Back to Journals » Cancer Management and Research » Volume 14
Efficacy and Safety of Sintilimab in Combination with Concurrent Chemoradiotherapy for Locally Advanced Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (SHARED): Study Protocol of a Prospective, Multi-Center, Single-Arm Phase 2 Trial
Authors Wei J, Lu X, Liu Q, Fu Y, Liu S, Li L, Liu F, Fan X, Yang J, Yang Y, Zhao Y, Guan W, Liu B
Received 18 January 2022
Accepted for publication 1 June 2022
Published 17 June 2022 Volume 2022:14 Pages 2007—2015
DOI https://doi.org/10.2147/CMAR.S355687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Jia Wei,1 Xiaofeng Lu,2 Qin Liu,1 Yao Fu,3 Song Liu,4 Lin Li,3 Fangcen Liu,3 Xiangshan Fan,3 Ju Yang,1 Yang Yang,1 Yang Zhao,5 Wenxian Guan,2 Baorui Liu1
1The Comprehensive Cancer Centre of Drum Tower Hospital, Medical School of Nanjing University & Clinical Cancer Institute of Nanjing University, Nanjing, 210008, People’s Republic of China; 2Department of General Surgery, Drum Tower Hospital, Nanjing, 210008, People’s Republic of China; 3Department of Pathology, Drum Tower Hospital, Nanjing, 210008, People’s Republic of China; 4Department of Radiology, Drum Tower Hospital, Nanjing, 210008, People’s Republic of China; 5Department of Biostatistics, Nanjing Medical University, Nanjing, 210029, People’s Republic of China
Correspondence: Baorui Liu, The Comprehensive Cancer Centre of Drum Tower Hospital, Medical School of Nanjing University & Clinical Cancer Institute of Nanjing University, Nanjing, 210008, People’s Republic of China, Tel +86 2583107081, Email [email protected]
Purpose: Concurrent chemoradiotherapy (cCRT) is the mainstay therapy of locally advanced gastric (G) and gastroesophageal junction (GEJ) cancers with a poor prognosis. Programmed cell death receptor-1 (PD-1) inhibitor has been approved and recommended to treat ≥ 3 line G/GEJ patients. A significant clinical benefit of PD-1 inhibitors in addition to cCRT has been observed in locally advanced lung cancer. Sintilimab, a humanized IgG4 monoclonal antibody with high affinity and specificity for PD-1, has shown promising efficacy with an overall response rate of 85% in combination with chemotherapy in gastric cancer in a phase Ib study (NCT02937116).
Patients and Methods: SHARED is a prospective, multicentre, single-arm Phase II trial in China, exploring the efficacy of sintilimab in combination with cCRT in locally advanced G/GEJ adenocarcinoma. According to a Simon optimal two-stage clinical design, 34 patients will be enrolled. All the eligible patients will receive one cycle of induction chemotherapy (S-1 plus nab-PTX) combined with sintilimab, followed by cCRT (radiotherapy plus nab-PTX) combined with sintilimab. Prior to the surgery, patients will receive another cycle of chemotherapy (S-1 plus nab-PTX) combined with sintilimab. In the adjuvant setting, all participants will be treated with 3 cycles of chemotherapy (S-1 plus nab-PTX) combined with sintilimab. The primary endpoint is the rate of pathological complete response (pCR). The secondary endpoints include disease-free survival (DFS), major pathological response (MPR), R0 resection rate, surgical AEs, overall survival (OS), event-free survival (EFS), and safety profile. Moreover, the prognostic value of tumor biomarkers and immune biomarkers will be explored.
Conclusion: SHARED is designed to primally evaluate the efficacy and safety of sintilimab in combination with cCRT in locally advanced G/GEJ cancers and to prospectively validate the prognostic value of tumor biomarkers and immune biomarkers.
