Back to Journals » International Journal of General Medicine » Volume 14
Efficacy and Safety of Regorafenib Monotherapy among Patients with Previously Treated Metastatic Colorectal Cancer in a Chinese Population: A Real-World Exploratory Study
Authors Wang RT, Zhao Y, Wang AL, Wang YT, Yin ZP, Chen K
Received 19 June 2021
Accepted for publication 12 August 2021
Published 7 September 2021 Volume 2021:14 Pages 5363—5373
DOI https://doi.org/10.2147/IJGM.S325545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Rui-Tao Wang, Yang Zhao, An-Lei Wang, Yu-Ting Wang, Zhong-Ping Yin, Kai Chen
Department of Oncology, Tianjin Fourth Central Hospital, Tianjin, 300060, People’s Republic of China
Correspondence: Kai Chen
Department of Oncology, Tianjin Fourth Central Hospital, Tianjin, 300060, People’s Republic of China
Tel +86 22-2624-9182
Email [email protected]
Background: Present study was condeucted to investigate the efficacy and safety of regorafenib for patients with previously treated metastatic colorectal cancer (mCRC) in a Chinese population and the prognostic implications of adverse reactions.
Methods: This retrospective study a total of 96 consecutive patients with mCRC who had failed standard chemotherapy regimens from June 2017 to December 2020. Patients received regorafenib at an initial dosage of 160 mg or 120 mg. The primary end point was progression-free survival (PFS), and secondary end points objective response rate (ORR), disease-control rate (DCR), overall survival (OS), safety, and associations between prognosis and adverse-reaction status.
Results: There were three patients with partial response, 49 with stable disease, and 44 with progressive disease. Consequently, the ORR and DCR of the 96 patients were 3.1% (95% CI 0.6%– 8.9%) and 54.2% (95% CI 43.7– 64.4%), respectively. Prognosis results showed that median PFS of the 96 patients was 2.5 (95% CI 1.98– 3.02) months and median OS 9.8 (95% CI 7.02– 12.59) months. Additionally, the most frequent adverse reactions during regorafenib treatment were hand–foot syndrome (HFS; 52.1%), hypertension (38.5%), and fatigue (33.3%). Interestingly, the relevance of prognosis to adverse-reaction status exhibited that median PFS of patients with HFS and patients without HFS was 3.3 months and 2.0 months, respectively (P=0.013). Similarly, median PFS of patients with hypertension and without hypertension was 3.6 months and 2.2 months, respectively (P=0.023).
Conclusion: Potential clinical benefit of regorafenib monotherapy was observed for patients with mCRC who had failed standard chemotherapy regimens. Hypertension and HFS induced by regorafenib therapy could be used as valuable biomarkers to predict the prognosis of regorafenib.
Keywords: colorectal cancer, regorafenib, efficacy, safety, biomarker
Introduction
Colorectal cancer (CRC) is one of the most common gastrointestinal tumors worldwide. In recent years, the incidence of CRC has been increasing significantly, and it is estimated that there are approximately 1.8 million new cases and 0.86 million deaths each year worldwide.1 Currently, there occur 0.38 million new cases and 0.19 million deaths in China annually.2 For metastatic CRC (mCRC)or unresectable CRC, standard first-line or second-line treatment involves a combination of fluorouracil-based chemotherapy (5-Fu plus either oxaliplatin or irinotecan) and molecularly targeted drugs, such as an anti-VEGF monoclonal antibody (bevacizumab) or an anti-EGFR monoclonal antibody (cetuximab and panitumumab).3 These combination regimens have proved to improve progression-free survival (PFS) and overall survival (OS), with a steady elevation in median OS to approximately 30 months in numerous clinical trials during the last two decades.4 Unfortunately, no standard treatment was available following exhaustion of supply of the aforementioned therapies until 2017. Therefore, patients with mCRC were in urgent need of effective targeted therapeutic drugs following the standard regimens.
