver disease and is difficult to treat. A meta-analysis showed that frequent HBV reactivation in dually infected chronic HBV and HCV patients receiving DAA therapy.68 The interaction between the two viruses and the antiviral treatment for only one of them may reactivate the two viruses, the choice of treatment methods becomes important.69 Before antiviral treatment, a comprehensive serological and virological evaluation is required to verify the activity of each virus and select the best antiviral scheme.70 In patients with active hepatitis C co-infection of HBV/HCV, HCV tends to be a priority target for control.70,71 That is to say, the general management method is to treat the dominant virus as a mono-infection, and then monitor the reactivation of another virus.70 HCV is usually dominant, whereas HBV shows a dominant or recessive pattern.69 Therefore, monitoring liver enzymes and markers indicating HBV/HCV replication before and during treatment is essential for early diagnosis and treatment of viral reactivation.
We adjusted the dosage of regorafenib according to the adverse reactions of the patients. Some previous studies also showed adverse reactions and dose adjustment of regorafenib.14,48,72 Among all patients in this study (n=76), 7.89% (n=6) at a daily dose of 160 mg regorafenib, 47.37% (n=36) at 120 mg, 36.84% (n=28) at 80 mg, and 7.89% (n=6) at 40 mg. We found that the largest number of patients received 120mg regorafenib dose, followed by 80mg, indicating that the TKI-related AEs of these drug doses were controllable and the clinical efficacy was acceptable Some patients in this study received an initial dose of 160mg of regorafenib, which was gradually reduced to 120mg or 80mg due to adverse reactions. Therefore, when patients can hardly tolerate TKI-related AEs, the dosage should be adjusted in time. It is possible that 120mg regorafenib per day is an appropriate dose for second-line treatment of advanced HCC, but more clinical studies are needed to confirm this.
Our study had some limitations. First, this was retrospective in design, and thus our findings need to be verified in prospective randomized trials of TACE-R-P versus TACE-R in advanced HCC after sorafenib resistance. Second, this study did not enroll patients who were resistant to lenvatinib as a first-line treatment. Thirdly, most of the HCC patients included in this study are infected with HBV virus. Therefore, for patients infected with HCV or infected with HCV/HBV at the same time, more research is needed to determine the HCV treatment method and the best plan for simultaneously managing the two viruses. Finally, the sample size of this study was limited.
In conclusion, TACE-R-P achieved better OS, PFS, ORR and DCR than TACE-R for HCC after sorafenib resistance. This study indicated the superiority of TACE-R-P to TACE-R in second-line treatment of HCC patients. These findings need to be confirmed in large sample, prospective randomized controlled trials.
Funding
This study was supported by the interventional radiology research project of Jiangsu Medical Association (1210012021081).
Disclosure
The authors report no conflicts of interest in this work. There is no competing interest associated with this study by any institution or organization.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
3. European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
4. Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7(10):3129–3140. doi:10.1158/1535-7163
5. Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi:10.1158/0008-5472
6. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
7. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
8. Finn RS, Zhu AX, Farah W, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: a systematic review and meta-analysis. Hepatology. 2018;67(1):422–435. doi:10.1002/hep.29486
9. Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a Phase III trial. J Hepatol. 2012;57(4):821–829. doi:10.1016/j.jhep.2012.06.014
10. Park JW, Koh YH, Kim HB, et al. Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol. 2012;56(6):1336–1342. doi:10.1016/j.jhep.2012.01.006
11. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
12. Fung AS, Tam VC, Meyers DE, et al. Second-line treatment of hepatocellular carcinoma after sorafenib: characterizing treatments used over the past 10 years and real-world eligibility for cabozantinib, regorafenib, and ramucirumab. Cancer Med. 2020;9(13):4640–4647. doi:10.1002/cam4.3116
13. Teufel M, Seidel H, Köchert K, et al. Biomarkers associated with response to regorafenib in patients with hepatocellular carcinoma. Gastroenterology. 2019;156(6):1731–1741. doi:10.1053/j.gastro.2019.01.261
14. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi:10.1002/ijc.25864
15. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
