Back to Journals » OncoTargets and Therapy » Volume 15
Efficacy and Safety of Regorafenib with or without PD-1 Inhibitors as Second-Line Therapy for Advanced Hepatocellular Carcinoma in Real-World Clinical Practice
Authors Liu K, Wu J, Xu Y , Li D , Huang S, Mao Y
Received 29 July 2022
Accepted for publication 10 September 2022
Published 1 October 2022 Volume 2022:15 Pages 1079—1094
DOI https://doi.org/10.2147/OTT.S383685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Kan Liu,1,2,* Jianbing Wu,1,2,* Yongkang Xu,1,2 Dan Li,1,2 Shenlang Huang,1,2 Ye Mao1,2
1Department of Digestive Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China; 2Jiangxi Key Laboratory of Clinical and Translational Cancer Research, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ye Mao, Email [email protected]
Background: Regorafenib is the first oral targeted drug as a second-line agent in patients with advanced hepatocellular carcinoma (HCC) who progressed on sorafenib treatment. Recently, several studies demonstrated that the combination of regorafenib and PD-1 inhibitors showed a synergistic effect. Our study aimed to evaluate the efficacy of regorafenib with PD-1 inhibitors (RP) and regorafenib alone (R) as second-line treatment for advanced HCC.
Methods: From October 2018 to January 2022, our retrospective study evaluated advanced HCC patients who received regorafenib with PD-1 inhibitors or regorafenib alone as a second-line treatment at the Second Affiliated Hospital of Nanchang University, China. The efficacy and safety were compared between RP and R groups.
Results: In total, 78 patients were enrolled in our study and were separated into two groups – RP group (48) and R group (30) – according to the criteria. The ORR of RP group and R group was 18.8% and 10%, respectively, and the DCR was 66.7% and 43.3%, respectively. The RP group had a longer mPFS (5.9 months vs 3.0 months, P< 0.001) and mOS (12.9 months vs 10.3 months, P=0.010) than the R group. Regorafenib monotherapy is an independent prognostic factor for OS and PFS. In OS, subgroup analysis showed that patients with AFP ≥ 400ng/mL, BCLC C stage and extrahepatic metastasis may benefit from RP, while in PFS, subgroup analysis showed that patients with BCLC C stage, AFP ≥ 400ng/mL, extrahepatic metastasis, ALBI ≥-2.60 and first-line treatment of sorafenib may benefit from RP. The incidence of grade 3/4 adverse reaction in the two groups was 22.9% and 23.3%, respectively, with no significant statistically difference (P=0.966).
Conclusion: In the second-line therapy of advanced HCC, compared to regorafenib alone, the combination of regorafenib and PD-1 inhibitors showed promising efficacy and tolerable drug toxicity.
Keywords: hepatocellular carcinoma, second-line therapy, immunotherapy, regorafenib, PD-1 inhibitor
Introduction
HCC, the primary tumor of the liver, accounting for 75–85% of primary liver tumors, is the fifth most common cause of cancer worldwide and the fourth leading cause of cancer-related death worldwide.1 The onset of HCC is insidious, and it is commonly diagnosed as locally advanced or distant metastases, which cannot be treated with surgery. The prognosis is poor, and the five-year survival rate is less than 20%.2,3 Currently, the systemic first-line therapy of advanced HCC is mainly atezolizumab plus bevacizumab (A+T), sorafenib and lenvatinib,4 and sintilimab plus bevacizumab analogs and Donafinib are also approved as first-line therapy in China.5,6 When the patient’s first-line therapy fails or cannot be tolerated, regorafenib, cabozantinib, ramucirumab, pembrolizumab and nivolumab are available as second-line therapy options.4 However, the efficacy of second-line targeted therapy for advanced HCC patients was unmet.7–10 The overall efficacy of immune checkpoint inhibitors has also not achieved very satisfactory results.11–13 Yasuda et al found that the combination of anti-VEGFR2 and anti-PD-1 antibody could enhance the production of interferon-γ, tumor necrosis factor-α and granzyme B, thus enhancing the immune response of tumor mice.14 Researches have indicated that anti-angiogenesis targeted treatment can reverse the immunosuppressive state of tumor microenvironment, normalize vascular permeability and immune environment, improve the immune response rate of immunosuppressants and reduce tumor drug resistance.15,16 There is no standard combination regimen in the second-line therapy of HCC, and regorafenib may be the best choice.