Recent years have seen the emergence of antiangiogenic targeted drugs exhibiting potential anticancer activity in the treatment of mCRC.5 Bevacizumab and ramucirumab were the most common antiangiogenic humanized monoclonal antibodies tested for the prevention of VEGF and VEGFR2 extracellular domain expression, and proved to significantly improve PFS and OS as first-line and second-line treatment for patients with CRC in the NO16966 and RAISE clinical trials, respectively.6,7 Regarding antiangiogenic small-molecule tyrosine-kinase inhibitors (TKIs) that modulate growth-factor signaling of angiogenesis, fruquintinib and regorafenib are the only available ones approved for the treatment of chemotherapy-refractory CRC as third-line therapy in China currently.8,9 Regorafenib is an orally available small-molecule TKI that targets signaling pathways involved in angiogenesis (VEGFR1–3), oncogenesis (Kit, RET, Raf1, and BRAF), and the tumor microenvironment (PDGFR and FGFR).10 The CONCUR trial of regorafenib in China included patients aged 50–66 years with mCRC and Eastern Cooperative Oncology Group (ECOG) 0–1 scores who had failed standard treatment, and indicated that regorafenib improved OS and PFS versus placebo, with median OS of 8.8 versus 6.3 months and median PFS of 3.2 versus 1.7 months, respectively.9 This trial suggested that regorafenib could be used as an important treatment option for patients whose disease had progressed after standard treatment.11 However, real-world evidence of regorafenib in patients with mCRC is relatively rare.
Overall response to targeted antiangiogenic drugs is comparatively low. In one study, objective response rates (ORRs) for regorafenib, fruquintinib, apatinib, and anlotinib monotherapy for mCRC were 4.4%, 4.7%, 8.3% and 6.5%, respectively,12 which suggested that great individual differences existed regarding the efficacy of targeted antiangiogenic drugs. Therefore, investigation of biomarkers that can predict the efficacy of regorafenib has been a research hot spot.13 A recent study that investigated the association between hand–foot skin reactions (HFSRs) and efficacy of regorafenib in the treatment of mCRC found that patients with CRC using regorafenib who experienced severe HFSRs had superior prognoses to those without severe HFSRs.14 Therefore, it could be of potential clinical significance to assess the correlation between adverse reactions induced by regorafenib and prognosis of the patients.
Consequently, the aim of the present research was to assess the efficacy and safety of regorafenib monotherapy for patients with previously treated mCRC in the real world and the prognostic implications of adverse-reaction status.
Methods
Design and Eligibility
Given that regorafenib was licensed in China over 3 years ago and many patients with mCRC have since been administered regorafenib after failing standard chemotherapy regimens, this study was designed retrospective. Consequently, consecutive patients with mCRC who had failed standard chemotherapy regimens at the Department of Oncology of Tianjin Fourth Central Hospital from June 2017 to December 2020 were included in this study. The main inclusion criteria were a histological diagnosis of stage IV colon cancer or rectal cancer confirmed by a pathological expert, age ≥18 years, ECOG performance status ≤2, and regorafenib administered for patients who had failed at least two lines of previous systemic chemotherapy, including fluoropyrimidine plus oxaliplatin or irinotecan regimens. Additionally, previous combination therapy with bevacizumab, cetuximab, or panitumumab was permitted but not mandatory, and at least one measurable target lesion according to the response evaluation criteria in solid tumors (RECIST 1.1) to assess tumor response and at least one measurement were required.15 The primary exclusion criterion was previous exposure to regorafenib (however, previous bevacizumab or other antiangiogenic TKI [apatinib, fruquintinib, or anlotinib] administration was permitted) another tumor or serious disease in the opinion of the investigator, and data on efficacy assessment not available. Eventually, a total of 96 patients with mCRC were recruited. A flowchart of the present study is shown in Figure S1). The primary end point was PFS, and secondary end points ORR, disease-control rate (DCR), OS, safety, and association between prognosis and adverse-reaction status. The study protocol was approved by the Ethics Committee of Tianjin Fourth Central Hospital, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from each enrolled patient.
Administration of Regorafenib and Assessment of Efficacy
Although 160 mg was the recommended dosage in the clinical trials of regorafenib for patients with CRC, numerous patients were given an initial dosage of 120 mg in view of the relatively high incidence of grade ≥3 adverse reactions (>50%) that were observed in the CONCUR clinical trial.9 Consequently, regorafenib was administered orally at an initial dosage of 160 mg or 120 mg per day with warm water and continuously on days 1–21 of each 28-day cycle until disease progression or intolerance to treatment. The dosage of 160 mg and 120 mg was up to the investigator and based on the physical condition of the patient. Most patients were treated with regorafenib 120 mg. Additionally, dosage reduction to either 120 mg or 80 mg once daily was permitted in cases of hematological or nonhematological toxicity. Treatment could be permanently discontinued due to severe adverse reactions.