16. Elseud YA, Shaaban A, Mohanty A, Albarrak J. Safety and tolerability of regorafenib: a real-life experience. J Gastrointest Cancer. 2022;53(1):187–191. doi:10.1007/s12029-020-00570-1
17. Lee MJ, Chang SW, Kim JH, et al. Real-world systemic sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Korea. Invest New Drugs. 2021;39(1):260–268. doi:10.1007/s10637-020-00977-4
18. Kim K, Jha R, Prins PA, et al. Regorafenib in advanced hepatocellular carcinoma (HCC): considerations for treatment. Cancer Chemother Pharmacol. 2017;80(5):945–954. doi:10.1007/s00280-017-3431-5
19. Ogasawara S, Ooka Y, Itokawa N, et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Japan. Invest New Drugs. 2020;38(1):172–180. doi:10.1007/s10637-019-00801-8
20. Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12(7):1322–1331. doi:10.1158/1535-7163
21. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
22. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
23. Hiraoka A, Kumada T, Tada T, et al. Therapeutic efficacy of ramucirumab after lenvatinib for post-progression treatment of unresectable hepatocellular carcinoma. Gastroenterol Rep. 2020;9(2):133–138. doi:10.1093/gastro/goaa042
24. Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018;11(1):39. doi:10.1186/s13045-018-0582-8
25. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–669. doi:10.1038/s41422-020-0343-4
26. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
27. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
28. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/S1470-2045(20)30011-5
29. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
30. Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers. 2020;12(5):1089. doi:10.3390/cancers12051089
31. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
32. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
33. Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878–2882. doi:10.3748/wjg.v10.i19.2878
34. Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103(4):914–921. doi:10.1111/j.1572-0241.2007.01712.x
35. Wang H, Xiao W, Han Y, et al. Study on safety and efficacy of regorafenib combined with transcatheter arterial chemoembolization in the treatment of advanced hepatocellular carcinoma after first-line targeted therapy. J Gastrointest Oncol. 2022;13(3):1248–1254. doi:10.21037/jgo-22-395
36. Han Y, Cao G, Sun B, et al. Regorafenib combined with transarterial chemoembolization for unresectable hepatocellular carcinoma: a real-world study. BMC Gastroenterol. 2021;21(1):393. doi:10.1186/s12876-021-01967-3
37. Huang J, Guo Y, Huang W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2022;9:157–170. doi:10.2147/JHC.S353956
38. Renzulli M, Peta G, Vasuri F, et al. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann Hepatol. 2021;22:100278. doi:10.1016/j.aohep.2020.10.006
39. Prajapati HJ, Xing M, Spivey JR, et al. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol. 2014;203(6):W706–W714. doi:10.2214/AJR.13.12308
40. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
41. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244. doi:10.1093/jnci/dju244
42. Liu K, Wu J, Xu Y, Li D, Huang S, Mao Y. Efficacy and safety of regorafenib with or without PD-1 inhibitors as second-line therapy for advanced hepatocellular carcinoma in real-world clinical practice. Onco Targets Ther. 2022;15:1079–1094. doi:10.2147/OTT.S383685
43. Hiraoka A, Kumada T, Kudo M, et al. Hepatic function during repeated TACE procedures and prognosis after introducing sorafenib in patients with unresectable hepatocellular carcinoma: multicenter analysis. Dig Dis. 2017;35(6):602–610. doi:10.1159/000480256
44. Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1α and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res. 2016;8(4):297–302. doi:10.14740/jocmr2496w
45. Liu P, Atkinson SJ, Akbareian SE, et al. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1α/VEGF signalling. Sci Rep. 2017;7(1):12651. doi:10.1038/s41598-017-12855-w
46. Freedman SJ, Sun ZY, Poy F, et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci USA. 2002;99(8):5367–5372. doi:10.1073/pnas.082117899
47. Zopf D, Fichtner I, Bhargava A, et al. Pharmacologic activity and pharmacokinetics of metabolites of regorafenib in preclinical models. Cancer Med. 2016;5(11):3176–3185. doi:10.1002/cam4.883
48. Rimassa L, Pressiani T, Personeni N, Santoro A. Regorafenib for the treatment of unresectable hepatocellular carcinoma. Expert Rev Anticancer Ther. 2017;17(7):567–576. doi:10.1080/14737140.2017.1338955
49. Wu RY, Kong PF, Xia LP, et al. Regorafenib promotes antitumor immunity via inhibiting PD-L1 and IDO1 expression in melanoma. Clin Cancer Res. 2019;25(14):4530–4541. doi:10.1158/1078-0432.CCR-18-2840
50. Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8(3):299–313. doi:10.2217/imt.15.126
51. Elovic AE, Ohyama H, Sauty A, et al. IL-4-dependent regulation of TGF-alpha and TGF-beta1 expression in human eosinophils. J Immunol. 1998;160(12):6121–6127.