Regorafenib is a small molecular multi-target tyrosine kinase inhibitor that inhibits rapidly accelerated fibrosarcoma 1(RAF-1), B-RAF, B-RAFV600E (B-RAF mutants), VEGFR1, 2 and 3, carcinogenic protein kit and RET, angiopoietin 1 receptor (TIE2), platelet-derived growth factor receptor (PDGFR), fibroblast growth receptor (FGFRs) 1 and 2.17–19 The RESORCE is a clinical trial of regorafenib as second-line therapy for advanced HCC, comparing regorafenib with placebo in patients with advanced HCC who failed to progress in first-line therapy with sorafenib. The results showed that regorafenib significantly prolonged mOS (10.6 months vs 7.8 months), mPFS (3.1 months vs 1.5 months), and improved ORR (11% vs 4%) and DCR (65% vs 36%).10 Studies have shown that regorafenib can 1) modulate macrophages to promote the proliferation and activation of CD8+T cells and improve antitumor immunity20–22; 2) inhibit colony-stimulating factor 1 receptor CSF1R to reduce tumor infiltration to macrophages and damage tumor immunity17,23; 3) reduce TAM infiltration, reverse vascular polarization from M2 tumor promoting phenotype to M1 tumor growth inhibitory phenotype24; 4) induce inhibition of STAT3 signal pathway and up-regulate NKG2D ligand, promote NK cells to recognize and kill HCC cells, which has the effect of anti-immunosuppression and promoting antitumor immunity.19,25 All these provide the theoretical principle for the combined strategy of regorafenib and PD-1 inhibitors.
The limitation of HCC monotherapy promotes the development of anti-angiogenesis targeted therapy and anti-PD-1/PD-L1 combination strategy, which have achieved encouraging achievements in first-line therapy. In the IMbrave150 trial, atezolizumab plus bevacizumab achieved better efficacy than sorafenib alone.26 ORIENT-2 studies show stronger efficacy of sintilimab plus bevacizumab analogs compared to sorafenib alone.5 All these provide an excellent practical basis for regorafenib combined with immunotherapy. Studies have shown that, compared with regorafenib or PD-1 inhibitor monotherapy, regorafenib/PD-1 inhibitor combination treatment can improve the survival rate and increase the survival benefit, and dual anti-PD-1/VEGFR-2 therapy can overcome the therapeutic resistance to either monotherapy, and increase the resistance to anti-PD-1 therapy, which has been confirmed in the model of HCC in mouse.22,27 In a recent Phase I b dose-escalation trial of regorafenib in combination with pembrolizumab (NCT03347292), ongoing results show that 30% of the evaluable patients showed partial responses, 61% had stable disease,28 and the remainder of the results are being tracked and have yet to be reported. Currently, a number of clinical studies, such as the I/II phase trial of regorafenib plus nivolumab (NCT04170556), the Phase II trial of regorafenib combined with tislelizumab (NCT04183088), and phase II trial of regorafenib plus sintilizumab (NCT04718909) et al,19 are in full swing in the first/second-line therapy of advanced HCC, showing the great potential of regorafenib plus PD-1/PD-L1 inhibitors in advanced HCC therapy.
Although multiple clinical trials of regorafenib combined with anti-PD-1/PD-L1 are underway, most of the final results have not yet been released, and the research results in real-world clinical practice are also limited. Therefore, in this study, we retrospectively analyzed the clinical data of advanced HCC in the second affiliated Hospital of Nanchang University in China, and compared the efficacy and safety of regorafenib plus PD-1 inhibitors and regorafenib monotherapy in the second-line treatment of HCC.
Patients and Methods
Patients
In this retrospective cohort study, we included patients who received regorafenib or regorafenib in combination with a PD-1 inhibitor at the Second Affiliated Hospital of Nanchang University, China, of consecutive HCC patients who developed disease progression or intolerance during first-line therapy (sorafenib or lenvatinib, etc.). The study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Second Affiliated Hospital of Nanchang University, China.
We included patients who met all of the following inclusion criteria: 1) at least 18 years old; 2) received regorafenib or regorafenib in combination with PD-1 inhibitor after failure of first-line therapy (sorafenib or lenvatinib, etc.); 3) HCC was radiologically or histologically confirmed according to American Association for the Study of Liver Disease criteria, with measurable disease based on the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1); 4) Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0–1. Patients were excluded from the study if they met any of the following exclusion criteria: 1) used targeted drugs other than regorafenib during second-line therapy; 2) history of severe underlying diseases and may not be able to tolerate treatment; 3) had a concurrent malignancy other than HCC.