Tumor response and progression status were assessed using RECIST 1.1 criteria.15 Changes in target lesions were evaluated with computed tomography or magnetic resonance imaging every 8 weeks or significant evidence of progression appeared. Adverse reactions during treatment were graded using Common Terminology Criteria for Adverse Events 4.03 to document toxicity profiles that might have been drug-related.16 Exploratory analysis of clinical implications of adverse reactions was mainly focused on the association between hypertension or hand–foot syndrome (HFS) status and PFS of the patients.
Each patient was followed up from enrollment. Firstly, patients were evaluated during regorafenib treatment and performed in the hospitalization. Demographic characteristics, previous treatment regimens, response status, adverse reactions, and progression status were obtained through the electronic medical records. Secondly, subsequent follow-up was mainly carried out on the telephone. Each subject was followed up every month for treatment after regorafenib progression and survival status. The last follow-up date of this study was April 30, 2021.
Statistical Analysis
All variables were analyzed using SPSS 25.0. The significance of proportional and continuous variables for hypertension and HFS status was assessed using χ2 and Mann–Whitney U nonparametric tests, respectively. ORR was the percentage of complete response (CR) and partial response (PR) in all patients. DCR was the percentage of CR and PR and stable disease in all patients.
PFS was defined as the interval from the onset of regorafenib treatment to disease progression or death, whichever occurred first. OS was defined as the interval from the onset of regorafenib treatment to death from any cause.17 For those without progression or death by the end of follow-up, survival end points were censored at the date of last follow-up. Kaplan–Meier curves were drawn using Stata 14.0 to compare differences in PFS according to hypertension or HFS status. Survival differences were calculated using log-rank test. Cox regression analysis was used for PFS in multivariate analysis. P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Baseline characteristics of the patients are presented in Table 1. Initial regorafenib dosage of 160 mg was observed in only 26 patients, with the rest receiving 120 mg. Dosage for each subgroup is exhibited in Table S1. It should be noted that HFS and hypertension were observed in 50 cases (52.1%) and 37 cases (38.5%), respectively. As shown in Table 1, baseline characteristics were similar and well-balanced for HFS status and hypertension status (P>0.05).
 |
Table 1 Baseline Characteristics of Patients with Metastatic CRC According to Hypertension and HFS Status |
Efficacy and Prognosis
Measurable target lesions were graded as per RECIST 1.1. The best overall response of the target lesion in each enrolled patient was recorded and efficacy assessed: CR in zero patients, PR in three, stable disease in 49, and PD in 44. Therefore, the ORR was 3.1% (95% CI 0.6%–8.9%) and DCR 54.2% (95% CI 43.7%–64.4%). A waterfall plot for the best percentage change in target-lesion size is shown in Figure 1. Magnetic resonance imaging of target lesions in one patient with liver metastasis after treatment with regorafenib is shown in Figure 2. Efficacy of regorafenib was satisfactory, and the target lesion was reduced dramatically.
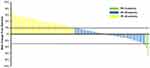 |
Figure 1 Waterfall plot of best percentage change in target-lesion size among atients with previously treated metastatic colorectal cancer. |
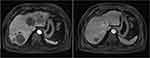 |
Figure 2 MRI scan results of change in target lesions in liver metastasis of one patient with metastatic colorectal cancer after treatment with regorafenib. |
Median follow-up was 9.5 (95% CI 0.2–24) months. At cutoff for analysis of the primary end point PFS, 90 incidents of progression or death had been observed (93.8%). As illustrated in Figure 3, median PFS was 2.5 (95% CI 1.98–3.02) months. OS was also analyzed in view of the relatively long follow-up. At cutoff for analysis of OS, 82 deaths had occurred (85.4%). As shown in Figure 3, median OS was 9.8 (95% CI 7.02–12.59) months. Subsequent therapeutic regimens are presented in Table S2 for 61 patients. Of these, chemotherapy was used in 19 (31.1%), antiangiogenic TKIs (fruquintinib, apatinib, or anlotinib) in 17 (27.9%), PD1 inhibitors in eleven (18%), and traditional Chinese medicine in 14 (23%).