Keywords: anti-PD-1, induction chemotherapy, chemoradiotherapy, immunotherapy
Introduction
Gastric and gastroesophageal junction (G/GEJ) cancer is the fifth most common cancer and the third most deadly cancer worldwide, with an estimated 1 million new cases and 783,000 death in 2018.1 In China, the incidence and mortality rate of G/GEJ cancer is substantially higher than that of the rest of the world. According to the data from Cancer Registry in China, the country has about 403,000 new cases and 291,000 death each year. Moreover, about 54% of patients are diagnosed with locally advanced (stage II and III) G/GEJ cancers2 which are characterized by tumor infiltration of the serosa and the presence of regional lymph node metastasis. At this stage, surgery alone might not be sufficient, and even with R0 surgical resection, the prognosis remains dismal.3 Regarding the patients developing T3-4 and N2-3 cancer, the 5-year survival rate after surgery is lower than 15%.4
Multimodality regimens, such as perioperative chemotherapy and adjuvant or neoadjuvant concurrent chemoradiotherapy (cCRT), have been developed to improve the poor prognosis in advanced G/GEJ cancers. Clinical trials have demonstrated that perioperative chemotherapy has specific advantages in degrading tumor stage, increasing R0 resection rate, and improving pathological complete response (pCR) rate. Thus, patients with operable stage III to IVa G/GEJ cancers can obtain significant survival benefits.5–7 The therapeutic benefits of preoperative cCRT, on the other hand, have been confirmed by the long-term follow-up results from CROSS and POET trials.8,9 In addition, the multidisciplinary team (MDT) guided cCRT has dominated the treatment for stage III–IV inoperable tumors in western countries, which provides a therapeutic window for radical surgery to extend the overall survival.
In addition to surgery, chemotherapy and radiation therapy, immunotherapy has become a new pillar of cancer therapy, improving the prognosis across a wide variety of solid tumors. One main driver behind this success is the development of immune checkpoint inhibitors (ICIs). Immunotherapy targeting PD-1/PD-L1 signalling is the most widely investigated regimen, and emerging clinical evidence has demonstrated its efficacy and safety. As a monotherapy regimen, anti-PD-(L)1 has conferred an approximately 12% objective response rate (ORR) in patients with advanced G/GEJ cancers, and an overall survival benefit was noted.10–12 In particular, the ORR in PD-L1 positive tumors was reported to be 15.5% versus 5.5% in PD-L1 negative ones in the KEYNOTE-059 study. Based on such results, in 2017 the US Food and Drug Administration (FDA) approved the use of pembrolizumab in the third-line treatment for patients with recurrent or advanced gastric cancer whose tumors express PD-L1. Also, in 2017 the Japanese Ministry of Health, Labor and Welfare approved nivolumab for the treatment of unresectable recurrent or advanced gastric cancer, which has progressed after chemotherapy.
Enormous combination studies have been carried out to improve the anti-tumor activities of the anti-PD-(L)1 regimen. In addition to inhibiting tumor proliferation, chemotherapy can modulate the immune system by enhancing tumor antigenicity, disturbing immune-suppressive pathways, inducing immunogenic cell death, and increasing effector T-cell functions.13,14 Therefore, it is hypothesized that the addition of chemotherapy to anti-PD-(L)1 may render superior benefits in advanced G/GEJ cancer patients. The global Phase 3 trial CHECKMATE-649 demonstrated a significant improvement in PFS (7.7 months versus 6.0 months in chemotherapy alone cohort) and OS (14.4 months versus 11.1 months in chemotherapy alone cohort) by combining chemotherapy with anti-PD-(L)1.15,16 As a result of this study, in April 2021 FDA approved nivolumab in combination with chemotherapy as the first-line treatment for advanced or metastatic G/GEJ and esophageal adenocarcinoma. Likewise, in the ongoing ATTRACTION-4 trials, nivolumab combined with chemotherapy has shown encouraging clinical activity in first-line treatment in Asian patients with advanced G/GEJ, with improved PFS and ORR and favourable safety profile.17
On the other hand, irradiation activates host immunity by triggering immunogenic cell death to release the damage-associated molecular patterns (DAMPs), which leads to dendritic cell activation, tumor antigens presentation and antigen-specific T cells priming.18 Based on the insights gained, there is a strong rationale to support the use of PD-(L)1 inhibitors with cCRT to convert the “cold” tumors into “hot” tumors. The therapeutic efficacy of such regimen has been demonstrated in the Phase III PACIFIC trial of the PD-L1 inhibitor durvalumab in locally advanced, unresectable NSCLC following platinum-based cCRT.19 Compared to the placebo, an improved PFS (from 5.6 to 16.8 months) with a similar safety profile was noted with durvalumab, and its efficacy was irrespective of tumor PD-L1 status. Based on that, in 2018 FDA approved durvalumab for patients with unresectable stage III NSCLC whose disease has not progressed following platinum-based cCRT. Moreover, the phase 3 CHECKMATE-577 trial is the first study to evaluate a checkpoint inhibitor in the adjuvant setting after cCRT and has demonstrated a statistically significant improvement in disease-free survival (DFS) from 11.0 months to 22.4 months in patients with resected esophageal and GEJ cancers.20 Despite these advances, the ideal combination regimen of checkpoint inhibitors and cCRT is yet to be optimized in patients with advanced G/GEJ cancers.