Baseline Data Collection
Record the baseline data of the patient after determining the baseline date, including age, sex, tumor stage, previous treatment of primary focus, extrahepatic metastasis, number of intrahepatic tumors, ECOG score, Child–Pugh score and stage, alpha-fetoprotein (AFP) level, liver biochemical indexes [alanine transferase (ALT), aspartate transferase (AST), alkaline cholinesterase (ALP)], total bilirubin level, albumin level and so on. The albumin–bilirubin classification (ALBI) was used to evaluate liver reserve function, ALBI=0.66 × log10 (bilirubin μmol/L)-0.085 × (albumin g/L), and divided into three grades according to the risk of death (<25%, 25%–90%, >90%): ALBI grade 1 (ALBI score ≤-2.60); ALBI grade 2 (ALBI score >-2.60 and ≤-1.39; ALBI grade 3 (ALBI score >-1.39). All CT and MRI analyses were based on independent assessments by two imaging physicians and final decisions were made by consensus when there was any ambiguity.
Treatment Methods
Patients in the RP group and R group were treated with 80–160mg regorafenib once a day for 21 days and then stopped for 7 days, 28 days as a cycle. To control treatment-related adverse reactions, regorafenib dose adjustment by the amount of 40 mg or transient interruption (<14 days) was allowed. Regarding immunotherapy, patients in the RP group were given the recommended dose of PD-1 inhibitor by intravenous drip on the first day of administration of regorafenib: toripalimab (240 mg) every 3 weeks, sintilimab or tislelizumab or camrelizumab (200 mg) every 3 weeks. Patients in the R group did not use PD-1 inhibitors.
Efficacy and Safety Assessment
The primary endpoint of this study is PFS, and the secondary endpoints are OS, ORR, DCR, adverse reactions. The date of the first regorafenib administration was assigned as the baseline date. The tumor was measured by computed tomography or magnetic resonance imaging every 2–3 treatment cycles, and according to the RECIST version 1.1 and mRECIST to evaluate the tumor response until the disease progresses or the treatment is changed. Tumor response was defined as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). ORR was defined as the optimal percentage of patients were evaluated the CR and PR. DCR was defined as the proportion of patients with the best overall response to CR, PR, or SD. PFS was calculated from the start of treatment until disease progression or death from any cause or the last follow-up in censored data. Toxicity assessments were based on the National Cancer Institute General Toxicity Criteria Version 5.0 (CTC5.0). The date of cutoff was March 20, 2022.
Statistical Analysis
The baseline demographic and clinical characteristics of different treatment groups were compared. In the inter-group comparison, independent sample t-test was used for continuous variables, and chi-square test or Fisher’s exact test was used for classified variables. The distribution of variables in normal distribution is described by mean standard deviation, and the distribution of variables in non-normal distribution is described by median and range. The Kaplan–Meier method was used to analyze OS and PFS, and drawn the survival curve. The difference of survival curve was tested by logarithmic rank test. Cox regression model was used for univariate and multivariate analysis, and hazard ratio (HR) and confidence interval (CI) were calculated. P<0.05 was considered statistically significant. All data were analyzed using IBM SPSS Statistics version 24 and GraphPad Prism 8.0.
Results
Study Population
We included 95 advanced HCC patients who received regorafenib with or without PD-1 inhibitors in the Second Affiliated Hospital of Nanchang University from October 2018 to January 2022, and finally 78 patients (RP group, n=48; R group, n=30) were enrolled in this study (Figure 1).
 |
Figure 1 Flowchart of patient screening. |
The baseline data show that 68.8% (33/48) patients in the RP group and 76.7% (23/30) patients in R group had HBV infection (P=0.045), 83.3% (40/48) patients in the RP group and 76.7% (23/30) patients in R group were BCLC C stage (P=0.467). The majority of targeted drugs used in first-line therapy in the R group were sorafenib (86.7%), while in the RP group, 64.6% of the patients used sorafenib and 35.4% used lenvatinib (P=0.032). Only 8.3% (4/48) of patients in the RP group had a Child–Pugh score of 7–9, while 30% (9/30) of the patients in the R group had a Child–Pugh score of 7–9 (P= 0.043). And, 93.8% and 76.3% of the patients previously received interventional therapy (TACE or HAIC) in RP and R groups, respectively (P=0.039). The initial doses of regorafenib in these two groups were 80 mg (RP group, n=28; R group, n=15), 120 mg (RP group, n=19; R group, n=13), and 160 mg (RP group, n=1; R group, n=2). The most common PD-1 inhibitors used in the RP group was camrelizumab (52.1%). The median duration of first-line treatment was 6.2 months (IQR, 4.0–10.1) in the RP group, while that in the R group was 8.2 months (IQR, 4.2–13.9) (P=0.305). The median cycle of second-line therapy was 6 (IQR, 4–10) in the RP group, and that in R group was 3 (IQR, 3–6) (P=0.353) (Table 1).