 |
Figure 3 Progression-free survivaland overall survival of patients with previously treated metastatic colorectal cancer. |
To investigate the influence of baseline characteristics on PFS, univariate analysis was carried out. As indicated in Table 2, age and ECOG score werw significantly associated with PFS: median PFS of patients aged <56 years was longer than those aged ≥56 years (3.5 vs 1.8 months, P=0.021), and median PFS of patients with ECOG scores of 0–1 was better than that of patients with a score of 2 score (3.8 vs 1.8 months, P=0.012). It should be noted that patients with right-sided colon cancer showed a trend of shorter PFS than those with left-sided CRC (1.8 vs 2.9 months), though the difference was not statistically significant (P=0.056). Similarly, adenocarcinoma conferred a trend of longer PFS than mucinous adenocarcinoma (2.5 vs 1.8 months, P=0.311).
 |
Table 2 Univariate PFS Analysis of Patients with Metastatic CRC According to Baseline Characteristics |
Safety Profile
No grade 5 adverse reactions were observed, though all patients experienced adverse reactions during treatment. The safety profile is shown in Table 3. Relatively serious grade ≥3 adverse reactions observed were HFS, hypertension, AST/ALT increase, diarrhea, oral mucositis, nausea, vomiting, fatigue, loss of appetite and proteinuria.
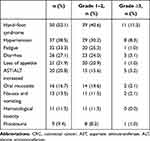 |
Table 3 Safety Profile of Patients with Metastatic CRC Treated with Regorafenib |
Prognostic Implications of Adverse-Reaction Status
As exhibited in Table 3, HFS and hypertension were the most common adverse reactions induced by regorafenib therapy. Therefore, the prognostic significance analysis was mainly concentrated on these. A total of 50 and 37 patients experienced HFS and hypertension during regorafenib monotherapy, respectively. On the other hand, as shown in Table 1, baseline characteristics of patients for HFS and hypertension status were well balanced. Consequently, subsequent analysis of clinical significance of hypertension and HFS was performed. PFS for HFS status is shown in Figure 4. Median PFS of patients with HFS was superior to those with no HFS (3.3 vs 2.0 months, χ2=6.23; P=0.013). Similarly, as illustrated in Figure 5, median PFS of patients with hypertension was longer than for those with no hypertension (3.6 vs 2.2 months, χ2=5.14; P=0.023).
 |
Figure 4 Progression-free survival of patients with previously treated metastatic colorectal cancer by hand–foot syndrome status. |
 |
Figure 5 Progression-free survival of patients with previously treated metastatic colorectal cancer by hypertension status. |
Multivariate analysis using Cox regression for PFS was performed on baseline characteristics that were significant on univariate analysis to adjust confounding factors. As illustrated in Table 4, statistically significant differences remained for HFS and hypertension, which suggested that HFS and hypertension were independent factors in PFS (HR 0.65, P=0.024; HR 0.67, P=0.035). ECOG score and age were also independent factors in PFS (HR 1.96, P=0.021; HR 1.72, P=0.033).
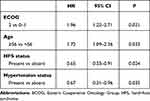 |
Table 4 Multivariate Cox Regression Analysis of PFS According to Baseline Characteristics and Adverse-Reaction Status |
Discussion
To the best of our knowledge, this study is the first to provide and highlight real-world evidence on the efficacy and safety of regorafenib for patients with mCRC who have failed standard regimens. Prognostic significance indicated that the most common adverse reactions of HFS and hypertension induced by regorafenib therapy could be used as potential biomarkers for prognostic prediction in patients receiving regorafenib therapy.