Sintilimab is a highly selective, monoclonal IgG4 antibody that inhibits interactions between PD-1 and its ligands, with a robust anti-tumor response.21 In vitro, compared to nivolumab or pembrolizumab, sintilimab has a higher binding affinity and is able to bind with more PD-1 molecules on CD3 + T cells, and has better T cell activating characteristics.21 In a phase 1b study (NCT03745170), sintilimab combined with oxaliplatin/capecitabine has shown promising efficacy in gastric cancer with an ORR of 85%22 which warranted a phase 3 ORIENT-16 study (NCT03745170) to further investigate the therapeutic potential of sintilimab in combination with chemotherapy in patients with advanced G/GEJ cancers.23 The interim results from ORIENT-16 study were orally presented on 2021 ESMO, in which sintilimab was demonstrated to be the first PD-1 inhibitor to show superior OS and PFS with an acceptable safety profile, in combination with chemo, in Chinese patients with G/GEJ cancer regardless of PD-L1 expression status.24
In addition, in Japan, D2 gastrectomy plus adjuvant S-1 is the standard treatment for locally advanced gastric cancer [18, 19], while nab-paclitaxel (nab-PTX) is an approved second-line gastric cancer treatment [23, 24]. The combination of S-1 with nab-PTX has proven to be an effective and safe first-line regimen in clinical [25, 26]. Furthermore, the interim analysis from the JACCRO GC-07 trial has demonstrated that the postoperative S-1 plus docetaxel is effective with few safety concerns in patients with stage III gastric cancer25 and similar benefits were also noted with S-1 plus nab-PTX in untreated patients with metastatic gastric cancer.26
Therefore, we propose to conduct the SHARED (Sintilimab plus chemoradiotherapy in gastric and GEJ adenocarcinoma) study, a phase 2 trial designed to evaluate perioperative sintilimab combined cCRT (S-1 plus nab-PTX) for patients with locally advanced G/GEJ adenocarcinoma.
Materials and Methods
Objectives
The primary objective is to demonstrate whether adding sintilimab to cCRT (a combination of nab-PTX and S-1 with radiotherapy) improves pathological complete response (pCR) rate in patients with locally advanced G/GEJ adenocarcinoma. The secondary objectives include disease-free survival (DFS), major pathological response (MPR), R0 resection rate, surgical adverse events (AEs), overall survival (OS), event-free survival (EFS), and safety profile. Moreover, the prognostic value of tumor biomarkers and immune biomarkers will be evaluated.