52. Myojin Y, Kodama T, Sakamori R, et al. Interleukin-6 is a circulating prognostic biomarker for hepatocellular carcinoma patients treated with combined immunotherapy. Cancers. 2022;14(4):883. doi:10.3390/cancers14040883
53. Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5(1):53. doi:10.1186/s40425-017-0257-y
54. Pinato DJ, Murray SM, Forner A, et al. Trans-arterial chemoembolization as a loco-regional inducer of immunogenic cell death in hepatocellular carcinoma: implications for immunotherapy. J Immunother Cancer. 2021;9(9):e003311. doi:10.1136/jitc-2021-003311
55. Kudo M. Combination cancer immunotherapy with molecular targeted agents/anti-CTLA-4 antibody for hepatocellular carcinoma. Liver Cancer. 2019;8(1):1–11. doi:10.1159/000496277
56. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi:10.1158/0008-5472
57. Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128(4):887–896. doi:10.1002/ijc.25397
58. Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi:10.1158/1078-0432
59. Kabashima A, Shimada S, Shimokawa M, Akiyama Y, Tanabe M, Tanaka S. Molecular and immunological paradigms of hepatocellular carcinoma: special reference to therapeutic approaches. J Hepatobiliary Pancreat Sci. 2021;28(1):62–75. doi:10.1002/jhbp.874
60. Shembrey C, Huntington ND, Hollande F. Impact of tumor and immunological heterogeneity on the anti-cancer immune response. Cancers. 2019;11(9):1217. doi:10.3390/cancers11091217
61. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197–218. doi:10.1038/s41573-018-0007-y
62. Sia D, Jiao Y, Martinez-Quetglas I, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017;153(3):812–826. doi:10.1053/j.gastro.2017.06.007
63. Shimada S, Mogushi K, Akiyama Y, et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine. 2019;40:457–470. doi:10.1016/j.ebiom.2018.12.058
64. Haanen JBAG. Converting cold into hot tumors by combining immunotherapies. Cell. 2017;170(6):1055–1056. doi:10.1016/j.cell.2017.08.031
65. Sagnelli E, Sagnelli C, Macera M, Pisaturo M, Coppola N. An update on the treatment options for HBV/HCV coinfection. Expert Opin Pharmacother. 2017;18(16):1691–1702. doi:10.1080/14656566.2017.1398233
66. World Health Organization. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection: Updated Version. Geneva: World Health Organization; 2016.
67. Jiang XW, Ye JZ, Li YT, Li LJ. Hepatitis B reactivation in patients receiving direct-acting antiviral therapy or interferon-based therapy for hepatitis C: a systematic review and meta-analysis. World J Gastroenterol. 2018;24(28):3181–3191. doi:10.3748/wjg.v24.i28.3181
68. Chen G, Wang C, Chen J, et al. Hepatitis B reactivation in hepatitis B and C coinfected patients treated with antiviral agents: a systematic review and meta-analysis. Hepatology. 2017;66(1):13–26. doi:10.1002/hep.29109
69. Mavilia MG, Wu GY. HBV-HCV coinfection: viral interactions, management, and viral reactivation. J Clin Transl Hepatol. 2018;6(3):296–305. doi:10.14218/JCTH.2018.00016
70. Liu CJ. Treatment of patients with dual hepatitis C virus and hepatitis B virus infection: resolved and unresolved issues. J Gastroenterol Hepatol. 2014;29(1):26–30. doi:10.1111/jgh.12421
71. Gish RG. HBV/HCV coinfection and possible reactivation of HBV following DAA use. Gastroenterol Hepatol. 2017;13(5):292–295.
72. Granito A, Marinelli S, Forgione A, et al. Regorafenib combined with other systemic therapies: exploring promising therapeutic combinations in HCC. J Hepatocell Carcinoma. 2021;8:477–492. doi:10.2147/JHC.S251729
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.