Regarding the efficacy of regorafenib, ORR and DCR were 3.1% and 54.2%, respectively, consistent with that the phase III CONCUR clinical trial of regorafenib for mCRC (ORR 4% and DCR 51% in the regorafenib arm). Actually, a relatively low response to regorafenib has been observed among clinical trials irrespective of ethnicity, with ORR of 0–6.4% and DCR of 15%–57%.18 Consequently, this highlights the common features of antiangiogenic small-molecule TKIs and the need to explore potential biomarkers to guide regorafenib therapy.19 However, median PFS in our study was 2.5 months. This was slightly shorter than the CONCUR trial (3.2 months). We speculated that the discrepancy could be attributed to the retrospective design of our study. Management of patients with mCRC in a retrospective study was not sufficient or normative compared with a well-designed phase III clinical trial, which was demonstrated in another retrospective study of regorafenib in mCRC.20 On the other hand, attention should be paid to the influence of ECOG scores. Patients with ECOG 2 in our study accounted for approximately 50%. As far as we know, ECOG score is one of the most significant prognostic factors in malignant tumors, and poor scores are associated with worse prognosis.21 Results of Cox regression analysis suggested that patients ECOG 2 was independently associated with worse PFS, consistent with the results of a previous study.22 Ducreux et al studied 1,037 patients with mCRC in a real-world study to investigate the safety and effectiveness of regorafenib in routine clinical practice,23 finding that median PFS was 2.9 (95% CI 2.8–3.0) months, consistent with the PFS in our study. The CONSIGN study explored the efficacy and safety of regorafenib for patients with mCRC who had progressed after standard therapy.24 Medical evidence from 2,872 patients showed that median PFS was 2.7 (95% CI 2.6–2.7) months, also consistent with our study. This suggests that regorafenib has potential efficacy for patients with previously treated mCRC to some extent. Interestingly, we also observed that patients with mucinous adenocarcinoma tended to have poorer PFS than those with adenocarcinoma, consistent with Mekenkamp et al.25 They studied 1,010 mCRC patients who had been treated with chemotherapy and targeted therapies in two phase III studies. Mucinous adenocarcinoma (99 patients) was associated with worse ORR, PFS, and OS. However, it should be noted that mucinous adenocarcinoma usually correlateds with higher proportion of deficient mismatch repair,26 which suggested that patients with mucinous adenocarcinoma might benefit from immunotherapy.
Notably, median OS was 9.8 (95% CI 7.02–12.59) months, longer than that observed in the CONCUR (8.8 months of regorafenib arm) and CORRECT trial (6.4 months of regorafenib arm) trials.9,27 We speculate that one explanation could be the difference in previous exposure to targeted drug treatment. In our study, 39.6% patients had been treated with targeted drugs (VEGF-targeted or EFGR-targeted or antiangiogenic TKIs). However, 60% and 100% patients had previously been given VEGF-targeted or EGFR-targeted biological drugs in CONCUR and CORRECT, respectively. Subgroup analysis of OS indicated that patients who were not treated with targeted biological therapy seemed to derive greater benefit from regorafenib than those who had been administered with previous targeted drug treatment.9 In a retrospective study of 29 patients with mCRC where 90% were treated with regorafenib therapy and had previous bevacizumab exposure, median OS of 6 (95% CI 5–8) months was observed.28 Therefore, previous targeted drug exposure could determine worse prognosis. However, this explanation should be interpreted with caution, given that no prospective study was available to confirm it. Another reason that longer OS was observed in our study could have been the continuous licensing since 2018 of immunotherapy drugs (pembrolizumab and nivolumab) and targeted antiangiogenic TKIs (fruquintinib), which can be effective as subsequent line treatment for patients with mCRC in China.8,9,29 Fruquintinib become a new standard third-line treatment for patients with mCRC in China in the FRESCO clinical trial.30 Consequently, immunotherapy and fruquintinib are available for patients with chemotherapy-refractory mCRC after progression with regorafenib, and contributed to survival benefit.31
Given the relatively high incidence (54%) of grade ≥3 adverse reactions from the 160 mg initial dosage of regorafenib in the phase III clinical trial into consideration, we chose 120 mg as an alternative initial regorafenib dosage. Similar initial dosage was also observed in another retrospective study and clinical practice.20 Common adverse reactions of patients with mCRC receiving regorafenib treatment were HFS, hypertension, AST/ALT increase, diarrhea, oral mucositis, nausea, vomiting, fatigue, loss of appetite, and proteinuria, consistent with a previous retrospective study of regorafenib.28 No new adverse reactions were detected during the study, which demonstrated that regorafenib at an initial dosage of 160 mg or 120 mg can be safe. Overall incidence of adverse reactions in our study was relatively lower than in the CONCUR trial, and we speculated that the difference might be attributed to the retrospective design of our study. A previous retrospective study indicated that adverse reactions had been documented poorly and insufficiently compared to a phase III trial.17 However, it should be noted that HFS incidence seem to be higher in Asian populations. All-grade HFS incidence in CORRECT (non-Asian population) was 47%, while all-grade HFS incidence in CONCUR (Asian population) was 73%. Another exploratory study among Japanese found that all-grade HFS incidence was 81.4%, and most of the patients had been administered 160 mg regorafenib.14 Though most patients in our study were administered 120 mg, all-grade HFS incidence was 52.1%. It would seem that HFS incidence is higher in Asian populations than that in non-Asian ones. However, this conclusion should be validated in large-scale prospective clinical trials.