Study Design and Participants
This is a prospective, multicentre, single-arm phase II trial in China. Eligible patients are aged 18 or older, histological or cytological confirmed locally advanced gastric or GEJ adenocarcinoma as defined according to 8th edition of American Joint Committee on Cancer (AJCC) classification of malignant tumors, a clinical-stage of III to IVa (T3N2-3M0, T4aN+M0 or T4bNanyM0) according to the 8th edition of Tumor, Node, Metastasis (TNM) classification, an Eastern Cooperative Oncology Group performance (ECOG) score ≤1, no prior cancer treatment, and one or more measurable lesions based on Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
Eligibility criteria on physiological parameters and organ function included adequate haematological function (absolute neutrophil count [ANC] ≥1.5×109/L, platelet count [PLT] ≥100×109/L, haemoglobin [Hb] ≥90 g/L, international normalized ratio (INR)/prothrombin time (PT) ≤ 1.5×upper limit of normal [ULN], and activated partial thromboplastin time (aPTT) ≤1.5×ULN), adequate hepatic function (plasma total bilirubin [PTB] ≤1.5×ULN, alanine transaminase [ALT], aspartate transaminase [AST] and alkaline phosphatase [AKP] concentration ≤2.5×ULN), and adequate renal function (creatinine concentration ≤1×ULN, albumin concentration ≥30g/L).
Pregnant, breastfeeding women or those positive in baseline pregnancy tests are not eligible, and all female patients will be on contraception during the study. Other exclusion criteria include patients diagnosed with gastric neuroendocrine tumors; patients with distant metastasis according to computed CT/MRI or endoscopic ultrasound (EUS); history of chemotherapy, radiotherapy or immunotherapy; patients with active malignant tumor in recent 5 years, except for basal cell or squamous cell cancer, superficial bladder cancer, and in-situ cervical or breast carcinoma; uncontrollable pleural effusion, pericardial effusion or ascites; severe cardiovascular disease within 12 months before recruitment, including coronary artery disease, ≥ grade 2 congestive heart failure, uncontrollable arrhythmias and myocardial infarction; patients with upper GI tract obstruction/bleeding or functional abnormality or malabsorption syndrome, which can affect absorption of S-1; uncontrollable concurrent infection and other concomitant diseases systemic diseases, or moderate or severe renal injury; allergic to any drug in this study; use of steroids or any other systemic immunosuppressive agents within 14 days before recruitment; use of corticosteroids (dosage >10mg/d prednisone or equivalent dose of other glucocorticoids) or other immunosuppressive agents within 4 weeks before recruitment, except patient treated with hormone for preventing allergy to contrast agents; receiving treatment from other studies within 4 weeks before recruitment; patients with autoimmune diseases or primary immunodeficiency; vaccinated with attenuated vaccine within 4 weeks before recruitment or plan to vaccinate during the study; active infections, including TB, HIV, HBV and HCV; and history of allogeneic stem cell transplantation or organ transplantation.
The study protocol and the informed consent forms have been reviewed and approved by institutional review boards and ethics committees at each institution. The study will be done in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines, and the written informed consent will be obtained from all enrolled patients. The study has been registered (ChiCTR1900024428).
Interventions
All patients will start with one cycle (3 weeks) of induction chemotherapy of S-1 (40mg/m2, PO, bid, D1 to D14) and nab-PTX (100–120mg/m2, IV, D1 and D8) in combination with sintilimab (200mg, IV, D1), followed by radiation therapy (45Gy/1.8Gy in 25 factions) with concurrent nab-PTX (80–100 mg/m2, IV, D1, D8, D15, and D22) and sintilimab (200mg, IV, D1 and D22) for 5 weeks. One to three weeks after the completion, patients will receive another cycle (3 weeks) of S-1 (40mg/m2, PO, bid, D1 to D14) and nab-PTX (100–120mg/m2, IV, D1 and D8) in combination with sintilimab (200mg, IV, D1). All patients will be preferably operated on within 1 to 3 weeks later. In case of any treatment-related adverse event (TRAE) that cannot be resolved shortly is noted, the patient will be urged to tumor assessment followed by the surgery. The adjuvant therapy will start in 4–6 weeks after the operation with 3 cycles (3 weeks) of S-1 (40mg/m2, PO, bid, D1 to D14 of each cycle), nab-PTX (100–120mg/m2, IV, D1 and D8 of each cycle) and sintilimab (200mg, IV, D1 of each cycle). Schematic diagram of SHARED regimen is in Figure 1.