 |
Table 1 Baseline Characteristics of Patients at Initiation of Second-Line Therapy |
Clinical Efficacy
The ORR of the RP group was higher than that of the R group (18.8% vs 10.0%, by mRECIST, P=0.353; 12.5% vs 6.7%, by RECIST 1.1, P=0.704). CR was the best therapeutic response, with 1 patient in group R showing complete response (by mRECIST). The DCR for the RP group was significantly higher than that in the R group (66.7% vs 43.3%, by mRECIST, P=0.042), and the patients with PD in the RP group were significantly lower than that in the R group (31.1% vs 56.7%, P=0.026) (Table 2).
 |
Table 2 Treatment Responses |
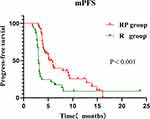
During the follow-up period, 70.8% (34/48) of the patients in the RP group and 86.7% (26/30) of patients in the R group had tumor progression. The mPFS time in the RP group was 5.9 months (95% CI:4.9–6.9), while that in the R group was 3.0 months (95% CI:2.8–3.2), the difference was statistically significant (P<0.001) (Figure 2).
Univariate and multivariate analyses were performed in this study (Table 3). The results showed that regorafenib monotherapy (HR=0.345, 95% CI:0.185–0.641, P<0.001) and AFP (HR=0.297, 95% CI:0.161–0.548, P<0.001) were independent prognostic factors for PFS. The PFS benefit analysis showed that there was a significant difference in PFS between the RP and R groups in patients with AFP ≥ 400ng/mL (P<0.001) (Figure 3B), ALBI ≥−2.60 (P=0.001) (Figure 3D), BCLC C stage (P<0.001) (Figure 3F), with extrahepatic metastasis (P<0.001) (Figure 3H), but there was no significant difference between the two groups in patients with AFP <400mg/mL (P=0.152) (Figure 3A), ALBI <-2.60 (P=0.177) (Figure 3C), BCLC B stage (P=0.076) (Figure 3E), without extrahepatic metastasis (P=0.157) (Figure 3G).
 |
Table 3 Univariate and Multivariate Analyses of Risk Factors for Progression-Free Survival |
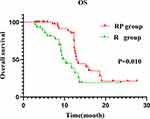
During the follow-up period, 41.7% (20/48) of patients in the RP group died and 56.7% (17/30) of patients in the R group died. Compared with the R group, the RP group had a longer median OS (12.9 months 95% CI:11.9–13.9 vs 10.3 months 95% CI:8.3–12.3) (P=0.010) (Figure 4). Univariate and multivariate analysis showed that regorafenib monotherapy (HR=0.436, 95% CI:0.191–0.966, P=0.049) was an independent prognostic factor for OS (Table 4).
 |
Table 4 Univariate and Multivariate Analyses of Risk Factors for Overall Survival |
Subgroup Analysis
According to the different variables to compare the risk of death and progression between the RP and R groups (Figure 5). The results showed that the RP group had a lower risk of progression than the R group in all subgroups and the RP group had a lower risk of death than the R group in almost all subgroups (except BCLC B stage and without extrahepatic metastasis). The RP group reduced the risk of death, and significant benefits were seen in patients with BCLC C stage (HR=0.288, 95% CI: 0.139–0.594), with extrahepatic metastasis (HR=0.273, 95% CI: 0.131–0.572) and AFP ≥400 ng/mL (HR=0.275, 95% CI: 0.105–0.716). Meanwhile, the RP group also reduced the risk of progression, with significant benefits in patients with BCLC C stage (HR=0.341, 95% CI:0.190–0.614), with extrahepatic metastasis (HR=0.324, 95% CI: 0.178–0.590), AFP ≥400 ng/mL (HR=0.067, 95% CI: 0.022–0.201), first-line treatment with sorafenib (HR=0.434, 95% CI: 0.239–0.787) and ALBI ≥-2.60 (HR=0.346, 95% CI: 0.177–0.675).