Interestingly, the prognostic significance of adverse reactions in our study suggested that hypertension and HFS induced by regorafenib could be used as potential biomarkers to predict the prognosis of regorafenib treatment, which is in line with ae previous study of the association between HFS and regorafenib efficacy in the treatment of mCRC.14 Similarly, a recent study using exploratory analysis to investigate the influence of HFS or hypertension on the efficacy of another antiangiogenic small-molecule TKI, anlotinib, for elderly patients with small-cell lung cancer32 demonstrated that hypertension and HFS might confer superior PFS to no hypertension and HFS, consistent with the clinical significance of hypertension and HFS in our study. To our knowledge, hypertension and HFS are the most common adverse reactions associated with antiangiogenic inhibitors affecting on the VEGF pathway, including bevacizumab, sorafenib, and sunitinib.33 A previous study indicated that the mechanism of hypertension could be attributed to the fact that inhibition of VEGFR in vascular endothelial cells might decrease the production of nitric oxide and prostacyclins, thus contributing to increased blood pressure.34 Analogously, HFS might result from decreased reconstruction of skin after restriction of angiogenesis induced by antiangiogenic therapy.35 Therefore, hypertension or HFS induced by regorafenib might reflect inherent host biology that causes differences in VEGF/VEGFR blockade to some extent, thus serving as potential biomarkers for efficacy prediction. However, this conclusion should be confirmed in large-scale prospective clinical trials. More importantly, we believe that focus should be placed on managing hypertension or HFS induced by regorafenib therapy instead of interruption of the treatment when these occur during clinical regorafenib administration.
Conclusion
This retrospective study provides real-world evidence of the superior efficacy and tolerable safety of regorafenib for Chinese patients with mCRC who have failed standard regimens. Hypertension and HFS induced by regorafenib therapy could be used as potential biomarkers to predict prognosis with regorafenib administration.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Bai M, Li ZG, Ba Y. Influence of KDR genetic variation on the efficacy and safety of patients with chemotherapy refractory metastatic CRC who received apatinib treatment. Int J Gen Med. 2021;14:1041–1055. doi:10.2147/ijgm.s300968
3. Ku G, Tan IB, Yau T, et al. Management of colon cancer: resource-stratified guidelines from the Asian Oncology Summit 2012. Lancet Oncol. 2012;13(11):e470–481. doi:10.1016/s1470-2045(12)70424-2
4. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, Phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi:10.1016/s1470-2045(14)70330-4
5. Papachristos A, Kemos P, Kalofonos H, Sivolapenko G. Correlation between bevacizumab exposure and survival in patients with metastatic colorectal cancer. Oncologist. 2020;25(10):853–858. doi:10.1634/theoncologist.2019-0835
6. Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized Phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26(12):2006–2012. doi:10.1200/jco.2007.14.9898
7. Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi:10.1016/s1470-2045(15)70127-0
8. Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO Randomized Clinical Trial. JAMA. 2018;319(24):2486–2496. doi:10.1001/jama.2018.7855
9. Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–629. doi:10.1016/s1470-2045(15)70156-7
10. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129(1):245–255. doi:10.1002/ijc.25864
11. Jing Z, Rui Z, Binglan Z. A comparison of regorafenib and fruquintinib for metastatic colorectal cancer: a systematic review and network meta-analysis. J Cancer Res Clin Oncol. 2019;145(9):2313–2323. doi:10.1007/s00432-019-02964-6
12. Wang F, Yuan X, Jia J, et al. Apatinib monotherapy for chemotherapy-refractory metastatic colorectal cancer: a Multi-centre, Single-Arm, Prospective Study. Sci Rep. 2020;10(1):6058. doi:10.1038/s41598-020-62961-5
13. Liu X, Qin S, Wang Z, et al. Correction to: early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol. 2018;11(1):5. doi:10.1186/s13045-017-0545-5