Dose modification of sintilimab is not allowed in this study. Sintilimab will be withheld to manage intolerable adverse event until toxicity resolves. Corticosteroids will be used to manage immune-related adverse events (irAEs) with Sintilimab discontinuation allowed for no more than 12 weeks. Sintilimab will be terminated upon completion of treatment, disease progression or intolerance. Guidance for sintilimab delay or discontinuation after adverse events is in Table 1.
 |
Table 1 Guidance for Sintilimab Delay or Discontinuation |
Before the operation, all patients will be assessed by the multidisciplinary committee, and surgical protocol will be decided according to the clinical judgment. A subject must be withdrawn from the study if a disease progression is noted. The surgical protocol includes transthoracic esophagectomy with resection of the proximal stomach and mediastinal and abdominal lymphadenectomy for type 1 GEJ cancers and gastrectomy with transhiatal distal oesophagectomy plus D2 lymphadenectomy for types 2 and 3 GEJ cancers. For the patients with gastric cancer, total or subtotal distal gastrectomy with D2 lymphadenectomy will be performed. For inoperable patients, the treatment options will be evaluated and tailored to the context of the patients’ situation and needs. Exploratory laparoscopy will be conducted to exclude peritoneal metastases, if necessary.
During the study, all patients will be assessed by physical examination, weight, ECOG performance status, vital signs, routine laboratory tests (blood routine, blood chemistry, blood coagulation routine, urine routine, stool routine for occult blood, and thyroid function), and 12-lead electrocardiogram (ECG) within 7 days before day one of the first cycle, one day before day one in subsequent cycles, one day before the surgery, one day after study completion or withdrawal, and at the first follow-up visit. Besides, all patients will be examined by echocardiography within 7 days before starting the study, one day before the surgery, one day before the first adjuvant cycle, and one day after study completion or withdrawal.
Tumor assessment by means of computed CT/MRI and EUS will be done at baseline (within 4 weeks before enrolment), every six weeks (±7 days) perioperatively, every nine weeks (±7 days) postoperatively, and every 3 months until the development of disease progression or the start of new anticancer treatment after treatment completion.
Follow-Up
All patients will be followed up on a monthly basis in the first 3 months for safety, and telephone visits will be conducted after the first in-person hospital visit. After completing safety follow-up, monthly telephone visits for a maximum of 2 years will be implemented until death from any cause, lost to follow-up, consent withdrawal, or sponsor’s election to terminate the study prematurely.
Assessment of Outcomes
Primary Endpoints
The primary endpoint is the rate of pCR, which is defined as the absence of viable tumor cells assessed by histological evaluation criteria after preoperative therapy.
Secondary Endpoints
DFS is defined as the time to postoperative recurrence or death from any cause. OS is defined as the time interval from enrolment to all-caused death. EFS is defined as the time from enrolment to recurrence or death from any cause. Patients (including those lost to follow-up) still alive at the time of analysis (DFS, OS and EFS) will be censored at the last disease assessment date.
MPR is defined as tumor residual cells ≤10% in the surgical specimen. R0 resection rate is defined as the rate of the complete surgical removal of any residual cancer cells in the tumor bed.
Adverse events (AEs) will be monitored and graded according to the US National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE, version 4.03). All AEs will be recorded from the first dose of the study drug until 90 days after the last dose of the study drugs. Serious adverse events (SAEs) are defined as death, hospitalization or prolonged hospitalization, permanent or severe disability, teratogenesis, or other significant clinical sequels. Surgical AEs are defined as the complication which happens during or in 30 days after the operation.
Exploratory Endpoints
Exploratory endpoints are assessments of potential tumor biomarkers and the relationship between immune biomarkers and clinical response.
Peripheral blood samples (10ml) will be collected at baseline and on the same day of sintilimab administration to evaluate serum levels of related tumor biomarkers (eg, CEA, AFP, CA19-9, CA72-4, and CA24-2).