Safety
The adverse events are shown in Table 5. Adverse events (AE) occurred in all patients and there were no treatment-related deaths. The frequent AEs of any grade in RP were hand-foot skin reaction (58.3%), proteinuria (56.3%), liver dysfunction (37.5%), diarrhea (31.3%), hyperbilirubinemia (25.0%), hypothyroidism (22.9%) and fatigue (20.8%). The common AE of any grade in group R were hand-foot skin reaction (40.0%), liver dysfunction (33.3%), diarrhea (26.7%), hyperbilirubinemia (23.3%) and proteinuria (20.6%). More than 5% of the grade 3/4 adverse reactions in the RP group were hand-foot skin reaction (n=3) and rash (n=3), and in the R group it was hand-foot skin reaction (n=5). The incidence of grade 3/4 adverse reactions was 22.9% in the RP group and 23.3% in the R group, with no significant difference between the two groups (P=0.966). In the RP group, 1 patient discontinued treatment due to gastrointestinal hemorrhage (grade 4), 1 patient stopped treatment because of fatigue (grade 3), and 1 patient discontinued PD-1 inhibitor because of rash (grade 4). In the R group, 1 patient also discontinued treatment because of hand-foot skin reaction (grade 4).
 |
Table 5 Adverse Events |
Discussion
Since sorafenib was first proved to prolong the survival time of patients with advanced HCC and was approved as the first-line therapy of HCC in 2007,29 the systemic therapy of anti-angiogenesis targeted therapy as an effective treatment of HCC now has significantly developed, especially in the second-line therapy, regorafenib, apatinib, cabozantinib, etc have been approved. However, in the clinical therapy of HCC, the low ORR, drug resistance and patient intolerance of anti-vascular targeted drugs force oncologists to constantly study new treatments modalities.30,31 In recent years, tumor immunotherapy based on immune checkpoint inhibitors has made significant progress. The ORR of about 20% and a considerable PFS have caught everyone’s eye.32 Based on the research findings of CheckMate-040 and KEYNOTE −224 trial, the US Food and Drug Administration (FDA) conditionally approved nivolumab and pembrolizumab as second-line treatments for advanced HCC.11,12 However, due to the special immune environment of the liver, the response rate of PD-1 inhibitors in different populations of HCC patients is low,33 and in the two pivotal confirmatory Phase III studies of nivolumab and pembrolizumab in 2019 the unexpected failure of CheckMate-459 and KEYNOTE224 to meet the prespecified primary endpoint.34,35 The exploration of combined therapy strategies of PD-1 inhibitors and anti-angiogenic targeted therapy has become the focus of research, and encouraging results have been achieved in the first-line treatment (bevacizumab plus atezolizumab).26 Due to its extensive inhibitory effect on tumor angiogenesis and favorable influences on macrophage polarization and cytotoxic CD8+ lymphocyte activity, regorafenib has become a strong candidate for combined therapy with PD-1 inhibitors in second-line therapy.19,36 There are no reports directly comparing RP and R, and our study shows that RP has a preferable survival benefit than R and does not increase the risk of adverse reactions.
In the recent retrospective study of Haidong Zhu et al, it mainly analyzed the efficacy and safety of regorafenib alone or in combination (including immune checkpoint inhibitors, TACE or triple therapy) in the first to fourth line of liver cancer. It is worth noting that more than half of the patients with Child–Pugh B grade were included. The results showed that regorafenib alone or in combination was effective, safe, effective and long-term in the treatment of advanced hepatocellular carcinoma. Especially in patients who received second-line treatment, the OS rates at 6 months and 12 months were 85.6% and 76.7%, respectively, which was similar to the main direction of our study. Our study further focused on the efficacy and safety of regorafenib alone or combined with PD-1 inhibitors in second-line therapy.37 Our results showed that in advanced HCC patients who failed to develop first-line therapy (sorafenib or lenvatinib), the regimen of regorafenib plus PD-1 inhibitors had better survival benefits than that of regorafenib alone in second-line therapy, and the mOS increased from 10.3 months to 12.9 months. Multivariate analysis showed that the combination of PD-1 inhibitors was an independent predictor of improving OS and PFS. This may be attributed to the fact that ORR, DCR and PFS in the RP group were significantly higher than those in the R group. In a recent phase I b dose-escalation trial (NCT03347292) of regorafenib plus pembrolizumab, current results showed that ORR was about 30% in assessable patients.28 In a retrospective study, the median OS and mPFS of regorafenib plus sintilimab in second-line therapy of advanced HCC were 13.4 months and 5.6 months, respectively, and the ORR was 36.2% (by mRECIST).38 In our study, the mOS of RP was 12.9 months and the mPFS was 5.9 months, compared with a lower ORR of 18.8%. The reason may be, first, in our study, due to various PD-1 inhibitors options, the efficacy of regorafenib plus PD-1 inhibitors for patients may be different.19,33,39 Therefore, we plan to enrol more samples of combined therapy in the next step and evaluate the effectiveness of different combinations. Second, the initial dose of regorafenib in most patients in our study was the lowest dose of 80 mg, and the dosage of regorafenib may also affect its optimal effect.40,41 In general, regorafenib plus PD-1 inhibitors is superior to regorafenib alone: First of all, regorafenib induces hypoxia-inducible factor 1 (HIF-1), which increases the expression of PD-L1 on MDSC and enhances its ability to inhibit T cell proliferation, leading to drug resistance, while PD-1 inhibitors may overcome the resistance of HIF-1-related anti-angiogenic targeted drugs;42–44 in addition, regorafenib also inhibits JAK1/2STAT1 and MAPK signal transduction, reduce the expression of PD-L1 in tumor,45 inhibit CSF1R damage tumor immunity,23 and regulate macrophages to increase the proliferation and activation of CD8+T cells by targeting VEGFR2/3,22 which may improve the efficacy of PD-1 inhibitors. Notably, in the R group, BCLC B stage HCC patient, who progressed on surgery, TACE and immunotherapy, was evaluated as mCR after a period of regorafenib and TACE treatment. At the end of cutoff date, the patient still on treatment. Regorafenib combined with interventional therapy may also have better efficacy, of course, this requires further research.