14. Kobayashi K, Kawakami K, Yokokawa T, et al. Association of hand-foot skin reaction with regorafenib efficacy in the treatment of metastatic colorectal cancer. Oncology. 2019;96(4):200–206. doi:10.1159/000495989
15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
16. Miller TP, Fisher BT, Getz KD, et al. Unintended consequences of evolution of the common terminology criteria for adverse events. Pediatr Blood Cancer. 2019;66(7):e27747. doi:10.1002/pbc.27747
17. Cheng JD, Chai LX, Zhao ZP, Hao YY, Li S. Efficacy and safety of anlotinib for patients with advanced NSCLC who progressed after standard regimens and the preliminary analysis of an efficacy predictor. Cancer Manag Res. 2020;12:5641–5650. doi:10.2147/cmar.s253366
18. Røed Skårderud M, Polk A, Kjeldgaard Vistisen K, Larsen FO, Nielsen DL. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a systematic review. Cancer Treat Rev. 2018;62:61–73. doi:10.1016/j.ctrv.2017.10.011
19. Suenaga M, Schirripa M, Cao S, et al. Gene polymorphisms in the CCL5/CCR5 pathway as a genetic biomarker for outcome and hand-foot skin reaction in metastatic colorectal cancer patients treated with regorafenib. Clin Colorectal Cancer. 2018;17(2):e395–e414. doi:10.1016/j.clcc.2018.02.010
20. Aljubran A, Elshenawy MA, Kandil M, et al. Efficacy of regorafenib in metastatic colorectal cancer: a Multi-institutional Retrospective Study. Clin Med Insights Oncol. 2019;13:1179554918825447. doi:10.1177/1179554918825447
21. Patel A, Sharma MC, Mallick S, Patel M, Bakhshi S. Poor performance status, urban residence and female sex predict inferior survival in pediatric advanced stage mature B-NHL in an Indian tertiary care center. Pediatr Hematol Oncol. 2018;35(1):23–32. doi:10.1080/08880018.2018.1424279
22. Kopeckova K, Buchler T, Bortlicek Z, et al. Regorafenib in the real-life clinical practice: data from the Czech Registry. Target Oncol. 2017;12(1):89–95. doi:10.1007/s11523-016-0458-1
23. Ducreux M, Petersen LN, Öhler L, et al. Safety and effectiveness of regorafenib in patients with metastatic colorectal cancer in routine clinical practice in the prospective, observational CORRELATE study. Eur J Cancer. 2019;123:146–154. doi:10.1016/j.ejca.2019.09.015
24. Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for Patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label Phase IIIb CONSIGN Study. Oncologist. 2019;24(2):185–192. doi:10.1634/theoncologist.2018-0072
25. Mekenkamp LJ, Heesterbeek KJ, Koopman M, et al. Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer. 2012;48(4):501–509. doi:10.1016/j.ejca.2011.12.004
26. Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39(1):13. doi:10.1186/s40880-019-0361-0
27. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi:10.1016/s0140-6736(12)61900-x
28. Calcagno F, Lenoble S, Lakkis Z, et al. Efficacy, safety and cost of regorafenib in patients with metastatic colorectal cancer in French clinical practice. Clin Med Insights Oncol. 2016;10:59–66. doi:10.4137/cmo.s38335
29. Franke AJ, Skelton WP, Starr JS, et al. Immunotherapy for colorectal cancer: a review of current and novel therapeutic approaches. J Natl Cancer Inst. 2019;111(11):1131–1141. doi:10.1093/jnci/djz093
30. Ganesh K, Stadler ZK, Cercek A, et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16(6):361–375. doi:10.1038/s41575-019-0126-x
31. Rawla P, Barsouk A, Hadjinicolaou AV, Barsouk A. Immunotherapies and targeted therapies in the treatment of metastatic colorectal cancer. Med Sci (Basel). 2019;7(8):83. doi:10.3390/medsci7080083
32. Song PF, Xu N, Li Q. Efficacy and safety of anlotinib for elderly patients with previously treated extensive-stage SCLC and the prognostic significance of common adverse reactions. Cancer Manag Res. 2020;12:11133–11143. doi:10.2147/cmar.s275624
33. Cabebe E, Wakelee H. Role of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol. 2007;8(1):15–27. doi:10.1007/s11864-007-0022-4
34. Tang JR, Markham NE, Lin YJ, et al. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287(2):L344–351. doi:10.1152/ajplung.00291.2003
35. Fischer A, Wu S, Ho AL, Lacouture ME. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2013;31(3):787–797. doi:10.1007/s10637-013-9927-x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