Tumor tissue samples will be collected at baseline and intrasurgery. TMB and MSI will be measured by FoundationOne CDx assay (Foundation Medicine, Cambridge, MA, USA). TMB-H will be defined as ≥10mut/Mb, and MSI-H will be defined as more than 30% of markers show instability in marker panels. PD-L1 expression will be measured by 22C3 pharmaDx assay (Agilent Technologies, Carpinteria, CA, USA), and PD-L1+ will be defined as PD-L1 CPS ≥1%. HER2 immunohistochemistry was performed on the BenchMark XT platform (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s recommendation. Normal gastric tissue will be collected concomitantly from each patient, and whole-exome sequencing (WES) will be performed to compare the transcriptome between tumor tissue and normal gastric tissue.
Statistical Analysis
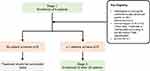
For the purpose of study design, a pCR rate at 15% (H0) in this setting will not be viewed as compelling for further study, while a pCR rate at 35% (Ha) or more will be considered of interest for further investigation. Based on this, a Simon optimal two-stage design will be employed with an H0=0.15 vs Ha=0.35, α=0.05 (one-sided) and power of 80%. Using these operating characteristics, the study plan is as follows (Figure 2):
- Stage 1: 9 patients will be accrued. If one or more patients demonstrate a pCR, the study will continue. Alternatively, if less than one patient responds, the study will be stopped.
- Stage 2: 25 additional patients will be accrued.
The sample size was calculated using the Power Analysis and Sample Size software program (PASS 16, NCSS Kaysville Utah USA).
EFS, DFS and OS will be analysed with the Kaplan–Meier method and the stratified Log rank test. The hazard ratio and its 95% confidence interval will be estimated with the Cox proportional hazards model. The incidences of the AEs and surgical AEs will be analysed using Fisher’s exact test. All comparisons are one-sided at the 0.05 level of significance. Data will be analysed with SAS version 9.4 statistical software (SAS Institute Inc., Cary, NC, USA).
Discussion
A number of clinical studies in patients with locally advanced G/GEJ cancers have evaluated the feasibility and efficacy of multimodal treatment approaches that center on a tumor-removing surgery with preoperative or postoperative chemotherapy or radiotherapy.9,27–31 Encouraged by the promising results from CHECKMATE-57720 the focus has been shifted towards the regimens that combine checkpoint inhibitors with chemotherapy in adjuvant or neoadjuvant settings (eg, ATTRACTION-5, KEYNOTE-585, MATTERHORN, and NCT04139135) for advanced G/GEJ cancers. The SHARED study is designed to begin with induction chemotherapy with anti-PD-1 to achieve maximum synergistic effects with subsequent cCRT. Given the potential of radiotherapy to revert the immune-suppressive tumor microenvironment, the addition of anti-PD-1 to cCRT is expected to provide a holistic solution for patients at a clinical-stage of T3N2-3M0, T4aN+ M0 or T4bNanyM0, a heterogenous population of both operable and inoperable patients.
Overall, there is a strong rationale warranting a phase 2 clinical trial to explore sintilimab combined with concurrent chemoradiotherapy in locally advanced G/GEJ cancers. The study aims are twofold, targeting to increase the pCR rate for operable patients to prolong the overall survival while ensuring that all patients, including inoperable patients, will gain more survival benefits from surgery. In addition, this study also has the capacity to prospectively evaluate the prognostic value of the status of TMB and MSI, PD-L1 expression level and tumor biomarkers, which can be applied to define eligibility criteria and stratification factors for further trials in locally advanced G/GEJ cancers.
Data Sharing Statement
The trial is ongoing, and no data is available.
Ethics Approval and Consent to Participate
The trial has been approved by the Ethics Committee of the Comprehensive Cancer Centre of Drum Tower Hospital. All patients are required to sign the written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
All investigators will not receive any remuneration. This research did not receive any grant from funding agencies in the public, commercial or not-for-profit sectors.
Disclosure
Innovent Biologics, Inc. provided the sintilimab, but had no role in study design, or writing of the protocol. The authors report no other conflicts of interest in this work.