Some studies have shown that AFP can promote HCC progression by inhibiting the HuR-mediated Fas/FADD apoptosis pathway, and the expression level of AFP is negatively correlated with the apoptosis rate of mouse and human hepatocellular carcinoma cells. Blocking AFP may be an effective strategy for treating advanced HCC.46 Our study indicated that the efficacy of RP was preferable than that of R group in patients with AFP ≥ 400 ng/mL, and the difference was statistically significant. In the subgroup analysis, the OS and PFS of RP in the subgroup with AFP≥400ng/mL were significantly better than those in the R group, which indicated that the RP group was more effective for advanced HCC with AFP ≥400ngmL. The presence of extrahepatic metastases and BCLC C stage are both indicative of advanced tumor stage, often indicating poor prognosis and shorter survival time.47 In our study, in patients with extrahepatic metastases and BCLC C stage, the RP group achieved a more significant benefit compared with the R group, and the difference was statistically significant, and this result was also reflected in the subgroup analysis. In patients with BCLC C stage and patients with extrahepatic metastasis, the OS and PFS of RP were significantly preferable than those of R group. These results suggest that patients with advanced HCC can benefit more from the RP regimen. However, whether patients without extrahepatic metastasis and BCLC B stage use the RP regimen have super-progressive drugs and whether they can benefit from it needs further research.48
In the RESORCE study, the mOS of the regorafenib group was 10.6 months.10 In our study, the mOS of the R group was 10.3 months, which is similar to our results. In the subgroup analysis, we also found an interesting phenomenon that the RP as second-line treatment in patients with first-line treatment of sorafenib can have a better PFS benefit than R, which has not been observed in patients with first-line therapy of lenvatinib. A retrospective study conducted in Korea and Italy showed that sorafenib-regorafenib treatment sequence reduced the risk of death and had a better OS.49 A retrospective study in Japan showed that advanced HCC patients treated with sorafenib were more likely to progress more slowly when they were converted to regorafenib.50 Some studies have shown that most of the targets of lenvatinib act on HCC are similar to regorafenib.19 Both regorafenib and lenvatinib can contribute to the treatment of HCC by affecting the TLR signal pathway,51 and the deregulation of Keap1/Nrf2 pathway after Keap1 gene inactivation in HCC will lead to drug resistance of human hepatocellular carcinoma cells to lenvatinib and regorafenib by up-regulating downstream Nrf2 genes and reducing ROS levels,52 Therefore, the selection of regorafenib after the use of lenvatinib may make patients more likely to develop drug resistance, and sequential therapy with sorafenib-regorafenib may be more suitable for HCC patients than lenvatinib-regorafenib,53 we suggest that patients who fail to use sorafenib in the first line can choose RP regimen as the second-line treatment, of course, this also requires retrospective and prospective studies with large samples to obtain higher medical evidence support.
Studies have shown that more than 50% of the patients with advanced HCC have no chance to receive second-line therapy, which is mainly due to impaired liver function, so effective tools to monitor liver function and liver reserve capacity are very important.53 Different from the traditional Child–Pugh score (CAP), ALBI grading is more objective and accurate, has wider applicability,54 and can better judge the prognosis and help clinical decision-making. In our study, patients with ALBI ≥-2.60 achieved a better PFS with RP than R, and the difference was statistically significant. This was also reflected in the subgroup analysis. One of the main problems in the application of PD-1 inhibitors in HCC is the lack of biomarkers that can predict reactions and hepatotoxicity,54,55 and ALBI may be the key to solving this problem. RP is tolerated in patients with ALBI ≥-2.60, may have a better benefit of PFS, which can be used as a choice for patients with poor liver function reserve after first-line treatment failure.