References
1. Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. doi:10.5114/pg.2018.80001
2. Zheng L, Wu C, Xi P, et al. The survival and the long-term trends of patients with gastric cancer in Shanghai, China. BMC Cancer. 2014;14(1):300. doi:10.1186/1471-2407-14-300
3. Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11(5):531–546. doi:10.6004/jnccn.2013.0070
4. Sites A. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014.
5. Ji J, Shen L, Li Z, et al. Perioperative chemotherapy of oxaliplatin combined with S-1 (SOX) versus postoperative chemotherapy of SOX or oxaliplatin with capecitabine (XELOX) in locally advanced gastric adenocarcinoma with D2 gastrectomy: a randomized phase III trial (RESOLVE trial). Ann Oncol. 2019;30:v877. doi:10.1093/annonc/mdz394.033
6. Kang YK, Yook JH, Park YK, et al. Phase III randomized study of neoadjuvant chemotherapy (CT) with docetaxel (D), oxaliplatin (O) and S-1(S) (DOS) followed by surgery and adjuvant S-1, vs surgery and adjuvant S-1, for resectable advanced gastric cancer (GC) (PRODIGY). Ann Oncol. 2019;30:v876–v877. doi:10.1093/annonc/mdz394.032
7. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–1957. doi:10.1016/s0140-6736(18)32557-1
8. Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–190. doi:10.1016/j.ejca.2017.04.027
9. Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi:10.1016/s1470-2045(15)00040-6
10. Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–726. doi:10.1016/S1470-2045(16)00175-3
11. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013–e180013. doi:10.1001/jamaoncol.2018.0013
12. Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/S0140-6736(17)31827-5
13. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi:10.1038/nri2216
14. Hato SV, Khong A, de Vries IJM, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. 2014;20(11):2831–2837. doi:10.1158/1078-0432.CCR-13-3141
15. Moehler M, Shitara K, Garrido M, et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study. Ann Oncol. 2020;31:S1191. doi:10.1016/j.annonc.2020.08.2296
16. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2
17. Boku N, Ryu MH, Oh DY, et al. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann Oncol. 2020;31:S1192. doi:10.1016/j.annonc.2020.08.2297
18. Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3(4):e28518. doi:10.4161/onci.28518
19. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937
20. Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–1203. doi:10.1056/NEJMoa2032125
21. Wang J, Fei K, Jing H, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. INMABS. 2019;11(8):1443–1451.
22. Xu J, Jin Y, Liu Y, Zhou H, Wang Y. Abstract CT213: ORIENT-16: sintilimab plus XELOX vs placebo plus XELOX as 1< sup> st</sup> line treatment for unresectable advanced gastric and GEJ adenocarcinoma. Cancer Res. 2019;79(13 Supplement):CT213. doi:10.1158/1538-7445.AM2019-CT213
23. Jiang H, Zheng Y, Qian J, et al. Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase Ib clinical trial. BMC Cancer. 2020;20(1):760. doi:10.1186/s12885-020-07251-z
24. Xu J, Jiang H, Pan Y, et al. LBA53 sintilimab plus chemotherapy (chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann Oncol. 2021;32:S1331. doi:10.1016/j.annonc.2021.08.2133
25. Yoshida K, Kodera Y, Kochi M, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–1304. doi:10.1200/jco.18.01138
26. He -M-M, Wang F, Jin Y, et al. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci. 2018;109(11):3575–3582. doi:10.1111/cas.13813
27. Ajani JA, Winter K, Fau Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24(24):3953–3958.
28. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi:10.1056/NEJMoa055531
29. De Paoli A, Navarria F, Torrisi E, et al. Neoadjuvant epirubicyn, oxaliplatin, capecitabine and radiation therapy (NEOX-RT) followed by surgery for locally advanced gastric cancer (LAGC): a phase II multicentric study. J Clin Oncol. 2019;37(15_suppl):4066. doi:10.1200/JCO.2019.37.15_suppl.4066
30. Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: a randomized, phase III trial of perioperative ECF chemotherapy with or without preoperative chemoradiation for resectable gastric cancer: interim results from an international, intergroup trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol. 2017;24(8):2252–2258.
31. Macdonald JS, Smalley SR, Benedetti J. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–730. doi:10.1056/NEJMoa010187
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.