Our study also has a number of limitations. Firstly, our study is a single-center retrospective study. Secondly, the RP group used four different PD-1 inhibitors in our study, which influenced the consistency of the treatment process. Thirdly, the sample size of this study is relatively small, the results of subgroup analysis need to be handled carefully, and extensive sample data necessary for further study. Fourth, the dose of regorafenib is not uniform, which further increases the heterogeneity of this study. Thus, the results of our study should be further extended to large-scale prospective studies according to different PD-1 inhibitors in order to obtain a higher level of medical evidence and clinical conclusions.
In summary, compared with regorafenib monotherapy, regorafenib plus PD-1 inhibitor has better therapeutic response, PFS and OS in the second-line therapy of HCC, and without increasing the incidence of AE, so it is tolerable, especially, patients with AFP ≥400ng/mL, BCLC C stage, with extrahepatic metastasis and ALBI ≥-2.60 seem to benefit more.
Data Sharing Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Approval
The study conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Second Affiliated Hospital of Nanchang University, China. In view of the nature of the retrospective study, this study does not require the patient’s informed consent. In any case, patient data confidentiality was guaranteed.
Acknowledgments
We thank our colleagues for their valuable efforts and comments on this manuscript. We also sincerely thank the editors and reviewers for their careful review and valuable opinions.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by National Natural Science Foundation of China (No. 82160602) and Youth Project of science and Technology Research Project of Jiangxi Education Department (No. GJJ200251).
Disclosure
The authors declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
References
1. Sung H, Ferlay J, Siegel RL., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (Concord-2). Lancet. 2015;385(9972):977–1010. doi:10.1016/S0140-6736(14)62038-9
4. Gordan JD, Kennedy EB, Abou-Alfa GK, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38(36):4317–4345. doi:10.1200/JCO.20.02672
5. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
6. Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled Phase II-III trial. J Clin Oncol. 2021;39(27):3002–3011. doi:10.1200/JCO.21.00163
7. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
8. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
9. Qin S, Li Q, Gu S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. lancet Gastroenterol hepatol. 2021;6(7):559–568. doi:10.1016/S2468-1253(21)00109-6
10. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
11. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
12. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
13. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/S1470-2045(20)30011-5
14. Yasuda S, Sho M, Yamato I, et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin Exp Immunol. 2013;172(3):500–506. doi:10.1111/cei.12069
15. Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8(3):299–313. doi:10.2217/imt.15.126
16. Eso Y, Seno H. Synergistic effects of anti-angiogenesis and immune checkpoint blockade - a new era of systemic chemotherapy for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2021;20(5):493–495. doi:10.1016/j.hbpd.2021.04.004
17. Zopf D, Fichtner I, Bhargava A, et al. Pharmacologic activity and pharmacokinetics of metabolites of regorafenib in preclinical models. Cancer Med. 2016;5(11):3176–3185. doi:10.1002/cam4.883
18. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int j Cancer. 2011;129(1):245–255. doi:10.1002/ijc.25864
19. Granito A, Marinelli S, Forgione A, et al. Regorafenib combined with other systemic therapies: exploring promising therapeutic combinations in HCC. J Hepatocell Carcinoma. 2021;8:477–492. doi:10.2147/JHC.S251729
20. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. doi:10.3389/fimmu.2020.583084
21. Zhou D, Luan J, Huang C, Li J. Tumor-associated macrophages in hepatocellular carcinoma: friend or foe? Gut Liver. 2021;15(4):500–516. doi:10.5009/gnl20223
22. Shigeta K, Matsui A, Kikuchi H, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e001435. doi:10.1136/jitc-2020-001435
23. Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5(1):53. doi:10.1186/s40425-017-0257-y
24. Abou-Elkacem L, Arns S, Brix G, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12(7):1322–1331. doi:10.1158/1535-7163.MCT-12-1162
25. Tai WT, Chu PY, Shiau CW, et al. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res. 2014;20(22):5768–5776. doi:10.1158/1078-0432.CCR-14-0725
26. Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. doi:10.1016/S1470-2045(21)00151-0
27. Shigeta K, Datta M, Hato T, et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology. 2020;71(4):1247–1261. doi:10.1002/hep.30889
28. El-Khoueiry A, Kim R, Harris W, et al. Phase Ib study of regorafenib (REG) plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2020;38:564. doi:10.1200/JCO.2020.38.4_suppl.564
29. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
30. Tang W, Chen Z, Zhang W, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5(1):87.
31. Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74(4):2264–2276. doi:10.1002/hep.31840
32. Foerster F, Galle PR. The current landscape of clinical trials for systemic treatment of HCC. Cancers. 2021;13(8):1962. doi:10.3390/cancers13081962
33. Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci. 2021;22(11):5801. doi:10.3390/ijms22115801
34. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, Phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
35. Yau T, Park JW, Finn RS, et al. LBA38_PR - CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874–v5. doi:10.1093/annonc/mdz394.029
36. Facciorusso A, Abd El Aziz MA, Sacco R. Efficacy of regorafenib in hepatocellular carcinoma patients: a systematic review and meta-analysis. Cancers. 2019;12(1):36. doi:10.3390/cancers12010036
37. Zhu H, Zhu X, Song Y, et al. Efficacy and safety of regorafenib alone or in combinations for advanced hepatocellular carcinoma: a multicenter real-world study. J Clin Oncol. 2022;40(16_suppl):e16122–e. doi:10.1200/JCO.2022.40.16_suppl.e16122
38. Huang J, Guo Y, Huang W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2022;9:157–170. doi:10.2147/JHC.S353956
39. Uson Junior PLS, Nagalo BM, Ahn DH, Bekaii-Saab T, Borad MJ. Combination immunotherapy for hepatocellular carcinoma: where are we currently? Semin Liver Dis. 2021;41(2):136–141. doi:10.1055/s-0040-1722646
40. Nakashima M, Ide K, Kawakami K. Comparison of standard initial dose and reduced initial dose regorafenib for colorectal cancer patients: a retrospective cohort study. Target Oncol. 2019;14(3):295–306. doi:10.1007/s11523-019-00642-8
41. Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18(9):2658–2667. doi:10.1158/1078-0432.CCR-11-1900
42. Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50(10):1–9. doi:10.1038/s12276-018-0159-1
43. Li Q, Ni Y, Zhang L, et al. HIF-1α-induced expression of m6A reader YTHDF1 drives hypoxia-induced autophagy and malignancy of hepatocellular carcinoma by promoting ATG2A and ATG14 translation. Signal Transduct Target Ther. 2021;6(1):76. doi:10.1038/s41392-020-00453-8
44. Wang J, Zhang N, Han Q, et al. Pin1 inhibition reverses the acquired resistance of human hepatocellular carcinoma cells to Regorafenib via the Gli1/Snail/E-cadherin pathway. Cancer Lett. 2019;444:82–93. doi:10.1016/j.canlet.2018.12.010
45. Wu RY, Kong PF, Xia LP, et al. Regorafenib promotes antitumor immunity via inhibiting PD-L1 and IDO1 expression in melanoma. Clin Cancer Res. 2019;25(14):4530–4541. doi:10.1158/1078-0432.CCR-18-2840
46. Chen T, Dai X, Dai J, et al. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11(10):822. doi:10.1038/s41419-020-03030-7
47. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
48. Muñoz-Martínez S, Iserte G, Sanduzzi-Zamparelli M, Llarch N, Reig M. Current pharmacological treatment of hepatocellular carcinoma. Curr Opin Pharmacol. 2021;60:141–148. doi:10.1016/j.coph.2021.07.009
49. Bang Y, Yoo C, Lonardi S, et al. Sequential treatment of sorafenib-regorafenib versus sorafenib-physician’s choice: a propensity score-matched analysis. Target Oncol. 2021;16(3):401–410. doi:10.1007/s11523-021-00797-3
50. Ogasawara S, Ooka Y, Itokawa N, et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Japan. Invest New Drugs. 2020;38(1):172–180. doi:10.1007/s10637-019-00801-8
51. Sasaki R, Kanda T, Fujisawa M, et al. Different mechanisms of action of regorafenib and lenvatinib on toll-like receptor-signaling pathways in human hepatoma cell lines. Int J Mol Sci. 2020;21(9):3349. doi:10.3390/ijms21093349
52. Zheng A, Chevalier N, Calderoni M, et al. CRISPR/Cas9 genome-wide screening identifies KEAP1 as a sorafenib, lenvatinib, and regorafenib sensitivity gene in hepatocellular carcinoma. Oncotarget. 2019;10(66):7058–7070. doi:10.18632/oncotarget.27361
53. Rimassa L, Wörns MA. Navigating the new landscape of second-line treatment in advanced hepatocellular carcinoma. Liver Int. 2020;40(8):1800–1811. doi:10.1111/liv.14533
54. Demirtas CO, D’Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3(5):100347. doi:10.1016/j.jhepr.2021.100347
55. Liu Z, Liu X, Liang J, et al. Immunotherapy for hepatocellular carcinoma: current status and future prospects. Front Immunol. 2021;12:765101. doi:10.3389/fimmu.2021.765101
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.